heterogeneous catalysis
(particulate, surface [solid] catalysis) is one of three different kinds of ▸▸▸catalysis; the others are ▸▸▸homogeneous and ▸▸▸biocatalysis. Het. catalysis (hc.) is defined as 1: Catalysis with the cat. in a different ▸▸▸phase than the reactants, and 2: With the cat. on solid, ▸▸▸porous material or impregnated in such material. (The term “solid” does not exclude mobile portions such as potassium cpds. in ▸▸▸steam reforming cats. or Cl in ▸▸▸isomerization cats; cf. ▸▸▸chlorinated alumina). The complexity of het. catalysts is depicted in Figure 1.
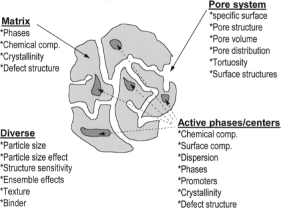
Whereas the cat. is solid, the reactant and the prod. phase may be liquid or gaseous, thus determining the ▸▸▸chemical engineering of the cat. reactions (cf. ▸▸▸catalytic reactors). The complexity of het. cat. results from its multi-dimensional nature in time and in length and the dynamics of the system catalyst-reactant (Figure 2).
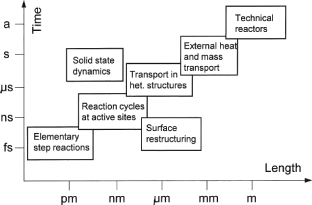
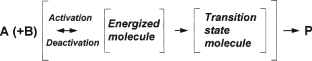
Het. cat. reactions take place on the ▸▸▸surface of the solid cats. The following sequences of phenomena are significant: ▸▸▸adsorption of the reactants (▸▸▸physisorption and eventually ▸▸▸chemisorption), mono- or bimolecular reaction of the chemisorbed reactants with other adsorbed species or with reactants in the gas phase, and the ▸▸▸desorption of the prods. (cf. ▸▸▸diffusion model of catalysis). Adsorbed molecules are activated by interaction with the cat. surface (which ought normally to be as great as possible) and thus reducing the energy barrier or ▸▸▸activation energy (cf. diagram under entry ▸▸▸activation energy; compare also ▸▸▸Langmuir-Hinshelwood kinetics, ▸▸▸thermodynamics in cat., ▸▸▸Arrhenius plot, ▸▸▸isotherms, ▸▸▸mass and ▸▸▸heat transfer, ▸▸▸single crystals, ▸▸▸active sites, etc.; Figure 3 for the reaction A+ B → P acc. to Wijngaarden et al.).
After desorption the active sites on the surface regenerate and are ready for a new ▸▸▸catalytic cycle.
Acc. to the ▸▸▸shape of the het. cats. (e.g., granules, ▸▸▸gauzes, ▸▸▸pellets, ▸▸▸extrudates, rings, etc.) various chemical engineering solutions of the problem of transporting reactants to the cat. particles and the prods. away from it have been realized (e.g., ▸▸▸fixed-bed, ▸▸▸slurry, ▸▸▸gauze, entrained, tubular ▸▸▸fluidized-bed, bubble columns, etc.) in ▸▸▸autoclaves, ▸▸▸CSTR, ▸▸▸batch, or other reactors. The interaction of gases, liquids, and surfaces on solids is extremely complex and thus difficult to treat with chemical ▸▸▸kinetics and reaction engineering. The understanding of het. cat. is fragmentary (cf., e.g., ▸▸▸pressure gap, ▸▸▸material gap) and needs experienced specialists. This is one of the important disadvantages of het. cat. The others are that the variation of electronic properties of the cats. (▸▸▸tailoring) is more or less empirical and that the mechanistic understanding of the cat. procs. on the surfaces is largely hypothetical. Novel techniques for tailoring of het. cats. take 3D ▸▸▸morphological methods including “▸▸▸nanoengineering” (also “colloidal nanoparticles”) into account. The belief that an optimal het. cat. for various synths. is also the optimal decomposition cat. is anti-quated.
Nevertheless, the use of het. cats. is essential: their ▸▸▸recycling (i.e., the start of a new cat. cycle) proceeds without any problems because the separation of products and cat. is simple (in contrast to ▸▸▸hom. cat.) and the catalyst ▸▸▸lifetimes are high. Examples of industrial procs. with het. cats. are compiled in Table 1 (see next page). Selected heterogeneous catalysts of industrial importance and the reactors applied are compiled in Table 2 (see next page).
The handling of het. cats. is normally simple also: a bunch of decisive reasons which explain why commercially approx. 80 % of all cat. procs. are run with het. cats. (cf. ▸▸▸catalysis).
There are various ways of merging ▸▸▸het. catalysis and ▸▸▸biocatalysis (▸▸▸enzyme catalysis), especially for the prod. of ▸▸▸bulk and ▸▸▸commodity chemicals from renewable feedstocks. A recent example is the ▸▸▸Cetus PO process which includes the enzymatically cat. ▸▸▸oxidation of ▸▸▸glucose and the heterogeneous (with Pd cats.) destruction of the ▸▸▸by-prod. hydrogen peroxide (cf. ▸▸▸hybrid catalyst).
The ww markets for het. cats. acc. to their application comprise 22 % (equal to 2.1·109 Euros per annum) for petrochemical uses, 24 % (2.3·109) for refinery techniques, 25 % (2.4·109) for ▸▸▸polymerizations, and 29 % (2.7·109) for environmental uses; cf. also ▸▸▸catalyst market.
F catalyse hétérogène; G heterogene Katalyse.
Table 1
| Basic conversion | Process and products |
| Addition reactions | Ethanol from ethylene |
| Alkylation | Ethyl benzene; polymeric olefins, poly gasolines; cumene |
| Amine syntheses | Aminations |
| Ammonolysis | |
| Amm(on)oxidation | SOHIO process, nitriles |
| Carbon monoxide conversions | Fischer-Tropsch synthesis |
| Methanation, methanol synthesis, steam reforming | |
| Catalytic reforming | Gasoline |
| Claus process | Removal of S |
| Conversion of hcs. | Steam reforming |
| Cracking | CC, DCC, catalytic cracking |
| Dehydration | Ethylene from ethanol |
| Dehydrogenation | Styrene from ethylbenzene, propylene from propane, paraffin d. |
| Environmental catalysis | Automotive exhaust cats. |
| DeNOx procs. | |
| Plastic waste work-up | |
| Hydrodealkylation, hydrodemetallation, hydrodenitrogenation, hydrodesulfurization, deep d., etc. | |
| Claus process | |
| Hydrogenation | Ammonia synthesis |
| H. of CO (methanol synthesis), | |
| H. of CO (Fischer-Tropsch synthesis), | |
| Aliphatic or aromatic aldehydes to alcohols (butanol), alkenes to hcs. Hydrocracking, HDS, HDN, fat hardening | |
| Isomerization | Butene isomerization, Toluene isomerization, Disproportionations |
| Oligomerization | Polymeric olefins, Polymer gasoline |
| Oxidation | Ethylene oxide, PSA from o-xylene or naphthalene, acrolein or acrylic acid from propene, ammoxidation (to acrylonitrile), formaldehyde from MeOH, sulfuric acid from SO2, sulfoxidation |
| Oxychlorination | Manuf. of vinyl chloride |
| Metathesis | Procs. of CdF, IFP, Phillips, Shell |
| Polymerization | Ziegler-Natta catalysis |
| Syngas chemistry | Fischer-Tropsch synthesis |
Table 2
| Catalyst (support/promoter) | Reactor type | Reaction |
| Ni (SiO2) | Slurry, batch | Hydrogenation of fats and oils |
| Ni (SiO2, Al2O3) | Fixed bed | Hydr. of aliphatic aldehydes |
| Trickle bed | Carbonyls ▸▸▸ alcohols | |
| Fluidized bed | Methanation | |
| Pt, Pd (carbon) | Slurry, batch | Prep. of fine chemicals |
| Pd (Al2O3) | Fixed bed | Hydrog. of alkynes, dienes |
| Fe (promoter) | Fixed bed | Ammonia synthesis |
| Fe | Slurry phase | Hydrog. of coal |
| Fe or Co (support) | Fixed bed or entrained bed | Fischer-Tropsch synthesis |
| Cu/ZnO (Al2O3) | Fixed bed | Methanol synthesis |
| Mo or W sulfides | Fixed bed | Hydrodesulfurization |
| Ni (support) | Fixed bed | Steam reforming |
| ZnO | Fixed bed | Dehydrogenation of sec. alcohols to ketones |
| Cr2O3 (Al2O3) | Fixed bed with regeneration | Lower alkenes from alkanes |
| Fe2O3 (promoters) | Fixed bed | Styrene from ethylbenzene |
| V2O5 (promoter, SiO2) | Fixed bed | Oxidation of SO2 |
| V2O5 (titania) | Fixed bed | Xylene ▸▸▸ carboxylic acid anhydrides; SCR cats. |
| Bi/Mo | Fixed bed | Propylene ▸▸▸ acrolein/acrylic acid Ammoxidation reactions |
| Clays, zeolites | Fluidized bed | FCC process |
| Pt/Pd/Rh | Gauze reactor | Oxidation of ammonia |
| Pt/Rh (honeycombs) | Fixed bed | Automotive exhaust cats. |
| Ag (SiO2 or Al2O3, promoter) | Fixed bed | Epoxidation of ethylene, Dehydrogenation of methanol |
| Pt (chlorided alumina) | Fixed bed | Isomerizations |
| HF, H2SO4 | Fixed bed, riser-tube, or multiphase | Alkylation |
| Metals (zeolites) | Fixed bed | Hydrocracking |
| Ion sieves | Fixed bed | Etherification |
| H3PO4 (support) | Fixed bed | Polymerizations of alkenes |
| Al2O3 | Fixed bed | Claus process |
| Al, Ti (support) | Batch | Polymerization |



