Tobacco
Abstract
January of 1983 was a turning point for plant biotechnology when tobacco was immortalized as a surrogate biological system for testing gene function at a conference on “Advances in Gene Technology: Molecular Genetics of Plants and Animals” hosted by the Miami Winter Symposia series. Although Arabidopsis has now become the system of choice for nuclear gene integration due to the ease of transformation and a short generation cycle, tobacco remains the only established system for plastid transformation. This review summarizes the use of tobacco in dissecting plant biology concepts pertaining to the three important compartments of the cell that harbor genetic material within them. Recent studies in N. benthamiana have brought the genus back to the limelight as an outstanding system for transient protein expression. Overall, this chapter also brings out the advantages and limitations of tobacco as a system for discovery in plant biology. As a nonfood and nonfeed crop tobacco retains a remarkable potential for use as a biofactory. Ironically, this genus with a notorious health reputation may prove to be indispensable for the production of medically relevant compounds.
1 Introduction
In January of 1983, the Miami Winter Symposia series hosted the conference on “Advances in Gene Technology: Molecular Genetics of Plants and Animals.” This conference would serve as a forum for unveiling one of the milestones in plant biology research—the introduction of a bacterial gene into a plant species. Two groups, one led by Mary-Dell Chilton and another led by Jeff Schell and Marc Von Montagu presented evidence that a bacterial gene for antibiotic resistance had been successfully integrated into the genome of tobacco plants. Since this seminal breakthrough, tobacco has become a widely used system for studies of gene function. It is the established system for plastid transformation; however, Arabidopsis has become the system of choice for nuclear gene integration due to the ease of transformation and a short generation cycle. This review summarizes the use of tobacco in dissecting plant biology concepts pertaining to the three important compartments of the cell that harbor genetic material within them. The chapter begins with familiarizing the reader with the sequence of events that led to the development of tobacco regeneration and transformation systems. Next, the utility of the transgenic tobacco system is discussed, detailing the seminal research that enlightened a new understanding of gene function based on important in planta observations.
2 Transgenic Research in Tobacco
2.1 Development of a Regeneration System and the Discovery of Cytokinins
Tobacco and carrot are the original model systems for plant tissue culture. While regeneration by somatic embryogenesis was first demonstrated with carrot cultures, the principles underlying regeneration via organogenesis were developed with tobacco. Tobacco tissue culture also has a historic role in hormone biology as the bioassay system used in the discovery of cytokinins in Folke Skoog's lab at the University of Wisconsin-Madison. Cell division factors could not have been discovered without a system in which their effects would be easily and unambiguously ascertained. Moreover, efficient regeneration is a prerequisite for transformation in plants. By virtue of its effective regeneration protocol, tobacco was the first plant to be transformed and has for many years been an important model plant for testing of transgenes.
2.1.1 Historical perspective: regeneration and the discovery of cytokinins
Early work on plant tissue culture focused on enticing cells to divide in culture. Important results were published by three researchers in the 1930s. Nobécourt (1938, 1939) and Gautheret 1939 in France reported on the maintenance of carrot callus cultures while White 1939a in the United States concentrated his research on tobacco. It should be noted that White's tobacco cultures were derived from the hybrid Nicotiana glauca × Nicotiana langsdorfii, which is prone to form tumors in planta. White 1939a showed that when these tumor tissues are placed in vitro, cell division could be maintained. Although this study only demonstrated the ability of naturally tumorous tissues to continue to divide in vitro, it did set the stage for further experiments using cultured tobacco tissues, eventually leading to the discovery of cytokinins and their role in organogenesis.
White 1939b observed the occasional formation of shoots on tumorous tissues submerged in liquid medium. Skoog, whose research had focused on auxins, showed that the tumorous tissues contained high levels of β-indoleacetic acid (IAA) and that addition of IAA to the liquid culture medium suppressed the sporadic formation of buds in these tissues (Skoog, 1944). Roots appeared in a few of the cultures, always attached to the basal end of shoots (Skoog, 1944). This was the first demonstration of complete plantlet formation from callus tissue.
Subsequent research was performed with Nicotiana tabacum W38, a genetically normal tobacco variety as opposed to the tumorous plants employed by White, which would infrequently form callus, roots, and buds on stem segments in culture. It was also observed that callus formation could be stimulated and bud formation suppressed by exogenous auxin (Skoog and Tsui, 1948). Interestingly, addition of adenine sulfate could overcome this inhibitory effect of IAA but to accomplish that, very high adenine levels were required (Skoog and Tsui, 1948). These observations led the authors to conclude that “formation of roots, buds, or undifferentiated growth of tissues can be obtained by the application of different proportions of auxin and adenine to the medium”. This was the first clear support for the concept that relative levels of growth factors control plant differentiation.
Pith tissue, which is rich in parenchyma but lacks vascular bundles, responded poorly to IAA but cell division was highly stimulated when natural extracts, such as coconut or malt extract, were added (Jablonski and Skoog, 1954). The biologically active fraction in yeast extract was isolated by Carlos Miller and found to be rich in purines (Skoog, 1994), which was consistent with the effects of adenine observed earlier. Further efforts by Miller in Skoog's lab focused on isolation of purines from natural substances and testing for their activity in tobacco cultures. The most potent substance was contained in autoclaved herring sperm DNA. The active compound was identified as N-furfuryladenine and its structure was subsequently confirmed by its chemical synthesis (Miller et al., 1955a, 1955b, 1956). The compound was given the name “kinetin” and was the first of a new class of plant growth regulators, named cytokinins for their ability to promote cytokinesis (Skoog et al., 1965).
The discovery of kinetin led to an upsurge in plant tissue culture research. Skoog and Miller 1957 showed in the classical experiment that kinetin and auxin together could give rise to shoots, roots, or callus, depending on the concentrations and ratios between the two types of compounds. High kinetin with low IAA resulted in shoot formation, low kinetin with high IAA in root formation, and high concentrations of both in callus formation. Since that time, this principle was found to apply generally to regeneration of many plant genera through organogenesis. Application of plant growth regulators to control cell division and differentiation is used as an essential tool for plant biotechnology, from micropropagation to transformation.
Related research resulted in further refinements in tissue culture methods. To optimize callus growth, a chemically defined formulation was devised based on analysis of ash from tobacco tissues (Murashige and Skoog, 1962). Further tests of organic nutrient requirements indicated that besides auxin and cytokinin, the only compounds necessary for tobacco tissue cultures, are sucrose, myo-inositol, and thiamine (Linsmaier and Skoog, 1965). This Murashige and Skoog (MS) mineral nutrient medium is still the most versatile and widely used plant tissue culture medium. Although many modified mineral media were developed after 1962 and given different names, they were often based on the MS medium. For more details on the development of the tobacco tissue culture system and the discovery of cytokinins, the reader is referred to Skoog 1994 and Armstrong 2002.
2.1.2 Development of a transformation system
Tobacco transformation was made possible by availability of a regeneration system as well as years of research on Agrobacterium, starting with the demonstration by Braun 1958 that tobacco crown gall tissues could grow in the absence of cytokinin and auxin even when Agrobacterium was eliminated from the tissues. This clearly showed that the tumor-inducing factors were maintained in the tissues even after many cell divisions. It should be noted that tobacco is an excellent host for Agrobacterium, forming large galls upon infection of stems, and has been used widely for crown gall research. An extensive treatise of Agrobacterium-based vector development is beyond the intention of this chapter and only a few of the important advances leading to tobacco transformation will be highlighted.
Two milestones on the road to transformation were the isolation of the Agrobacterium Ti plasmid (Zaenen et al., 1974) and the demonstration that bacterial DNA was inserted in the DNA of host cells (Chilton et al., 1977). They formed the basis for the identification of the essential genes inside as well as outside the transferred region and for the design of modified vectors for plant transformation. Also crucial to development of suitable gene vectors was incorporation of selectable markers, such as resistance to kanamycin (Bevan et al., 1983; Fraley et al., 1983; Herrera-Estrella et al., 1983), which made it possible to delete the tumor-forming genes. These and many other important contributions culminated in production of transformed tobacco and petunia plants that developed normally and formed progeny segregating for the transgene in a Mendelian fashion (De Block et al., 1984; Horsch et al., 1984). The procedures used to generate the transformants involved co-cultivation with protoplasts.
The tobacco protoplast transformation system was soon replaced by a much simpler system involving incubation of leaf discs with Agrobacterium (Horsch et al., 1985, 1988). Tobacco leaf discs respond very well to cytokinin and auxin, just like the pith or callus tissues used by Skoog and Miller 1957. Shoots, roots, and callus can be obtained on appropriate combinations of the two hormones (Figure 1). Shoot formation at optimal growth regulator concentrations is very prolific, which is essential to efficient transformation. Moreover, callus formation is limited at concentrations used for shoot formation and thus the occurrence of somaclonal variants is minimized.
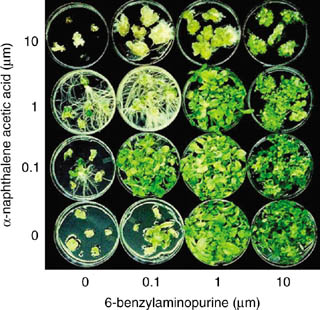
Interaction between α-naphthaleneacetic acid (NAA) and N6-benzyladenine (BAP) in formation of roots, shoots, and callus from N. tabacum leaf discs
At about the same time as Agrobacterium-mediated systems were established, success was also reported on tobacco transformation by direct gene transfer with small plasmids (Paszkowski et al., 1984). Direct gene transfer methods have, however, lower success rates and insertion into plant DNA is less precise, accompanied with more rearrangements. For practical purposes Agrobacterium-mediated transformation of leaf discs has remained the method of choice. An exception is transformation of chloroplasts, for which direct transformation is applied (discussed in Section 2.5).
2.1.3 The use of tobacco haploids to obtain lines homozygous for multiple inserts
N. tabacum is a disomic tetraploid, meiotically and genetically behaving like a diploid. As with most other species, there is an inherent problem when the objective is to generate transformed lines with multiple inserts (Smith et al., 1994; Lee et al., 2003). Although lines with more than one insert are often found after tobacco transformation, as indicated by ratios of 15:1, 63:1, etc., in the T1, only one locus needs to be homozygous to render a T2 completely resistant to the selective agent. Such lines are generally still segregating for the other insert loci and several further generations need to be characterized by extensive Southern analyses to identify completely homozygous lines for three or four insert loci.
Transformation of haploid tobacco followed by doubling of the chromosome number is an alternative and much quicker method for obtaining homozygous lines with multiple inserts. Haploid plants can be obtained through several routes (Nitsch and Nitsch, 1969; Burk et al., 1979) and haploid materials are usually maintained as micropropagated shoots. Leaf discs from these shoots can be used for Agrobacterium-mediated transformation as described before for tetraploid tobacco, resulting in formation of transformed haploid shoots. The plants obtained after rooting of these shoots are normal but sterile. Interestingly, cultured midrib segments from fully expanded leaves can give rise to doubled haploid shoots whereas those from younger leaves only yield haploid shoots (Kasperbauer and Collins, 1972). Most of the plants (about 70%) obtained from the older leaves have doubled chromosome numbers and can be distinguished from haploids by their fertility. Progeny from each fertile plant should be homozygous for all transgenes.
The only disadvantage of this method resides in the fact that the number of independent inserts is initially unknown; however, Southern analyses after digestion with a restriction enzyme that does not cut the insert will quickly determine the number of independent inserts in each line. Overall, this procedure results in significant savings in time and effort.
2.2 Transgenic Tobacco and Light-Regulated Gene Expression
Not long after the introduction of transgenes into tobacco was published, several groups aggressively began functional characterization of promoter sequences in planta. Of particular interest were regulated promoters, especially those where activity was modulated by light. Light is essential for plant metabolism; therefore, it is little wonder that light also has significant roles in regulating plant growth and development. In this way plant form and composition are best adjusted to capture and utilize ambient light energy. Because of the clear importance of light in affecting plant biology, scientists have carefully studied the effects of specific wavelengths, intensities, and photoperiods on plant processes of agricultural or research interest. Before the widespread availability of molecular tools and techniques, hundreds if not thousands of careers were well spent characterizing the physiology associated with light developmental processes, the biochemical and spectral properties of regulatory pigments and the components that comprises transduction schema. While these scientists postulated the properties of various photosensors and signaling networks, their efforts preceded the availability, utility, and agility of Arabidopsis as a transformable genetic system.
In these cases, transgenic tobacco served as an important transitional system, owing to its ease of transformability and ability to produce ample resources for meaningful study in a short time. Transgenic tobacco was the structure–function workhorse, the best system to test various hypotheses of gene form and function throughout the 1980s and into the 1990s. With the advent of simple “floral dipping” (Clough and Bent, 1998) transformation of Arabidopsis, it then became possible to test constructs and heterologous gene expression in this useful genetic system, and the tests in tobacco fell from favor. Still, tobacco's utility is realized in a contemporary context (Pierik et al., 2004). The following pages present a historical account of experiments where tobacco served as a surrogate for gene constructs devised to dissect the roles of photoreceptors and their signaling mechanisms in regulating photomorphogenic phenomena.
2.2.1 Photoreceptors and photophysiology
The transgenic tobacco system was valuable in early characterization of photoreceptor contributions to plant growth and development. With Arabidopsis photomorphogenic mutants just being characterized, overexpression of photosensor constructs in a tobacco background allowed gain-of-function analyses that would allow description of receptor function. These were valuable because they could complement the many careful studies of action spectra or absorption spectra that described physiological phenomena and the receptors that guided them.
Phytochrome is a pigment that activates developmental, molecular, and biochemical processes upon absorption of red light (Quail, 2002; Casal and Yanovsky, 2005). The red light activated receptor is toggled off by far-red light. Under natural conditions the ratio of red to far-red is dictated by the position of the sun and the amount of atmosphere it travels through, so the red/far-red ratio is an accurate indicator of time and perhaps season. Far-red light is readily transmitted through, and reflected from plant tissue, therefore the red/far-red ratio is an important barometer of plant neighbor density or plant position within a canopy (Kim et al., 2005; Vandenbussche et al., 2005).
While the physiology and biochemistry of phytochromes was advanced in the early 1980s, little was known about the molecular mechanisms of phy signaling. In the absence of a simple transgenic genetic system like Arabidopsis, it was necessary to exploit the most agile systems of the day, in this case the recently defined transgenic tobacco system. More importantly, such a system would allow study of plant gene expression and characterize the regulation of genes and light-sensing mechanisms from monocotyledonous species. Although critical to agriculture, efficient transformation of monocots has always lagged behind that of eudicots, such as tobacco. Because of this barrier, several early studies validated tobacco as an effective system to study regulation of light-regulated gene expression using constructs obtained from monocots (Lamppa et al., 1985). Later, a rice type-I phytochrome (most likely phytochrome A by recent comparisons) was introduced into tobacco for studies of phy effects on circadian rhythms and plant growth. These studies were important because type-I phytochromes are typically absent from mature tissues and transgenic studies provided a platform to test the effects of type-I on various processes of biological interest. Tobacco plants containing the rice type-I phy cassette expressed the protein highly in leaves. The protein was abundant and associated correctly with its chromophore. Since there was ample evidence of expression, assembly, and photoactivation, the effect of the overexpressor was assessed in a transgenic context. When the mRNA levels of the rhythmically-expressed chlorophyll a/b binding protein (cab) gene were assessed, it became clear that phy overexpressors had greater amplitude of cab expression under free-run conditions, indicating a role for the receptor itself in maintenance of circadian rhythms. In a subsequent study, the effects of rice type-I phy overexpression on plant stature were evident, as phy overexpressors were short as seedlings and mature plants (Nagatani et al., 1991). The change was attributed to a difference in cell elongation and not cell number.
A series of reports from Harry Smith's laboratory utilized transgenic tobacco to analyze the role of specific phytochrome receptors in mediating various plant responses to light. Transgenic lines of N. tabacum and Nicotiana plumbaginifolia were constructed, overexpressing the phytochrome A (PhyA) gene from oat (Avena sativa) (Keller et al., 1989). The initial characterizations showed that overexpressors generated a spectrally active chromoprotein of the proper size. The transgenic overexpressors exhibited dwarfish growth, a loss of apical dominance, and dark green leaves. The same plants were used by McCormac et al. 1992 to describe the relationship between far-red fluence rate and various plant responses. The study demonstrated that oat phyA was able to function in a tobacco background, as transgenic seedlings possessed hypocotyls that were shorter than wild type when grown under a given fluence rate of far-red light. More importantly, wild type seedlings exhibited a loss of sensitivity to far-red whereas transgenic overexpressors did not, reinforcing a well-described theme of transcriptional down-regulation of PhyA upon illumination. Additional characterization of Avena PhyA-overexpressing tobacco seedlings was performed, measuring cotyledon angle in response to extremely low fluence pulses of red light (Casal et al., 1994). The study confirmed that phyA is the photoreceptor mediating response to the most minor red irradiances.
These studies were expanded by assessment of phy degradation patterns in transformed cells in culture. Here McCormac et al. 1993 established liquid suspension cultures of tobacco cells overexpressing the oat phyA. The overexpressed phyA was the predominant form detected and was analyzed for degradation patterns following red or far-red irradiation, as well as for degradation dependence on chromophore association. In vitro changes in gene expression mirrored those induced in vivo. This work established and validated a single-cell system where phytochrome activation, signaling, response, and degradation could be further studied. Tobacco PhyA overexpressors also were used to study the effect of PhyA expression on germination. Tobacco and Arabidopsis differ in their spectral response to germination. Whereas Arabidopsis germination is induced with all wavelengths (Shinomura et al., 1996), tobacco exhibits far-red induced inhibition of germination, much like that observed in Borthwick's classical experiments with “Grand Rapids” lettuce (Borthwick et al., 1952). However, when harboring a PhyA transgene, seeds were less sensitive to the far-red block of germination (McCormac et al., 1993).
The set of phyA studies in transgenic tobacco culminates with studies showing the effect of phyA overexpression on traits relevant to agriculture. Robson and Smith 1996 showed that strong overexpression of phyA results in a complete suppression of shade avoidance phenotypes, changes in architecture that render the plant elongate and hyponastic. High overexpression of phyA induced a proximity-conditional dwarf phenotype whereas some lines with lower expression levels exhibited normal phenotypes in white light but shade avoidance defects when the red/far-red ratio was lowered (Schmitt et al., 1995). In PhyA overexpressors, photosynthate was allocated away from stems in these plants, increasing assimilates in leaf tissue. The report concludes that overexpression of PhyA may be useful in other crops to increase harvest index. Similar neighbor insensitivity was noted by Casal and Sanchez 1994 when they carefully assessed the roles of red/far-red ratio on plant growth, concluding that overexpression of phyA would cause a lag in response to detection of neighbors. A study was designed to test if these effects had ecological ramifications. The adaptive plasticity hypothesis, the notion that environmentally induced changes in plant form and composition would actually have an effect on fitness, was tested using these same lines (Schmitt et al., 1995). Analysis of PhyA overexpression and wild-type lines showed that the ability to take on shade avoidance characters allowed plants to better compete, as evidenced by a greater dry mass in wild-type plants upon harvest. These findings support the adaptive plasticity hypothesis.
The studies of Avena phyA indicate a role for phytochrome in mediating responses to a shade environment, directed as a decrease in red/far-red ratio. Blue light and the gaseous hormone ethylene also have a role in regulating shade avoidance response (Pierik et al., 2004). A report showed shade avoidance responses in transgenic tobacco bearing an overexpressed mutated copy of the Etr1 gene, the gene encoding the ethylene receptor. These plants exhibit a dominant insensitivity to ethylene, which accumulated to high levels in dense stands of plants. The ethylene response was shown to be independent of the red/far-red ratio and was related to blue light fluence rates. This study demonstrates that shade avoidance symptoms are not simply affected by the red/far-red ratio and phytochrome activity.
Later, oat phyA, and Arabidopsis phytochrome B (phyB) and phytochrome C (phyC) were introduced independently to a tobacco background to probe the roles of individual phytochromes in various light responses (Halliday et al., 1997). This study reiterated the respective sensitivities of phyA and phyB to far-red and red light, as well as demonstrated a role for phyC for the first time. Furthermore, this study showed the long-term effects of phy overexpression on photoperiodic flowering. A cultivar normally insensitive to a night break on short days could be made sensitive with phy overexpression, and a cultivar normally exhibiting a delay in flowering after a night break showed an increased delay. This study provided a mechanistic complement to the plethora of photophysiological studies that tied phytochrome to regulation of photoperiodic flowering.
Studies in blue light signaling utilized the tobacco system as well. The aforementioned Avena PhyA overexpressor showed enhanced hypocotyl growth inhibition phenotypes under blue light (Casal and Sanchez, 1994). Transgenic tobacco systems were also used to characterize newly discovered blue light sensors. The hy4 Arabidopsis mutant was identified as a seedling with defects in its response to blue and white light (Koornneef et al., 1980). The mutants possessed long hypocotyls and poorly developed cotyledons, indicating a major role for the HY4 protein in regulation of photomorphogenic development. Later, the hy4 mutant would be found to encode a protein with homology to microbial photolyases (Ahmad and Cashmore, 1993), the light-activated flavoproteins that catalyze repair of thymidine dimers in DNA caused by ultraviolet damage. The photolyases absorb optimally in the blue and UV-A portion of the spectrum, correlating well with the wavebands that produce conspicuous phenotypes in the hy4 mutant. These findings led to the compelling conclusion that Hy4 encoded a flavin-based photoreceptor that regulated stem elongation during early light development. Although identified genetically in Arabidopsis, the transgenic tobacco system was used because of its ability to rapidly produce plants that could be used in further description of this new light sensor's function, as well as uncover structure–function relationships.
An important study by Lin et al. 1995 provided the overexpression data that complemented the Arabidopsis loss-of-function genetic studies. Transgenic tobacco seedlings were hypersensitive to UV-A, blue and green light treatment, growing with shorter stems than wild-type seedlings. Hy4 overexpressing seedlings were not distinguishable from wild-type seedlings in darkness, red light, or far-red light. Here tobacco served as a rapid means to test the effect of a photoreceptor in a heterologous system, showing that the receptor, as well as the transduction mechanism, is conserved between the two different species representing two different plant families.
2.2.2 Elucidating light signal transduction mechanisms
The use of transgenic tobacco provided a foundation to study the mechanism of phytochrome signal transduction. Studies of Sinapis cab promoter activity in transgenic tobacco demonstrated that the promoter's activity is dictated by the host context, as tobacco has contrasting sensitivity to phytochrome induction compared to mustard (Kretsch et al., 1995). Initial studies of cell fractionation and immunocytochemical analyses provided evidence that PHYA and PHYB were localized to the nucleus upon light activation (Mosinger et al., 1988; Sakamoto and Nagatani, 1996). These observations led to the exciting hypotheses repartitioning of the photoreceptor itself may constitute an important step in light signaling. However, cell fractionation studies were inconclusive due to the fact that phytochrome is a notoriously “sticky” protein, and may be improperly ascribed to a given location or condition. To bypass this problem, Kircher et al. 1999 studied the kinetics of photoreceptor repartitioning in transgenic tobacco. The authors created a fusion protein between phytochrome B and green fluorescent protein (GFP), allowing them to nondestructively track the movement of the protein through time following light treatment. The results indicate that the photoreceptor::GFP fusion is detected in the cytosol in dark-grown plants and then reapportioned to the nucleus following illumination, although PHYA and PHYB translocate with different kinetics and spectral sensitivities. The movement of PHYB to the nucleus is driven by red light, is far-red reversible, and has a low-fluence illumination threshold. Import of PHYA is much more light sensitive and occurred in response to both red and far-red light. These studies in tobacco illustrated that photoreceptor localization to, and removal from, the nucleus plays a central role in the regulation of gene expression by light, a theme that would form the foundation of further investigations.
2.2.3 Analyses of light-regulatory sequences
As useful as tobacco was to understand the physiology of photosensor function and the mechanisms of transduction, tobacco first served to establish a basis for light-regulated promoter studies. The first efforts appear in the literature from N.H. Chua's laboratory in the mid-1980s, where efforts centered upon the promoters of two light-regulated nuclear genes—cab and the small subunit of ribulose 1-5 bisphosphate carboxlase/oxygenase (rbcS). These studies allowed a primary glimpse into the structural elements that coupled ambient light conditions to regulation of gene expression in plants. A study by Lamppa et al. 1985 indicated that the wheat cab genes were properly regulated in the heterologous tobacco system. Next Nagy et al. 1986 demonstrated the phytochrome reversibility of wheat cab-1 in wheat, and showed that a 1.8kb promoter fragment could faithfully execute phytochrome-mediated induction in transgenic tobacco. A follow-up study pared down the wheat cab regulatory regions to a 286bp promoter region as that conferring phytochrome responsiveness, and showed that phytochrome did not affect transcript stability (Nagy et al., 1987). A related study of the light-regulated 2.4kb pea rbcS promoter showed that it could be properly regulated in the heterologous context, and that a significant deletion construct leaving only 352bp was still sufficient to confer a phytochrome response. Kuhlemeier et al. 1989 used transgenic tobacco to characterize both positive and negative regulators of rbcS activation. The use of protein synthesis inhibitors in transgenic tobacco provided evidence indicating that light control of cab transcripts was due to labile factors (Lam et al., 1989).
The utility of tobacco as a transgenic system was realized in the definition of sequence motifs that narrowed the analyses of large expanses of light-responsive promoters. One element with demonstrated light regulatory capacity is the GT-1 motif. A study by Lam and Chua 1990 utilized tobacco to illustrate that the GT-1 motif of the pea rbcS promoter could confer light-regulated activity to a normally constitutive promoter. Proper induction was seen in cells that contained chloroplasts, suggesting a signal from the organelle was necessary. While necessary, the GT-1 motif was not sufficient to confer responsiveness (Cuozzo-Davis et al., 1990). The GT-1 motif was later shown to be specifically part of a phytochrome responsive element (Gilmartin and Chua, 1990) and that the precise spacing between GT-1 motifs was critical for full activity (Gilmartin and Chua, 1990). A DNA binding activity designated 3AF1 bound to the light responsive promoter, but the elements bound did not confer light regulation to a constitutive promoter (Lam and Chua, 1990). Later transgenic suspension cultures would be used to illustrate that phytochrome induction of light-regulated genes could be induced by addition of calmodulin and inhibited by appropriate calmodulin inhibitors (Zhou et al., 2001).
Analysis of regulatory regions of other light-responsive genes raised exciting new questions about the role of post-transcriptional processes in the maintenance of transcript abundance. In etiolated seedlings, the pea ferredoxin 1 (Fed1) gene exhibited unusual induction kinetics relative to cab, rbcS, and other light-regulated transcripts (Kaufman et al., 1985, 1986). These findings prompted Elliott et al. 1989 to express the modular components of the pea Fed1 gene in transgenic tobacco. Long (∼2kb) and short versions of the Fed1 promoter were still active when driving a reporter gene, but no light regulation was observed. Conversely, the cauliflower mosaic virus 35S (CaMV 35S) promoter::Fed1 transcript was strongly regulated by light, indicating that the regulatory sequences were downstream of the transcriptional start. Attachment of the Fed1 sequence to a CaMV 35S-driven β-glucuronidase (GUS) construct conferred light regulation to the normally constitutive GUS transcript. Later analyses in transgenic tobacco showed that the upstream regulatory regions from pea were most active during the dark-light transition and that transcript regulation was more evident in the green tissues (Gallo-Meagher et al., 1992).
To further dissect the regulatory mechanisms Petracek et al. 1997 studied accumulation of CaMV 35S-driven Fed1 transcripts in response to the electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), as well as Fed1 association with polysomes in light-grown and dark-adapted tissues. DCMU blocked accumulation of Fed1 transcripts indicating an association with active photosynthesis. In light-grown tissue, Fed1 transcripts were associated with polyribosomes, whereas such relationships were not observed in darkness, in subthreshold illumination or after DCMU treatment. Later it would be shown that stability is associated with specific sequences in the 5′-UTR and coding region that would promote polysome association in light conditions, stabilizing the transcript (Dickey et al., 1998). The stability could be broken with the introduction of termination codons into the coding region suggesting that active translation is required for optimal stability (Petracek et al., 2000).
Regulation of another gene associated with photosynthesis was similarly studied using the transgenic tobacco system. A deletion series of the pea plastocyanin promoter (petE) was fused to GUS and introduced into transgenic tobacco (Pwee and Gray, 1993). The results described a host of positive and negative regulatory elements that occurred between −784 and +3 relative to the transcription start, and also required a plastidic signal. However, the light induction observed from truncated promoters never was as robust as that exhibited by the endogenes, leading the authors to conclude that other sequences located 3′ to the transcription start may modulate gene expression. This hypothesis, initiated from a study in transgenic tobacco, revealed a post-transcriptional mechanism that would later be described in Arabidopsis with further resolution (Brown et al., 2005).
Key enzymes of the phenylpropanoid pathway are regulated by light, and they too became targets for promoter analyses. A 1.1kb fragment of the Phaseolus vulgaris phenylalanine lyase (PAL2) promoter was placed upstream of a GUS reporter and introduced into tobacco (Liang et al., 1989). The resulting plants indicated that the promoter was active in specific tissues and could be regulated in a wound- and light-inducible manner. Similarly the promoter that drives the parsley 4CL-1 gene (encoding 4-coumarate:coenzyme A ligase) was placed upstream of the GUS reporter, and its activity was tested in a deletion series (Hauffe et al., 1991). Transient assays in protoplasts isolated the regulatory regions to a 210bp fragment, and activity was shown to be tissue specific in mature plants. The “G-box” motif is present in the promoters of many plant genes, and it was shown to be a functional part of the parsley chalcone synthase gene when activated by UV light (Kaulen et al., 1986).
Although functional gene studies in tobacco have fallen from favor, it is important to remember that Nicotiana species still maintain many advantages over Arabidopsis. The large plants and organs are more amenable to biochemical techniques. Recently Nicotiana benthamiana and N. tabacum have been used for transient assays of transgenic protein function, as well as in vivo protein–protein interaction studies (Clemente, 2006). Plus, tobacco is a solanaceous crop, serving as a useful intermediate for translational genomics studies of light signaling in other solanaceous crop species, such as tomato. Although not a central system in the study of light-mediated processes, it is important for us to bear in mind the advantages of this system in elucidation of light-signaling mechanisms and their translation to relevant crop species.
2.3 Tobacco Transformation Studies for Biotic and Abiotic Stresses
Establishment of transgenic technology in tobacco ushered in an era of research to engineer resistance to various forms of stress. Crops are constantly being challenged by biotic and abiotic factors. Tobacco became a host to study and test the genes that could protect important crops in the future.
2.3.1 Biotic stress
2.3.1.1 Insect resistance
It has been well documented through pioneering research in tobacco that expression of Bacillus thuringiensis (Bt) endotoxin genes, as well as proteinase inhibitor (PI) genes, confer insect resistance. However, it must be noted that novel sources of resistance are continuing to be explored for future use in insect control.
Most Lepidoptera species are susceptible to the Cry 1 and Cry 2 insecticidal crystal proteins produced by Bt. The first studies to examine insect resistance following transfer of Bt genes were in tobacco in 1987 (Barton et al., 1987; Vaeck et al., 1987). These transgenic tobacco plants carried truncated versions of either cry1Aa or cry1IAb, respectively, and were resistant to the larvae of Manduca sexta (L.). However, Cry protein levels were low (less than 0.001% of leaf soluble proteins) and this initially led to the use of synthetic cry genes whose expression levels were higher in plant cells. The codons of these bacterial cry1Ab and cry1Ac genes, as well as other features, were optimized for usage in plants and this raised the level of expression in transgenic tobacco (0.02–1% of leaf soluble proteins) (Perlak et al., 1991). In these examples, the cry genes were driven by the constitutive CaMV 35S promoter. However, Williams et al. (1993) used the promoter from the pathogenesis-related PR-1a gene to control expression of cry1Ab. More recently, cry2Aa2 was placed behind the promoter from the Solanum tuberosum leaf- and stem-specific (ST-LS1) gene to allow expression in green tissues of tobacco (Zaidi et al., 2005). In this study, CRY2AS2 levels reached 0.21% of leaf soluble protein and plants were highly resistant to the larvae of Heliothis virescens. Another approach to increase cry gene expression (and improve transgene containment) has been to insert these genes into the tobacco chloroplast genome. Integration of a cry1Ac gene (McBride et al., 1995), cry2Aa2 (Kota et al., 1999) and cry1Ia5 (Reddy et al., 2002) into the chloroplasts of tobacco led to high levels of expression (3–5% of leaf soluble proteins), and resistance to a number of economically important lepidopterean pests without deleterious effects on plant growth. De Cosa et al. 2001 expressed the cry operon in chloroplast transgenic tobacco resulting in accumulation of Cry protein to 46.1% of tsp with devastating effect on the lepidopteran pests. Recently, Chakrabarti et al. 2006 showed that expression of a truncated cry9Aa2 in tobacco chloroplasts resulted in resistance to the potato tuber moth (PTM), Phytorimacea operculella as measured by leaf bioassays. Levels of CRY9AA2 were extremely high (10% of leaf soluble proteins); however, such high expression led to delayed development.
Other microbial genes also have proved useful. The Agrobacterium tumefaciens isopentenyl transferase (ipt) gene, which is important for cytokinin production, was introduced into tobacco under the control of the potato PI II gene promoter (Smigocki et al., 1993). This wound-inducible system resulted in decreased feeding of M. sexta and increased mortality for the peach potato aphid, Myzus persicae. However, plant development was negatively altered resulting in poor root systems and a reduction in chlorophyll levels.
Viral genes also have been introduced to curb insect feeding. Enhancin genes from Trichoplusia ni or Helicoverpa armigera baculoviruses encoding metalloproteases that breakdown mucin, which compromises the peritrophic membrane, were introduced into tobacco. T. ni larvae allowed to feed directly on transgenic leaves showed reduced growth and development as well as increased mortality (Cao et al., 2002). Long-term feeding studies using transgenic tobacco expressing the T. ni baculovirus enhancin gene in artificial diets showed similar results with Pseudaletia separata and Spodoptera exigua larvae (Hayakawa et al., 2004).
Plant PIs have been quite effective in reducing insect growth both in vitro and in vivo. The PIs are classified as inhibiting serine, cysteine, metallo or aspartyl proteases. The first PI gene shown to be effective against insects, cowpea trypsin inhibitor (CpTI), a serine PI, was introduced into tobacco in 1987 and displayed resistance to M. sexta and H. virescens (Hilder et al., 1987). Since that time, numerous serine PI genes, including those expressing potato (PPI-II), tomato (TI-II), and sweet potato (spTI-1) PIs have been introduced into tobacco and shown to slow the growth of several species (Johnson et al., 1989; McManus et al., 1994; Yeh et al., 1997). In 2000, three soybean genes (KTi3, C-II, and PI-IV) coding for serine PIs were introduced into tobacco (Marchetti et al., 2000). In this study, 100% mortality was achieved for S. littoralis larvae fed on certain tobacco transgenic lines.
Lectins, a group of carbohydrate-binding proteins, have shown insecticidal properties. Tobacco expressing a pea lectin was toxic to H. virescens (Gatehouse et al., 1992), while those expressing the snowdrop lectin (GNA) have displayed resistance to the peach potato aphid M. persicae (Hilder et al., 1995). More recently, tobacco plants expressing the Pinellia ternata agglutinin transgene (pta) also reduced the growth of M. persica (Yao et al., 2003). Therefore, the pta gene could be used in conjunction with or pyramided with the snowdrop lectin gene (gna) to control aphids.
A CaMV 35S tobacco anionic peroxidase gene was able to provide broad-spectrum insect resistance particularly to caterpillars, aphids, and whiteflies in both greenhouse (Dowd and Lagrimini, 1997) and field studies (Dowd and Lagrimini, 2006). However, the mode of action of the peroxidase is believed to be indirect and is not well understood.
Gamma-aminobutyrate (GABA), a nonprotein amino acid, accumulates in plants following various abiotic stresses. GABA is an inhibitory neurotransmitter that acts at insect neuromuscular junctions, and therefore may contribute to insect resistance. MacGregor et al. 2003 showed that tobacco transgenics expressing a glutamate decarboxylase complementary DNA (cDNA) and accumulating large amounts of GABA were not preferred by H. virescens compared to nontransgenics. Tryptamine is another neuroactive agent that results in insect antifeeding behavior. Transgenic tobacco plants expressing the tryptophan decarboxylase 1 gene leading to increased accumulation of tryptamine reduced the feeding and slowed the growth of M. sexta larvae (Gill et al., 2003).
Caffeine was produced in tobacco via insertion of three coffee N-methyltransferase genes (Uefuji et al., 2005). In preference studies, S. litura larvae fed on wild-type tobacco leaf discs rather than those of the transgenics. Therefore, caffeine may act as a feeding deterrent.
To date, genes derived from insects have conferred only low levels of insect resistance when expressed in tobacco. Chitin is a structural polysaccharide that is a component of the insect exoskeleton, as well as the peritrophic membrane, which separates food from midgut tissue. Genes coding for chitinases, normally produced by insects during moulting, have been cloned and introduced into plants for insect control. A M. sexta chitinase was expressed in transgenic tobacco, and when purified and fed to merchant grain beetle, Oryzaephilus mercator, resulted in toxicity (Wang et al., 1996). A chitinase cDNA from M. sexta, introduced into tobacco, reduced feeding damage and growth of H. virescens larvae, but not of M. sexta larvae (Ding et al., 1998). A synthetic gene containing multiple copies of the trypsin modulating and oostatic factor (TMOF) from Aedes aegypti (Aea-TMOF) was introduced into tobacco (Tortiglione et al., 2002). H. virescens larvae fed with transgenic leaves expressing this gene showed a reduction in growth compared to those fed with control plants. These genes may be more effective when used in conjunction with other control strategies.
Genes from animals also affect insectivory. Expression of a bovine spleen trypsin inhibitor (SI) gene in transgenic tobacco leaves was effective in decreasing both survival and growth of H. armigera larvae (Christeller et al., 2002). When expressed at high levels, both biotin-binding proteins conferred a high level of insect resistance on transformed tobacco plants to larval PTM, P. operculella (Zeller) (fam. Gelechiidae), and on apple plants to larvae of the lightbrown apple moth (LBAM), Epiphyas postvittana (Walker) (fam. Tortricidae). More than 90% of PTM larvae died on tobacco plants expressing either avidin or streptavidin genes within nine days of inoculation (Marwick et al., 2003).
The venom toxin, ω-ACTX-Hv1a (Hvt), from the Australian funnel web spider (Hadronyche versuta), acts as a calcium channel antagonist. Transgenic tobacco expressing Hvt were effectively protected with 100% mortality of H. armigera and S. littoralis larvae (Khan et al., 2006).
2.3.1.2 Virus—pathogen- and nonpathogen-derived resistance
There are two main approaches to engineering plants for viral resistance. One approach is pathogen-derived resistance (PDR) where a portion or a complete viral gene is inserted into the plant conferring resistance. This was first demonstrated in transgenic tobacco plants containing the coat protein (CP) gene of tobacco mosaic virus (TMV) (Powell-Abel et al., 1986). PDR has been studied and used extensively for a wide variety of viral genes, viruses, and hosts since that seminal report (see Dasgupta et al., 2003 for review). Likewise, tobacco transgenics were used in the discovery that post-transcriptional gene silencing (PTGS) was the result of a diffusible signal. When viral RNA is the elicitor or target of PTGS, the mechanism is referred to as virus-induced gene silencing (VIGS) (see Robertson, 2004 for review). Transgenic tobacco studies (particularly using N. benthamiana) have been used extensively to understand the RNA silencing mechanisms at work in VIGS. The other approach is nonpathogen-derived resistance that uses host resistance genes to confer virus resistance. Once again, tobacco has been a model organism to understand the resistance mechanisms and to generate transgenic virus resistant plants. The classic example is the tobacco N gene that provides resistance to TMV. It is a disease resistance gene (R) member of the toll-interleukin (TIR)-leucine-rich repeat (LRR) family.
2.3.1.3 Fungi—pathogenesis-related proteins
There are five families of PR proteins (PR-1 to PR-5) that have members displaying antifungal activity. In the first report of fungus-resistant transgenic plants, Broglie et al. 1991 expressed a bean chitinase gene in tobacco as well as in Brassica napus that displayed resistance to Rhizoctonia solani. Since that time, transgenic tobacco has been used in numerous studies to examine the antifungal effect of various PR-proteins expressed alone or in combination (e.g., Broglie et al., 1991; Alexander et al., 1993; Nielsen et al., 1993; Vierheilig et al., 1993; Liu et al., 1994; Zhu et al., 1994; Velazhahan and Muthukrishnan, 2003).
2.3.1.4 Bacteria
Magainin is one of the earliest reported antimicrobial peptides isolated from skin secretions of the African clawed frog Xenopus laevis and is thought to function as a natural defense mechanism against infection. The engineered magainin analog peptide, Myp30, was found to inhibit spore germination of the oomycete, Peronospora tabacina (Adam) in vitro, and the growth of a bacterial pathogen Erwinia carotovora subsp. carotovora (Jones). Transgenic tobacco (N. tabacum L.) plants expressing Myp30 were evaluated for resistance to these pathogens. The expression of the peptide only to an extracellular location resulted in significant reduction in sporulation and lesion size due to P. tabacina infection. A significant increase in resistance to the bacterial pathogen was also observed regardless of the targeting location of the peptide (Li et al., 2001).
2.3.2 Abiotic stress
Plant growth and productivity are greatly influenced by environmental conditions such as drought, cold, and salinity, which lead to water stress. Different kinds of stresses often trigger similar responses in plants as a result of cellular dehydration, which causes osmotic stress. In addition, the production of reactive oxygen species increases damage to cellular structures and impacts metabolism. Many stress-associated genes have been identified, including those involved in osmolyte biosynthesis, fatty acid metabolism, free radical detoxification, and signal transduction as well as molecular chaperones, ion and water transporters, and transcription factors. Tobacco transformation has played an important part in studies to evaluate the roles of these genes in stress tolerance.
To date, most studies have looked at constitutive overexpression of stress-related proteins using the CaMV 35S promoter. While this strategy allows elucidation of protein function with relative ease, it results in transgene expression in all parts of the plant at all developmental stages, which may have undesirable consequences for plant growth (Holmström et al., 1996; Kumria and Rajam, 2002; Kasuga et al., 2004). The use of stress-inducible promoters has demonstrated the potential to more effectively transfer genes or pathways for stress tolerance into other important plant species (Nelson et al., 1998; Kasuga et al., 2004; Khodakovskaya et al., 2006). Expression of foreign proteins has also been controlled by the addition of targeting sequences (Shen et al., 1997; Nuccio et al., 1998).
2.3.2.1 Biosynthesis of osmolytes and osmoprotectants
Tobacco has provided a good model system for understanding the role of compatible solutes in osmotic stress. In response to a variety of stresses, compatible solutes accumulate in cells at high concentrations, facilitating the retention of water, and thereby stabilizing the structure of macromolecules, without interfering with cytoplasmic functions. Introduction of novel pathways for the biosynthesis of compatible solutes in tobacco has resulted in increased stress tolerance of transgenic plants. Common nontoxic solutes include quaternary ammonium compounds, such as glycine betaine, amino acids, amino acid derivatives, sugars, and sugar alcohols.
2.3.2.2 Quaternary ammonium compounds—glycine betaine
Glycine betaine (GlyBet) is a quaternary ammonium compound occurring naturally in a variety of plants, animals, and microorganisms (Rhodes and Hanson, 1993). It is a very efficient compatible solute that accumulates in response to stress conditions. Transgenic approaches in tobacco and other higher plants that do not normally accumulate GlyBet have provided evidence for the physiological role of this osmoprotectant. GlyBet is synthesized in higher plants by the two-step oxidation of choline, catalyzed by a ferredoxin-dependent choline monooxygenase (CMO) and a NAD+-dependent betaine aldehyde dehydrogenase (BADH) (Rhodes and Hanson, 1993). In mammalian cells and Escherichia coli, GlyBet is synthesized by another two-step reaction involving a NAD+-dependent choline dehydrogenase (CDH) in combination with BADH (Landfald and Strøm, 1986). By contrast, the soil bacterium Arthrobacter is able to synthesize GlyBet directly from choline in a one-step reaction catalyzed by choline oxidase (COD) (Ikuta et al., 1977).
Tobacco has been used extensively as a model for the introduction of GlyBet synthesis into a nonaccumulator. Engineering GlyBet synthesis in tobacco has been achieved by changing the CMO/BADH pathway, the CDH/BADH pathway, or the COD pathway (Sakamoto and Murata, 2000). Transfer of the CMO/BADH pathway from natural GlyBet accumulators to tobacco was attempted by introduction of a BADH cDNA from either spinach (Spinacia oleracea) or sugarbeet (Beta vulgaris) under control of the CaMV 35S promoter (Rathinasabapathi et al., 1994). BADH was targeted to the chloroplasts of transgenic plants and levels of the enzyme were similar to those in spinach following salt stress. Transgenic plants accumulated GlyBet to high levels, but only in the presence of the exogenously supplied precursor betaine aldehyde, demonstrating that increased expression of BADH alone was not sufficient for GlyBet synthesis in tobacco (Rathinasabapathi et al., 1994). High levels of BADH expression were also achieved following transformation of tobacco with barley (Hordeum vulgare) BADH cDNA (Ishitani et al., 1995). GlyBet accumulation was not reported; however, BADH transcripts accumulated in a stress-responsive manner. Li et al. 2003 introduced BADH from the halophyte Suaeda liaotungensis into tobacco, under control of the CaMV 35S promoter. Levels of GlyBet were much lower in transgenic plants than in S. liaotungensis, which accumulates GlyBet to high levels. Despite not accumulating GlyBet to physiologically relevant levels, some transgenic plants survived on media containing 200mM NaCl and suffered less membrane damage than wild-type plants (Li et al., 2003). Expression of GlyBet in tobacco has also been shown to increase tolerance to high temperature stress (45°C) during seedling growth by maintaining the activation of Rubisco, enhancing photosynthesis (Yang et al., 2005). These authors introduced the spinach BADH gene under control of the CaMV 35S promoter and transgenic plants were able to accumulate low levels of GlyBet, up to 4.6μmolg−1 fresh weight, mainly in chloroplasts.
Since non-GlyBet-accumulating plants show some BADH activity, a spinach CMO gene was introduced into tobacco under control of the CaMV 35S promoter, to investigate the potential for GlyBet production by engineering synthesis of this enzyme alone (Nuccio et al., 1998). CMO was successfully targeted to the chloroplasts, but transgenic plants accumulated levels of GlyBet far below those of natural accumulators, possibly limited by the endogenous choline supply. In a similar approach, Huang et al. 2000 introduced the COD gene from Arthrobacter pascens into tobacco under control of the CaMV 35S promoter. Transgenic plants accumulated too little GlyBet to significantly affect osmoregulation, despite a moderate increase in salt tolerance (Huang et al., 2000). In contrast to these studies, the COD gene from Arthrobacter globiformis (codA), targeted to the chloroplasts under control of the CaMV 35S promoter, conferred freezing tolerance (−2°C for 24h) on transgenic tobacco plants (Konstantinova et al., 2002). Choline oxidase protein was detected in the homozygous line analyzed, although the expression level remained stable under nonstress or stress conditions. Transgenic plants were also able to survive freezing stress under field conditions (Konstantinova et al., 2002).
In an effort to remove the constraint placed by insufficient choline on GlyBet synthesis, Mcneil et al. 2001 employed a spinach cDNA encoding phosphoethanolamine N-methyltransferase (PEAMT), a key enzyme in choline biosynthesis. The PEAMT coding sequence was introduced under control of the strongly constitutive figwort mosaic virus 34S promoter, into tobacco plants already expressing spinach CMO and sugarbeet BADH. Transgenic plants contained up to 50-fold more free choline than control plants and GlyBet synthesis was enhanced up to 30-fold, without affecting plant growth (Mcneil et al., 2001).
Attempts to engineer the bacterial GlyBet biosynthesis pathway into tobacco produced similar results to those targeting the plant pathway. Introduction of the second enzyme of the E. coli GlyBet pathway, betB, encoding BADH, was not sufficient for GlyBet production in tobacco (Holmström et al., 1994). By comparison, the first enzyme of the E. coli GlyBet pathway, betA, encoding CDH, under control of the Arabidopsis RbcS1A promoter allowed GlyBet accumulation in transgenic tobacco plants (Holmström et al., 2000). Plants expressing betA had enhanced salt tolerance, measured as reduction in fresh weight, compared to controls (Lilius et al., 1996; Holmström et al., 2000), and also showed greater resistance to photoinhibition under salt stress and low temperature conditions (Holmström et al., 2000). When transgenic tobacco plants expressing betA were crossed with those expressing betB, effectively completing the biosynthesis pathway, GlyBet accumulation was two- to threefold higher than in plants only producing CDH (Holmström et al., 2000). Transgenic lines producing both CDH and BADH, however, did not appear to have improved stress tolerance over those producing CDH alone.
2.3.2.3 Amino acids—proline
Proline accumulates to very high levels in plants under stress, protecting them against osmotic and oxidative stresses. In E. coli, proline biosynthesis from glutamate is controlled by a three-gene operon, proB (γ-glutamyl kinase), proA (glutamic γ-semialdehyde dehydrogenase), and proC (Δ1-pyrroline-5-carboxylate reductase); however, in plants, proline can be synthesized directly from glutamate under stress conditions by Δ1-pyrroline-5-carboxylate synthetase (P5CS) (Delauney and Verma, 1993). An alternative proline biosynthesis pathway exists in plants, involving transamination of ornithine, catalyzed by ornithine-δ-aminotransferase (δ-OAT), to two possible intermediates, both of which can be reduced to proline. Engineering the proline biosynthesis pathway in tobacco has demonstrated that overproduction of proline in plants can confer increased tolerance to osmotic stress.
Kavi Kishor et al. 1995 introduced the mothbean (Vigna aconitifolia) P5CS gene into tobacco under control of the CaMV 35S promoter. Transgenic plants produced high levels of P5CS and synthesized 10- to 18-fold more proline (830–1590μgg−1 fresh weight) than control plants. Osmotic potential was maintained in leaf cells of transgenic plants and wilting was delayed under drought treatment, likely due to constitutive levels of proline (Kavi Kishor et al., 1995). Transgenic plants also showed enhanced biomass and flower development under salt stress conditions (Kavi Kishor et al., 1995). Under nonstress conditions, Konstantinova et al. 2002 observed only a threefold higher proline content compared to wild-type plants in tobacco lines transformed with the same mothbean PC5S gene. This level increased to 15-fold, however, during chilling and freezing stresses (−2°C for 24h) (Konstantinova et al., 2002). The same authors also introduced a P5CS gene from Arabidopsis into tobacco. There was no difference in proline content between these transformed lines and wild-type plants under normal conditions, but proline content increased five- to eightfold during chilling and freezing stresses (Konstantinova et al., 2002). Plants harboring either PC5S gene were also able to survive freezing stress under field conditions (Konstantinova et al., 2002). Tobacco plants engineered to express enzymes from the E. coli proline biosynthesis pathway are able to accumulate similar levels of proline under salt-stress conditions (Sokhansanj et al., 2006).
Since P5CS is under feedback inhibition by proline, which may be lost under stress conditions, Hong et al. 2000 compared proline levels in transgenic tobacco plants expressing a wild-type mothbean P5CS enzyme and a mutated form, whose feedback inhibition was removed, both under control of the CaMV 35S promoter. Under nonstress conditions, plants expressing the mutated P5CS enzyme accumulated twofold more proline than plants expressing the wild-type P5CS and had higher germination rates on media containing 200mM NaCl. In addition, higher proline levels were associated with lower malondialdehyde (MDA) levels, a major cytotoxic product of lipid peroxidation used as an indicator of free radical production (Hong et al., 2000).
To investigate the possibility of increasing osmotolerance in plants by manipulating the ornithine-dependent proline biosynthesis pathway, Roosens et al. 2002 introduced the Arabidopsis ornithine-δ-amino transferase (δ-OAT) cDNA into tobacco, under control of the CaMV 35S promoter. Transgenic plants overexpressing δ-OAT synthesized approximately threefold more proline than control plants and had increased biomass and germination frequency under osmotic stress conditions. In another alternative strategy to increase proline biosynthesis, Yonamine et al. 2004 introduced the tobacco NtHAL3a gene, involved in the coenzyme A biosynthetic pathway, into cultured tobacco BY2 cells under control of the CaMV 35S promoter. Transgenic plants contained approximately four- to fivefold more proline under nonstressed and salt-stressed conditions and showed improved tolerance to 100mM and 140mM NaCl (Yonamine et al., 2004). Kolodyazhnaya et al. 2006 introduced an antisense suppressor of proline dehydrogenase from Arabidopsis into tobacco, leading to transgenic plants with increased proline content and elevated salt tolerance.
2.3.2.4 Amino acid derivatives—polyamines
Polyamines (PAs) are nitrogenous cellular compounds, formed by the decarboxylation of amino acids, which accumulate under a variety of abiotic and oxidative stress conditions. Examples include putrescine, spermidine, and spermine. The role of PAs in plants has been studied by overexpression of S-adenosylmethionine decarboxylase (samdc), a key enzyme in spermidine and spermine biosynthesis, in tobacco. Transgenic tobacco plants expressing a carnation (Dianthus caryophyllus L.) samdc cDNA under control of the CaMV 35S promoter accumulated 2.2–3.1 times more soluble total PAs than wild-type plants (Wi et al., 2006). Transgenic plants had an increased number and weight of seeds, increased net photosynthetic weight, and suffered less chlorophyll degradation after salt, cold, acidic stress, and abscisic acid (ABA) treatment. In addition, transcription of antioxidant enzymes was induced more significantly in transgenic plants than controls, following stress treatment (Wi et al., 2006). Increaed PA biosynthesis in transgenic tobacco was also achieved by introduction of a human samdc gene under control of the CaMV 35S promoter (Waie and Rajam, 2003). Transgenic plants had increased spermidine and putrescine levels and exhibited tolerance to salt stress (250mM NaCl) and PEG-induced drought stress. Kumria and Rajam 2002 expressed the mouse putrescine synthesis gene ornithine decarboxylase (ODC) in tobacco under control of the CaMV 35S promoter to up-regulate PA metabolism. Transgenic plants had higher mouse ODC activity, but reduced activity of plant ODC and the alternative putrescine synthesis gene arginine decarboxylase (ADC). PA levels were two- to threefold higher in transgenic plants compared to controls and plants were more tolerant to salt stress (300mM NaCl) (Kumria and Rajam, 2002).
PAs share a common precursor, S-adenosylmethionine (SAM), with ethylene, resulting in metabolic competition between ethylene and PA biosynthesis. Antisense expression of ethylene biosynthetic genes is expected to shift the competition for the SAM precursor in favor of PA biosynthesis. Wi and Park 2002 introduced antisense constructs of carnation cDNAs encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase or ACC oxidase into tobacco. Transgenic lines had higher PA contents and increased number and weight of seeds, in addition to reduced chlorophyll loss following oxidative stress, high salinity, acid conditions, or ABA treatment (Wi and Park, 2002).
2.3.2.5 Sugars and sugar alcohols
Compounds related to sugar metabolism also accumulate in plants during responses to water stress and osmotic adjustment. These include sucrose, trehalose, fructans, mannitol, and D-ononitol. The role of these osmolytes in protecting against abiotic stress has been investigated by engineering tobacco with enzymes in their biosynthetic pathways.
Sucrose and hexose concentrations in the cytoplasm of plant leaf cells can be increased by inhibition of sugar transportation to the sink organ. Transgenic tobacco plants expressing an apoplastic yeast invertase maintained constant photosynthetic activity and higher turgor pressure under 300mM salt stress, whereas wild-type plants showed marked photoinhibition and a greater increase in osmotic pressure (Fukushima et al., 2001).
The nonreducing disaccharide trehalose protects biomolecules from environmental stress in many microorganisms. Most plant species, however, do not appear to accumulate trehalose to detectable levels. Increasing trehalose concentrations in plants could therefore enhance drought and salinity tolerance (Penna, 2003). Trehalose is produced from UDP-glucose and glucose-6-phosphate, via trehalose-6-phosphate, catalyzed by the enzymes trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase (Goddijn and Van Dun, 1999). Transgenic tobacco plants expressing the yeast trehalose-6-phosphate synthetase (TPS1) gene under control of the Rubisco small subunit (rbcS) promoter accumulated low concentrations of trehalose, ranging from 0.08% to 0.32% of the dry weight, in leaves and roots (Holmström et al., 1996). Plants exhibited significant growth retardation and detached leaves showed reduced water loss compared to controls, when subject to air-drying. In a similar experiment, transgenic tobacco plants expressing the yeast trehalose-6-phosphate synthetase (TPS1) gene under control of the CaMV 35S promoter accumulated trehalose up to 0.17mgg−1 fresh weight in leaves and showed increased drought tolerance (Romero et al., 1997).
These plants also exhibited phenotypic changes including stunted growth and lancet-shaped leaves, as well as reduced sucrose content. In this case, water loss from detached leaves was not significantly affected by trehalose accumulation. Given also that trehalose concentrations in transgenic plants were too low for a conventional osmoprotectant effect, these authors suggested that sugar synthesis had resulted in altered sugar metabolism (Romero et al., 1997). Recently, a trehalose-6-phosphate gene from Arabidopsis (AtTPS1) was introduced into tobacco under control of the CaMV 35S promoter (Almeida et al., 2005). Transgenic lines displayed higher germination frequencies on media containing 0.5M mannitol or 0.2M NaCl or under temperature stress at 15°C or 35°C. In accordance with earlier experiments, water loss in transgenic plants or detached leaves did not vary significantly between transgenic and wild-type plants (Almeida et al., 2005).
Overexpression of the E. coli trehalose-6-phosphate synthetase (otsA) and trehalose-6-phosphate phosphatase (otsB) genes in tobacco plants also enhanced trehalose synthesis (0.11mgg−1 fresh weight) with concomitant changes in leaf morphology (Goddijn et al., 1997). Despite the low-level accumulation of trehalose in transgenic tobacco expressing both otsA and otsB, an increase in dry mass and improved photosynthesis under drought stress was observed (Pilon-Smits et al., 1998). The low levels of trehalose accumulation observed in transgenic plants overexpressing genes of the trehalose biosynthetic pathway may result from the activity of an endogenous trehalase enzyme, which breaks down trehalose to glucose (Goddijn et al., 1997). When the potent trehalase inhibitor validamycin A was added to transgenic plants in vitro or growing hydroponically, trehalose accumulation increased up to 0.41mgg−1 fresh weight in tobacco leaves (Goddijn et al., 1997).
Fructans are soluble, polymers of fructose, used as storage sugars in many plants, but not tobacco. It has been suggested that, due to their solubility, fructans may also play a role in osmotic adjustment (Pilon-Smits et al., 1995). The SacB gene encoding levansucrase from Bacillus subtilis, fused to the carboxypeptidase Y vacuolar sorting signal from yeast, was introduced into tobacco under control of the CaMV 35S promoter to produce transgenic plants that accumulate bacterial fructans (Pilon-Smits et al., 1995). Fructan-producing plants performed significantly better under polyethylene-glycol-mediated drought stress, exhibiting 55% higher growth rates than wild-type plants resulting in 33% more fresh weight and 59% more dry weight (Pilon-Smits et al., 1995). This difference in weight was most pronounced in the roots. In addition to polyethylene-glycol-mediated drought stress, Konstantinova et al. 2002 found that tobacco plants carrying the SacB gene were able to recover from freezing stress (−2°C for 24h), whereas wild-type tobacco plants were not. Plant survival under freezing field conditions was also increased, allowing earlier planting. Fructan accumulation increased in the transgenic line during freezing stress and decreased after recovery (Konstantinova et al., 2002).
The sugar alcohol mannitol occurs widely in plants and animals, but is not normally synthesized in tobacco. Tarczynski et al. 1992 overexpressed the E. coli mannitol-1-phosphate dehydrogenase (mtlD) gene in tobacco, under control of the CaMV 35S promoter, resulting in biosynthesis of mannitol. Mannitol concentrations were greater than 6μmolg−1 fresh weight in the leaves and roots of some transformed plants (Tarczynski et al., 1992). Transgenic plants showed increased tolerance to prolonged high salt conditions (30 days of 250mM NaCl) (Tarczynski et al., 1993). Fresh weight loss in plants from mtlD lines was less than in control lines and transgenic plants increased in height by an average of 80% compared to 22% in control plants. However, since plants containing mannitol often produced new roots and leaves, the relative increases in fresh weight and height in these plants are likely due to new growth rather than a reallocation of resources (Tarczynski et al., 1993). Karakas et al. 1997 introduced the same mtlD cassette into tobacco and saw only marginal increases in dry mass under salt stress. Transgenic plants were 20–25% smaller than wild-type tobacco plants, but whereas salt stress decreased the dry weight of wild-type plants by 44%, transgenic plants suffered no reduction in dry weight. Since mannitol was shown to be a relatively minor osmolyte in transgenic tobacco, the slower growth of transgenic plants and not the presence of mannitol per se, may have resulted in greater salt tolerance. Shen et al. 1997 targeted mtlD to the chloroplasts by addition of an aminoterminal transit peptide. Mannitol accumulated at concentrations ranging from 2.5 to 7μmolg−1 fresh weight, with one line accumulating approximately 100mM mannitol in chloroplasts. These plants had increased resistance to methyl-viologen-induced oxidative stress, as demonstrated by increased retention of chlorophyll in leaves following treatment (Shen et al., 1997). The location of mannitol in chloroplasts appeared to increase the hydroxyl radical-scavenging capacity of cells, thereby reducing oxidative damage.
Transgenic tobacco plants with increased tolerance to salt and drought conditions were produced by introducing a myo-inositol O-methyltransferase (IMT1) cDNA from ice plant (Mesembryanthemum crystallinum) into tobacco under control of the CaMV 35S promoter (Sheveleva et al., 1997). In the halophytic ice plant, IMT1 methylates myo-inositol to form D-ononitol, a cyclic polyol, in a stress-inducible manner, likely resulting in decreased osmotic potential in the cytoplasm and regulation of sodium accumulation in the vacuole (Nelson et al., 1998). Transgenic tobacco plants accumulated D-ononitol in amounts exceeding 35μmolg−1 fresh weight, experienced less inhibition of photosynthetic CO2 fixation and recovered more quickly from salt or drought stress. One day after rewatering drought-stressed plants, photosynthesis had recovered 75%, compared to 57% in wild-type plants (Sheveleva et al., 1997).
2.3.2.6 Ectoine
Ectoine is a tetrahydropyrimidine that functions as a compatible solute in halophilic bacteria, where it is synthesized from aspartic β-semialdehyde by three successive enzyme reactions. The three ectoine biosynthetic genes, ectA, ectB, and ectC, from Halomonas elongate were each placed under control of the CaMV 35S promoter and introduced together into tobacco cv. Bright Yellow 2 (BY2) cells (Nakayama et al., 2000). Transgenic BY2 cells accumulated ectoine to low levels (14 to 79nmolg−1 fresh weight) and displayed tolerance to hyperosmotic shock (620mM mannitol or 500mM NaCl for 20min) (Nakayama et al., 2000). The ectoine biosynthesis genes were used to create transgenic tobacco plants that accumulated ectoine under salt stress conditions (Moghaieb et al., 2006). Transgenic plants showed less reduction in dry weight, photosynthetic rate, and impairment of stomatal conductance than wild-type plants, as well as increased Na+ concentrations in leaves and roots and increased osmotic adjustment (Moghaieb et al., 2006). Ectoine was suggested to improve maintenance of root function, so that water is taken up consistently under saline conditions and to increase transpiration and protect Rubisco proteins from salt, thereby increasing photosynthetic rate (Moghaieb et al., 2006).
2.3.3 Protective proteins
2.3.3.1 LEA and LEA-related proteins
Osmotic stress induces late embryogenesis-abundant (LEA) proteins in plant's vegetative tissues, a large and diverse group of stress-responsive proteins conferring dehydration tolerance (Bartels and Sunkar, 2005). Dehydrins are group-2 LEA proteins that respond to water stress in plants. Members of the LEA/dehydrin superfamily have been expressed in tobacco, to investigate their role in dehydration stress, including plant responses to freezing. Kaye et al. 1998 introduced cDNA sequences encoding the spinach CAP160 and CAP85 cold-acclimation proteins into tobacco, under control of the CaMV 35S promoter. Transgenic plants containing each sequence were crossed to obtain progeny expressing both proteins. The CAP160 sequence had very limited homology to the Arabidopsis rd29A and rd29B stress-regulated proteins and CAP160 mRNA expression was increased by low temperature and water stress (Kaye et al., 1998). Whilst the temperature at which 50% of cells were killed by freezing stress did not vary between transgenic and wild-type tobacco, plants expressing the spinach proteins suffered slightly less electrolyte leakage, suggesting a small reduction in freeze damage (Kaye et al., 1998). Citrus (Citrus unshiu Markov.) dehydrin (CuCOR19) cDNA was introduced into tobacco under control of the CaMV 35S promoter (Hara et al., 2003). Transgenic plants suffered less electrolyte leakage than control plants following 3h freezing stress at −4°C and also exhibited better seedling growth and earlier germination at 15°C. MDA content was lower in transgenic lines than control lines following freezing stress, indicating reduced lipid peroxidation (Hara et al., 2003). The CuCOR19 protein prevented peroxidation of soybean liposomes in vitro, suggesting that this dehydrin may act as a radical scavenging protein, protecting plant membranes.
2.3.3.2 Osmotin
Osmotin is a protein involved in adaptation to low water potential, but not induced by osmotic shock in cultured tobacco cells (Singh et al., 1989). Synthesis of mRNA encoding osmotin is either induced or stabilized by ABA, but accumulation of the protein is induced by adaptation to low water potentials via a post-translational control mechanism (Singh et al., 1989). The osmotin gene was overexpressed in tobacco plants under control of the CaMV 35S promoter (Sokhansanj et al., 2006). Transgenic plants were able to produce shoots on medium containing 320mM NaCl and showed comparable chlorophyll a content to wild-type plants (Sokhansanj et al., 2006).
2.3.3.3 Heat shock proteins
Maintenance of protein functional conformation and prevention of aggregation is important for cell survival following abiotic stress. Heat shock proteins (HSPs) are synthesized upon exposure to high temperature stress and assist in protein refolding under stress conditions (Wang et al., 2004). Overexpression of HSPs in tobacco has been used to improve understanding of HSP function during temperature stress.
A tobacco class I cytosolic small HSP gene, TLHS1, which showed a strong molecular chaperone activity in vitro, was overexpressed in tobacco under control of the CaMV 35S promoter (Park and Hong, 2002). Transgenic plants were less affected by heat stress at 40°C for 4h or 45°C for 1h, as measured by almost two times higher cotyledon opening rate in transgenic compared to control plants. A mitochondrial small HSP (MT-sHSP) cDNA from tomato (Lycopersicon esculentum) was introduced into tobacco under control of the CaMV 35S promoter (Sanmiya et al., 2004). Transgenic plants constitutively produced the MT-sHSP protein, accumulating higher levels after heat stress and were able to survive treatment at 48°C for 2h, in contrast to wild-type plants. Transgenic tobacco plants, expressing tobacco HSP70 (NtHSP70-1) under control of the CaMV 35S promoter, produced significantly higher levels of HSP70 in leaves than control plants, under nonstress conditions (Cho and Hong, 2006). Three-week-old, HSP70 overexpressing seedlings were able to maintain leaf turgidity following 2 weeks drought stress, whereas control plants showed severe wilting and stress symptoms. Expression of the CaERD15 (early responsive to dehydration) gene was considerably reduced in HSP70 overexpressing lines following drought stress, suggesting increased dehydration stress tolerance (Cho and Hong, 2006).
Alvim et al. 2001 transformed tobacco with the CaMV 35S promoter driving expression of the soybean (Glycine max) chaperone binding protein (BiP), a constitutively expressed HSP70 (HSC70) homolog and an important component of the endoplasmic reticulum stress response. Transgenic tobacco plants harboring a sense-orientation BiP gene displayed enhanced tolerance to the glycosylation inhibitor tunicamycin, which promotes the accumulation of unfolded proteins in the endoplasmic reticulum. Sense transgenic plants were able to maintain leaf turgidity under progressive dehydration stress, whereas antisense transgenic plants showed increased sensitivity to water stress (Alvim et al., 2001). BiP may act to alleviate oxidative stress, since levels of the antioxidant enzyme superoxide dismutase (SOD) were increased in control and antisense plants, but not in sense transgenic plants (Alvim et al., 2001).
2.3.3.4 Membrane damage—fatty acid metabolism genes
Membrane damage under cold stress may be dependent upon the degree of unsaturated fatty acids present in phosphatidyl-glycerol membranes. Chilling-sensitive plants possess a high degree of saturated fatty acids, whereas cold-tolerant plants contain a high proportion of unsaturated fatty acids. During acclimation to cold temperatures, desaturase enzymes increase the proportion of unsaturated fatty acids allowing membranes to remain fluid (Iba, 2002). Cold stress also increases production of free radicals leading to lipid peroxidation. This causes loss of unsaturated fatty acids, increase in membrane rigidity, and membrane degradation. Transgenic tobacco experiments have contributed to understanding the relationship between low temperature tolerance and membrane rearrangement and synthesis.
The effect of unsaturated membrane lipid levels on chilling tolerance was demonstrated by the introduction of a cDNA encoding glycerol-3-phosphate acyltransferase from chilling-sensitive squash into tobacco, under control of the CaMV 35S promoter (Murata et al., 1992; Moon et al., 1995). The transgenic plants showed increased saturation of chloroplast thylakoid membrane lipids, resulting in a slightly decreased ability of leaves to recover from low-temperature photoinhibition. Ishizaki-Nishizawa et al. 1996 introduced a broad-specificity Δ9 desaturase gene (des9) from the cyanobacterium Anacystis nidulans into tobacco under control of the CaMV 35S promoter. This desaturase, which was targeted to plastids, introduces a cis-double bond at the Δ9 position of both 16 and 18 carbon-saturated fatty acids. Transgenic plants had reduced saturated fatty acid contents in most membrane lipids and displayed tolerance to prolonged chilling (11 days at 1°C or 52 days at 10°C).
Tobacco was transformed with an Arabidopsis chloroplast ω-3 fatty acid desaturase gene (FAD7) under control of the CaMV 35S promoter to produce plants with increased levels of trienoic (16:3 and 18:3) fatty acids (Kodama et al., 1994). Transgenic plants displayed enhanced cold tolerance compared to wild-type plants, which showed inhibition of leaf growth after 7 days at 1°C. Khodakovskaya et al. 2006 introduced the FAD7 gene into tobacco under control of the cold-inducible Arabidopsis cor15a promoter. Compared to wild-type plants, transgenic plants showed greater survival under prolonged low temperature stress (44 days at 0.5, 2, or 3.5°C), more stable levels of trienoic fatty acids and stability of chloroplast membrane ultrastructure (Khodakovskaya et al., 2006). Conversely, transgenic tobacco lines in which the FAD7 gene was silenced contained lower levels of trienoic fatty acids and were able to acclimate better to higher temperatures (45 days at 36°C or 3 days at 47°C) (Murakami et al., 2000). Hamada et al. 1996 introduced a tobacco microsomal ω-3 fatty acid desaturase gene (NtFad3) in sense and antisense orientations under control of the CaMV 35S promoter. In one of the sense lines, the 18:3 content increased by about 1.5-fold in root tissues and 1.1-fold in leaf tissues, whereas in antisense lines, the 18:3 content was decreased to about 80% in root tissues and 70–80% in leaf tissues, compared to control plants (Hamada et al., 1996). Recently transgenic tobacco plants were produced that express the transcript of a double-stranded RNA (dsRNA) of the tobacco plastid ω-3 fatty acid desaturase gene NtFAD7 (Hamada et al., 2006). Compared to control plants, 16:3 and α-18:3 fatty acid contents decreased to less than 2.7% and 7.5–10.4%, respectively, in leaves of transgenic plants. Increased drought tolerance was observed in tobacco plants overexpressing cytosolic B. napus FAD3 or plastidic Arabidopsis FAD8 ω-3 fatty acid desaturase genes under control of the CaMV 35S promoter (Zhang et al., 2005). Plants expressing FAD3 showed a large increase in the ratio of linolenic (18:3) to linoleic (18:2) acids, whereas in FAD8 expressing plants, this increase was smaller and mainly associated with plastidic lipids.
2.3.4 Ionic stress
2.3.4.1 Transporter proteins
Maintaining intracellular ion homeostasis is critical to cell survival, affecting enzyme activities and membrane potentials. Exposure to high salt environments imposes sodium toxicity in addition to osmotic stress. Plants employ three mechanisms to prevent excess Na+ accumulation in cells (Zhu, 2003; Bartels and Sunkar, 2005). Na+ transporters restrict the entry of Na+ into cells; intracellular Na+ is compartmentalized into the vacuole; plasma membrane Na+/H+ antiporters transport cytosolic Na+ back into the external medium or the apoplast. Whilst many of the genes involved in ion homeostasis have been identified and their regulatory mechanisms elucidated in Arabidopsis, tobacco has provided a background to compare these genes with those of the halophyte Thellungiella halophila.
Gao et al. 2006 introduced vacuolar H+-pyrophosphatases (PPases) from T. halophila (TsVP) and Arabidopsis (AVP1) individually into tobacco, under control of the CaMV 35S promoter. These enzymes function to pump Na+ into vacuoles. At 300mM NaCl, transgenic plants had 60% greater dry weight than wild-type tobacco and higher viability of mesophyll protoplasts (Gao et al., 2006). Transgenic lines expressing TsVP were able to accumulate 25% more solutes than wild-type plants under nonstress conditions and 20–32% more Na+ under salt stress. Cell membrane damage and MDA content were reduced in TsVP-expressing plants, suggesting that compartmentalization of Na+ in vacuoles reduces its toxic effects on cells (Gao et al., 2006). Salt tolerance in tobacco was also improved by overexpression of the GhNHX1 cDNA, a putative tonoplast Na+/H+ antiporter from cotton (Gossypium hirsutum), under control of the CaMV 35S promoter (Wu et al., 2004).
2.3.4.2 Calcineurin
Calcineurin (CaN) is a Ca2+- and calmodulin-dependent protein phosphatase (PP2B) that is an integral intermediate of a salt stress transduction pathway in yeast effecting NaCl tolerance through regulation of Na+ influx and efflux. A truncated form of the catalytic subunit and the regulatory subunit of yeast CaN were co-expressed in tobacco, under control of the CaMV 35S promoter, to reconstitute a constitutively active phosphatase in vivo (Pardo et al., 1998). Transgenic lines exhibited increased tolerance to NaCl as seedlings in vitro or as plants in hydroponics. The protective effects of CaN in transgenic tobacco appeared to result from the preservation of root integrity during salt shock (Pardo et al., 1998). These results provided evidence that modulation of a signal transduction pathway in plants could be used to improve stress tolerance.
2.3.4.3 Calcium binding protein
Pandey et al. 2002 produced transgenic tobacco plants expressing a novel calcium binding protein from Entamoeba histolytica (EhCaBP), under control of the CaMV 35S promoter. Seeds of transgenic plants showed enhanced germination rates under nonstress conditions and seedlings produced 20–37% more dry weight than wild-type plants. Transgenic seeds were also able to germinate and grow on 200mM NaCl and produced 55–100% more dry weight compared to wild-type plants (Pandey et al., 2002).
2.3.5 Oxidative stress—detoxification
Abiotic stress often leads to severe cellular damage in plants as a result of oxidative stress. Antioxidant enzyme systems protect plant cells by either suppressing the production of, or by scavenging reactive oxygen intermediates (Mittler, 2002). Generation of transgenic tobacco plants overexpressing components of reactive oxygen-scavenging systems has been important in understanding how plants defend themselves against oxidative stress (Allen, 1995).
2.3.5.1 Reactive oxygen intermediate scavenging
SOD acts as a first line of defense against oxidative stress, converting superoxide anion radicals into hydrogen peroxide. Sen Gupta et al. 1993 introduced a chloroplastic Cu/Zn SOD from pea (Pisum sativum) into tobacco under control of the CaMV 35S promoter. During chilling stress at moderate light intensity, transgenic plants maintained photosynthetic rates approximately 20% higher than wild-type plants and were able to retain almost 90% of their photosynthetic capacity following chilling stress at high light intensity. Transgenic plants also exhibited reduced levels of light-mediated cellular damage following treatment with the active oxygen generator methyl viologen (Sen Gupta et al., 1993). These results demonstrated that SOD is an important component of the active-oxygen scavenging system in plants; however, a petunia chloroplastic Cu/Zn SOD introduced into tobacco under control of an rbcS promoter fragment increased SOD levels, but did not increase tolerance to ozone stress (Pitcher et al., 1991).
Mitochondrial manganese superoxide dismutase (MnSOD) from N. plumbaginifolia was introduced into tobacco under control of the CaMV 35S promoter, either as a full-length cDNA or with its mitochondrial leader sequence replaced by a chloroplast transit sequence (Bowler et al., 1991). High-level production of MnSOD in chloroplasts significantly reduced oxidative damage induced by methyl viologen in the light, whereas, under dark conditions, transgenic plants expressing either chloroplast- or mitochondria-targeted MnSOD were significantly more resistant to methyl viologen than control plants. In addition, transgenic plants overexpressing MnSOD in chloroplasts displayed enhanced tolerance to ozone damage (Van Camp et al., 1994). Chloroplastic iron superoxide dismutase (FeSOD) from Arabidopsis coupled to a chloroplast-targeting sequence was introduced into tobacco under control of the CaMV 35S promoter (Van Camp et al., 1996). Expression of FeSOD in transgenic plants was able to protect both the plasmalemma and photosystem II against oxidative damage caused by methyl viologen treatment. This is in contrast to overexpression of MnSOD, which only enhanced protection against ion leakage (Slooten et al., 1995).
Hydrogen peroxide resulting from SOD activity is scavenged by ascorbate peroxidases (APX), glutathione peroxidase (GPX) or catalase. APX and GPX require an ascorbate (AsA) or glutathione (GSH) regenerating cycle, involving the oxidation of AsA to monodehydroascorbate (MDA) or GSH to oxidized glutathione (GSSG). The role of APX in protection against oxidative stress was demonstrated through the production of transgenic tobacco plants expressing antisense copies of the tobacco cytosolic APX gene (Örvar and Ellis, 1997). Transgenic plants exhibited reduced APX activity and significantly higher levels of injury following high-level ozone exposure. Torsethaugen et al. 1997, however, observed that transgenic tobacco plants overproducing a chloroplast-targeted cytosolic APX isoform from pea showed no increase in protection against ozone-induced stress, compared to wild-type plants. Badawi et al. 2004 transformed tobacco with an Arabidopsis APX cDNA fused to a chloroplast transit sequence from Arabidopsis glutathione reductase (GR), under control of the CaMV 35S promoter. Transgenic plants showed increased tolerance to the active oxygen-generators methyl viologen and sodium sulfite, as well as enhanced tolerance to salt and water stress, as determined by net photosynthesis. Overexpression of the pepper (Capsicum annum) APX-like 1 gene (CAPOA1) in tobacco, under control of the CaMV 35S promoter, also resulted in transgenic plants with increase in total peroxidase activity (Sarowar et al., 2005). Transgenic plants exhibited increased tolerance to methyl-viologen-mediated oxidative stress, in addition to significantly increased growth. These results suggest that the tobacco active oxygen scavenging system can be enhanced by overproduction of APX.
Overexpression of a tobacco glutatione S-transferase with glutathione peroxidase activity (GST/GPX) in transgenic tobacco, under control of the CaMV 35S promoter, enhanced seedling growth under stress conditions (Roxas et al., 1997). Transgenic plants contained higher levels of GSSG and AsA and were able to maintain metabolic activity under stress conditions (Roxas et al., 2000).
Yoshimura et al. 2004 introduced a Chlamydomonas glutathione peroxidaselike protein, targeted to either the chloroplasts or cytosol, into tobacco under control of the CaMV 35S promoter. Transgenic plants showed increased tolerance to methyl-viologen-induced oxidative stress, chilling stress under high light conditions, and salt stress. Lipid hydroperoxidation was suppressed in leaves of transgenic plants compared to wild-type plants, under stress conditions, leading to maintenance of membrane integrity (Yoshimura et al., 2004).
Protection against methyl-viologen-induced oxidative stress could be increased by overexpressing both SOD and APX in chloroplasts of tobacco plants (Kwon et al., 2002). Chloroplast-targeted Cu/Zn SOD, MnSOD, and APX from pea, all under control of the CaMV 35S promoter, were introduced alone or in combination. Tolerance to oxidative stress was only slightly increased in transgenic plants expressing a single SOD isoform; however, the combination of SOD with APX resulted in greater protection (Kwon et al., 2002).
MDA radicals produced by APX are converted back to AsA through reactions with ferredoxin or MDA reductase. MDA can also spontaneously disproportionate to AsA and DHA, which is reduced to AsA in a reaction catalyzed by DHA reductase (DHAR), using GSH as the electron donor. GSSG is converted back to GSH by GR. Transgenic tobacco plants expressing a chloroplast-targeted human DHAR cDNA, under control of the CaMV 35S promoter, exhibited higher levels of AsA and GSSG than wild-type plants (Kwon et al., 2001). In addition to increased DHAR activity, GR activity was also increased in transgenic T0 and T1 plants (Kwon et al., 2001, 2003). Leaf discs of transgenic plants showed a reduction in membrane damage following treatment with methyl viologen or hydrogen peroxide compared to wild-type plants as well as enhanced tolerance to low temperature and salt stress. Eltayeb et al. 2006 introduced cytosolic DHAR from Arabidopsis into tobacco, under control of the 35S promoter. Transgenic plants exhibited greater tolerance to ozone, drought, salt, and polyethylene glycol (PEG)-induced stress than wild-type plants, manifested by higher net photosynthesis. A GR gene from E. coli was introduced into tobacco under control of the CaMV 35S promoter and targeted to the chloroplast (Aono et al., 1993). Increased GR activity in leaves of transgenic plants was correlated with increased tolerance of photooxidative stress induced by paraquat and sulfur dioxide.
Apoplastic acorbate oxidase (AAO) catalyzes the oxidation of AsA to MDA using oxygen. A tobacco AAO cDNA was introduced into tobacco plants in both sense and antisense orientation, under control of the CaMV 35S promoter (Yamamoto et al., 2005). In sense orientation, overexpression of AAO led to severe inhibition of germination by high salinity. In contrast, germination frequency, photosynthetic activity, root length, and seed yields were higher in antisense plants at high salinity, than in either wild type or sense plants (Yamamoto et al., 2005). The authors suggest antisense suppression of AAO leads to a relatively low level of hydrogen peroxide accumulation and a high redox state of apoplastic and symplastic ascorbate under salt stress.
Antisense technology in tobacco also provided evidence for an alternative defense mechanism that can compensate for the lack of APX and chloramphenicol acetyltransferase (CAT) (Rizhsky et al., 2002). Double antisense plants were less sensitive to oxidative stress than were transgenic plants lacking either APX or CAT. Increased protection against oxidative stress was correlated with reduced photosynthetic activity, the induction of MDAR, metabolic genes belonging to the pentose phosphate pathway and IMMUTANS, a chloroplast homolog of mitochondrial alternative oxidase (Rizhsky et al., 2002).
2.3.5.2 Lipid peroxidation
Free radical-mediated lipid peroxidation is accompanied by the generation of highly reactive aldehyde degradation products. Aldose/aldehyde reductases reduce a range of aldehydes and ketones to alcohols, whereas aldehyde dehydrogenases are important enzymes in the conversion of toxic aldehydes to less reactive carboxylic forms. Despite their potential roles in detoxification pathways, functional information on both types of enzyme is currently lacking. Transgenic overexpression in tobacco has provided insights into detoxification of reactive aldehyde species.
A stress-activated alfalfa gene (MsALR) encoding a novel plant NADPH-dependent aldose/aldehyde reductase was expressed in transgenic tobacco plants under control of the CaMV 35S promoter (Oberschall et al., 2000). Transgenic plants had greater tolerance to paraquat-induced oxidative stress and reduced accumulation of lipid peroxidation-derived reactive aldehydes. Plants expressing this enzyme were also able to recover from prolonged drought stress and showed tolerance to heavy metal treatment (Oberschall et al., 2000). Rodrigues et al. 2006 transformed tobacco with a soybean cDNA (GmTP55) encoding an ALDH7 aldehyde dehydrogenase, under control of the CaMV 35S promoter. Transgenic plants accumulated GmTP55 mRNA in leaves and showed tolerance to salinity (200mM NaCl) during germination and to water stress during plant growth. Transgenic plants also exhibited enhanced tolerance to H2O2- and paraquat-induced oxidative stress, associated with reduced concentrations of lipid peroxidation-derived reactive aldehydes (Rodrigues et al., 2006).
Enzymes of the glyoxalase pathway are required for glutathione-based detoxification of methylglyoxal (MG), a potent cytotoxic compound produced during lipid and carbohydrate metabolism and salt stress. Overexpression of glyoxylase I (glyI) from Brassica juncea, both under control of the CaMV 35S promoter, conferred tolerance to MG and high salinity in transgenic tobacco (Veena et al., 1999). When these plants were further transformed with the glyoxylase II (glyII) gene isolated from rice, transgenic plants exhibited even greater tolerance to high MG and NaCl concentrations and were able to grow, flower, and set seed under continuous salinity stress (Singla-Pareek et al., 2003). Increased levels of glutathione-related antioxidative enzymes enabled plants to resist an increase in MG levels under salinity stress, maintaining a higher GSH:GSSG ratio (Yadav et al., 2005).
2.4 Generalities on Plant Mitochondrial Genomes and Function
Mitochondrial biogenesis and functioning depend on both nuclear and mitochondrial DNA (mtDNA) encoded subunits. The mitochondrial (mt) genome in higher plants is larger and more complex than in other eukaryotes (Schuster and Brennicke, 1994). With some exceptions, plant mitochondrial genomes are maternally inherited, as in most animals. According to cosmid mapping, they may be represented as a single circular map, the so-called master molecule (Palmer and Shields, 1984), which can give rise to subgenomic circles by recombination between inverted or direct repeats (Backert et al., 1997). Subgenomic molecules are often in substoichiometric amounts, and can serve as reservoir for mitochondrial genome evolution (Small et al., 1989). Mitochondrial genomes are quite variable, ranging from 218 to 2500kb (Palmer and Herbon, 1988). Within one family, the Cucurbitaceae, mitochondrial genome sizes vary between 330kb and 2,500kb (Ward et al., 1981). However, the numbers of proteins specified by the smallest and largest of the mitochondrial genomes of the family are roughly similar (Stern and Newton, 1985).
In contrast to animal mitochondria, much of the mtDNA in plants appears to be noncoding. Indeed, all of the known genes account for only 10–20% of mtDNA of various species (Palmer et al., 2000). The noncoding mtDNA includes introns, pseudogenes, nonfunctional chloroplast sequences, and retrotransposons of nuclear origin. The coding part of the Arabidopsis thaliana mitochondrial genome consists of about 57 open reading frames (Unseld et al., 1997). Known genes found so far in all the sequenced plant mitochondrial genomes include those for ribosomal RNAs, tRNAs, and several mitochondrial proteins. The mtDNA proteins include several subunits of the complexes of the electron transfer chain: Complex I (NAD 1, 2, 3, 4, 4L, 5, 6, 7 and usually NAD9), Complex III (COB), Complex IV (COX 1, 2, 3), and Complex V (ATP 1, 6, 8, and 9). In addition, the mitochondrial genomes include genes for proteins involved in the biogenesis of cytochrome c (ccmB, FN, FC, and usually ccmC), as well as those for several ribosomal proteins.
Plant mtDNA mutants are scarce, as mtDNA usually has a slow rate of nucleotide substitution, although there are some exceptions (Palmer et al., 2000). Thus, most of the well-studied mitochondrial mutations derive from rearrangements and deletions. In some cases, these rearrangements are neutral and do not confer any marked phenotype, as in the Nicotiana sylvestris U mutant (Vitart et al., 1992; Albert et al., 2003). In contrast, several mtDNA reorganizations have been reported to be associated with cytoplasmic male sterility (CMS) that is thought to be caused by the expression of mitochondrial encoded chimeric proteins deleterious to pollen development. Nuclear genes, designated restorers of fertility (Rf) impair the production of CMS-associated polypeptides (discussed in Schnable and Wise, 1998; Budar et al., 2002; Wise and Pring, 2002). An important class of Rf genes consists of PPR transcription factors that are involved in the control of both chloroplastic and mitochondrial gene expression (Lurin et al., 2004). RNA editing in plant mitochondria, which involves the substitution of some C residues present in the initial transcript for U residues has also been associated with CMS in several species (Schuster and Brennicke, 1994). In contrast to CMS, mtDNA deletion mutants are impaired in both vegetative and reproductive development. They are generally heteroplasmic, with cells harboring a mixture of normal and deleted mtDNA molecules, and are not stably transmitted through sexual reproduction. In maize, mtDNA deletion mutations resulting in abnormal growth are termed nonchromosomal stripe (NCS). A number of maize NCS mutants have been analyzed and shown to result from deletions of portions of essential mitochondrial genes (Newton et al., 1989, 1990; Marienfeld and Newton, 1994). The heteroplasmic plants exhibit distorted growth, attributed to the loss of the mitochondrial protein synthesis in mutant sectors (Hunt and Newton, 1991; Roussell et al., 1991; Newton et al., 1996), but they can shed pollen from normal sectors. The Arabidopsis maternally inherited distorted leaf (MDL) phenotype has been associated with rearrangements and deletions in rps3/rpl16 (Sakamoto et al., 1996). In contrast, an abnormal growth mutant of cucumber, designated MSC (mosaic), is paternally transmitted (Malepszy et al., 1996). Near-homoplasmic deletion mutants stable by sexual reproduction have been reported in N. sylvestris, conferring growth retardation and partial male sterility (Li et al., 1988; Lelandais et al., 1998).
A major function of plant mitochondria is oxidative phosphorylation that couples electron transport through the respiratory chain to proton translocation from to the matrix to the intermembrane space, generating an electrochemical gradient necessary for ATP synthase (Siedow and Umbach, 1995). In addition to the main electron transport chain consisting of complexes I–IV, mitochondria in plants and some fungi possess nonproton-pumping respiratory enzymes encoded by the nuclear genome: various external and internal NAD(P)H alternative dehydrogenases by-passing Complex I (Rasmusson et al., 1999, 2004; Moller, 2001) and a cyanide-resistant terminal oxidase (AOX) branching at the level of the ubiquinone pool (Lambers, 1982; reviewed in Vanlerberghe and Mcintosh, 1997). In contrast to Complexes I, III, and IV, alternative respiratory enzymes are not directly involved in energy production. Hence, it has been proposed that AOX may prevent over-reduction of the respiratory chain, either in situations of excessive NADH supply as a consequence of a high TCA activity or a high ubiquinone reduction level during inhibition of the COX pathway (Lambers, 1982). Another role for alternative respiratory enzymes may be to minimize the formation of reactive oxygen species (ROS) by over-reduced electron transport chain components (Wagner and Krab, 1995; Purvis, 1997; Moller, 2001), particularly at the ubiquinone pool, and to limit oxidative stress (Popov et al., 1997; Maxwell et al., 1999). Accordingly, several studies have revealed new important functions for plant mitochondria, as the control of cell redox homeostasis and their importance for chloroplastic metabolism (Dutilleul et al., 2003a, 2003b). Plant mitochondria, like animal mitochondria seem to be involved in resistance to biotic and abiotic stresses and cell death control (Purvis and Shewfelt, 1993; Ordog et al., 2002; Tiwari et al., 2002); however, experimental evidence is scarce and precise mechanisms remain to be determined.
In addition to oxidative phosphorylation, mitochondria play numerous roles in plant metabolism (Douce and Neuburger, 1989; Mackenzie and McIntosh, 1999). They are directly involved in the synthesis of nucleic acids, pantothenate, and several amino acids as methionine, glycine, serine, and proline and indirectly, through S-adenosyl methionine, in all methylation reactions. They also contain branched-chain amino acid transaminases (BCATs) catalyzing the last step of the synthesis and/or the initial step of the degradation of leucine, isoleucine, and valine (Aubert et al., 1996; Anderson et al., 1998; Diebold et al., 2002). Plant mitochondria are involved in the C2 oxidative photosynthetic carbon cycle (Mouillon et al., 1999; Rébeillé et al., 2006) and possess all the necessary enzymatic equipment for de novo synthesis of tetrahydrofolate and lipoic acid, serving as cofactors for glycine decarboxylase and serinehydroxymethyltransferase functioning. The final steps of biotin and folate synthesis involve several mitochondrial proteins (Picciocchi et al., 2003). Folates are crucial intermediates for a set of reactions that involve the transfer of single-carbon units (C1 metabolism). Plant mitochondria also contain the enzymatic equipment necessary to transform malonate into the two main building units for fatty acid synthesis, malonyl- and acetyl-acyl carrier protein (ACP), a component of complex I (Gueguen et al., 2000), and have been reported to contribute to leaf lipid synthesis in Arabidopsis (Wada et al., 1997).
All the above processes are carried out by nuclear-encoded enzymes and thus manipulation of mitochondrial function might involve transformation of both nuclear and mitochondrial genomes. However, the only photosynthetic organism of which the mitochondrial genomes can be easily manipulated is Chlamydomonas reinhardtii (Remacle et al., 2006). Thus, in higher plants transgenesis essentially involves nuclear genes. For example, antisense repression of the mitochondrial NADH-binding subunit of complex I in transgenic potato plants affects male fertility (Heiser et al., 1997). In addition, the introduction of mitochondrial genes in the tobacco nuclear genome has been reported (Hernould et al., 1993; Pineau et al., 2005).
2.4.1 Nicotiana mtDNA mutants and transgenics
Tobacco species are a valuable model system for investigating the genetic interaction between mitochondria, chloroplasts, and nucleus of the plant cell. Indeed, the tobacco mtDNA is fully sequenced (Sugiyama et al., 2005), and stable mtDNA deletion mutants have been obtained by protoplast culture (reviewed in Vedel et al., 1999).
2.4.1.1 N. sylvestris mtDNA deletion mutants
In N. sylvestris, the female ancestor of the cultivated N. tabacum, protoplast culture has given rise to stable mutants with either neutral mtDNA rearrangements (Vitart et al., 1992; Albert et al., 2003) or mtDNA deletions (Li et al., 1988; Chétrit et al., 1992). These deletion mutants, designated CMSI and CMSII due to their partial male sterility, do not exhibit the leaf variegation described above for the other abnormal growth mutants, and their morphological defects are stably transmitted through sexual and somatic generations. This probably reflects the near-homoplasmic mutant composition of their mtDNAs that contain large deletions including the nad7 sequence encoding the NAD7 Complex I subunit (Pla et al., 1995; Gutierres et al., 1997; Lelandais et al., 1998). Respiration measurements on mitochondria isolated from CMS leaf tissues showed near complete lack of activity of Complex I and impaired oxidation of glycine, a major respiratory substrate in photosynthetic cells of C3 plants (Douce and Neuburger, 1989). In contrast, the oxidation rate of tricarboxylic cycle substrates and exogenous NADH were increased. Total leaf respiration measured by gas exchange experiments (IRGA) was higher in the mutant than in the WT (Duranceau et al., 2000; Sabar et al., 2000; Dutilleul et al., 2003a). Nonproton-pumping respiratory pathways maintain normal in vivo respiration levels in leaves of NAD7-deficient CMS I and II, but these rates are decreased in pollen (Sabar et al., 1998). Survival of these plants depends on the activation of nuclear-encoded internal and external alternative NAD(P)H dehydrogenases, which bypass Complex I (Rasmusson et al., 1999, 2004).
Cyanide-resistant respiration and alternative oxidase (AOX) protein levels were shown to be induced in CMS I and II (Gutierres et al., 1997; Sabar et al., 2000) as in all the maize NCS plants (Karpova et al., 2002). Because alternative oxidase is nucleus encoded and its induction can be seen at the RNA level, the mutant mitochondria must produce a signal to induce AOX gene expression. Furthermore, because AOX is also induced by electron transport inhibitors, such as antimycin A (Vanlerberghe and Mcintosh, 1997), it is apparent that dysfunction of the mitochondrial respiratory chain results in a strong signal for induction of AOX. However, use of oxygen isotope fractionation to measure the in vivo AOX activity (Guy et al., 1989) showed that in vivo partitioning through the AOX and the cytochrome (COX) pathways was similar in CMSII and wild-type leaves (Priault et al., 2007). This is not related to marked differences in the redox state of the protein, rather to either expression of a specific AOX gene and/or impaired metabolic control (Vidal et al., 2007). The higher respiration of mature CMSII leaves was supported exclusively by enhanced COX activity (Priault et al., 2007; Vidal et al., 2007). Enhanced activity of the proton-pumping COX route in the mutant can thus be viewed as a compensation for the lack of the first coupling site of the respiratory chain.
Complex I activities are also strongly reduced in a N. sylvestris nuclear mutant, termed NMS1, deficient for the NAD4 subunit (De Paepe et al., 1990; Brangeon et al., 2000). Alternative oxidase transcripts and proteins were induced, although to a lesser extent than in CMS, and external NAD(P)H dehydrogenase activities were not increased (Sabar et al., 2000). These results show that lack of NAD4 and NAD7 has different biochemical and physiological consequences. The relationships with the phenotype, that is more affected in NMS1 than in CMS plants, remain however to be determined.
In contrast to respiration, photosynthesis is affected in the N. sylvestris CMSI, CMSII, and NMS1 mutants (Sabar et al., 2000). It was demonstrated that mitochondrial Complex I activity is required for optimal photosynthetic performance and is necessary to avoid redox disruption of photosynthesis (Dutilleul et al., 2003a). CMSII plants are also impaired in acclimation to high light (Priault et al., 2006a), but have higher photorespiration (Priault et al., 2006b). These studies show the importance of mitochondria for chloroplast biogenesis and function, previously revealed by physiological and inhibitor studies (reviewed in Krömer, 1995; Raghavendra and Padmasree, 2003).
Moreover, the metabolite profile of CMSII leaves is enriched in amino acids with low C/N, and depleted in starch and 2-oxoglutarate (2-OG). The accumulation of nitrogen-rich amino acids was not accompanied by increased expression of enzymes involved in nitrogen assimilation (Dutilleul et al., 2005). Analysis of pyridine nucleotides showed that both NAD and NADH were increased by twofold in CMS leaves as compared to wild type, providing strong evidence that pyridine nucleotide availability exerts a crucial influence in the integration of ammonia assimilation and the anaplerotic production of carbon skeletons.
The N. sylvestris CMSII mutant was further exploited to explore the role of plant mitochondria in the regulation of cellular redox homeostasis and stress resistance. Acclimation in response to loss of Complex I function is associated with marked spatial and temporal reorganization of antioxidant and defense metabolism that affords enhanced protection to oxidative stress (Dutilleul et al., 2003b). The overall cellular redox state is maintained, as evidenced by lower H2O2, and ascorbate and glutathione redox states similar to the wild type. The reorganization of the antioxidant system, both inside and outside the mitochondria, is associated with enhanced resistance to ozone and TMV (Dutilleul et al., 2003b) and with altered reaction to harpin, a bacterial elicitor of the hypersensitive response (Boccara et al., 2001; Garmier et al., 2002).
To test whether N. sylvestris CMS defects directly result from the deletion of nad7, CMS transgenic plants carrying an edited nad7 cDNA fused to the CAMV 35S promoter and to a mitochondrial targeting sequence, were generated (Pineau et al., 2005). The nad7 sequence was transcribed, translated, and the NAD7 protein directed to mitochondria in CMS transgenics therefore termed CMSIInad7, which recovered both wild-type morphology and growth features. Blue-native/SDS gel electrophoreses and enzymatic assays showed that a functional complex was present in CMSIInad7 mitochondria, demonstrating that lack of complex I in CMSII was indeed the direct consequence of the absence of the NAD7 subunit. Hence, NAD7 is necessary for complex I assembly in plants. The reversion of the AOX expression pattern observed in the transgenic CMSnad7 was another indication for the restoration of the wild type properties of the respiratory chain. Furthermore, CMSnad7 plants harbored amino acid contents similar to the wild type (Dutilleul et al., 2005). Taken together, these results also show that allotopic expression from the nucleus can fully complement the lack of a mitochondria-encoded complex I gene.
2.4.1.2 N. tabacum mutants for mtRNA editing
Transgenic tobacco plants transformed with an unedited copy of the mitochondrial atp9 gene were partially male sterile (Hernould et al., 1993), whereas the expression of the antisense atp9 RNA abolished the effect of the unedited chimeric gene, by strongly decreasing the abundance of edited atp9 transcripts (Zabaleta et al., 1996). These results clearly indicate the importance of RNA editing for plant fertility.
2.4.2 Tobacco transgenics of nuclear genes for mitochondrial function and biogenesis
2.4.2.1 Respiratory chain enzymes
To date a few studies of plant transgenics for nuclear genes encoding respiratory proteins have been reported, possibly because most genes encoding main chain subunits are not single copy, and that double mutants are lethal. In Nicotiana species, the most extensive studies have concerned the alternative respiratory enzymes, AOX and alternative NAD(P)H dehydrogenases.
2.4.2.1.1 Alternative oxidase
The AOX protein is a homodimer with the two polypeptides linked by a disulfide bridge (Andersson and Nordlund, 1999; Juszczuk and Rychter, 2003). The reduced form is more active than the covalently linked oxidized form in in vitro assays (Umbach and Siedow, 1993; Day et al., 1994) and the enzyme is further activated by pyruvate and other α-ketoacids (Millar et al., 1993). However, the AOX activation state in vivo is not well understood (Millenaar et al., 2002). AOX genes belong to a multigene family, comprising at least two subfamilies, termed AOX1 and AOX2, with only limited nucleotide homology (Considine et al., 2002)
Sense and antisense DNA constructs of the N. tabacum Aox1 gene were introduced into tobacco, and transgenic plants with both increased and decreased levels of the AOX protein were analyzed (Vanlerberghe et al., 1994, 1995). Antisense cultured cells could not survive in the presence of inhibitors of the cytochrome pathway (Vanlerberghe et al., 1997, 2002), confirming that a critical function of AOX may be to support respiration when the main pathway is impaired.
Tobacco AOX sense and antisense transgenics were further used to demonstrate the critical role of plant mitochondria in response against abiotic and biotic stresses, including cell death (Maxwell et al., 1999; Ordog et al., 2002; Robson and Vanlerberghe, 2002; Gilliland et al., 2003; Amirsadeghi et al., 2006). In particular, antisense AOX transgenics were reported to be more susceptible to cell death inducers. However, subsequent measurement of AOX activity by oxygen isotope discrimination indicated that the in vivo AOX activity measured by oxygen isotope discrimination was similar in transgenics with either low or high protein amounts as in the wild type (Guy and Vanlerberghe, 2005), showing that complex regulations of whole mitochondrial metabolism rather than AOX activity per se was involved in the previously observed stress responses.
2.4.2.1.2 NAD(P)H dehydrogenases
Overexpression of the potato NDB1 gene in transgenic N. sylvestris demonstrated that this gene encodes an external dehydrogenase specific for NADPH and dependent on calcium for activity. Transformed plants had increased protein levels for alternative oxidase and uncoupling protein, indicating crosstalk for the different categories of energy-dissipating proteins that bypass oxidative phosphorylation (Michalecka et al., 2004)
2.4.2.2 TCA cycle enzymes
2.4.2.2.1 Isocitrate dehydrogenase
Transgenic N. tabacum plants overexpressing NADP+-dependent mitochondrial isocitrate dehydrogenase (mtICDH) displayed a measurable increase in the reductive activation of AOX in comparison with wild type, implicating this enzyme in the redox activation of AOX (Gray et al., 2004). These results support the hypothesis that mtICDH may be a regulatory switch involved in tricarboxylic acid cycle flux and the reductive modulation of AOX.
2.4.2.2.2 Aconitase
In animals, aconitase is a bifunctional protein that is involved in both TCA cycle functioning and RNA processing. A similar role was recently demonstrated in Arabidopsis and N. benthamiana transgenics. Aconitase-silenced plants displayed a delayed hypersensitive response (HR), suggesting that aconitase might play a role in mediating oxidative stress and regulating cell death (Moeder et al., 2007).
2.4.3 Other mitochondrial functions in relation with stress tolerance
Transgenic tobacco cells accumulating free proline by silencing proline dehydrogenase expression were tolerant to osmotic stress (Tateishi et al., 2005). Transgenic tobacco plants overexpressing the tomato mitochondrial small heat-shock protein exhibited normal morphology and growth rates, but showed higher tolerance to heat stress, whereas antisense plants were more susceptible (Sanmiya et al., 2004).
2.4.4 Concluding remarks
Nicotiana mtDNA mutants and nuclear transgenics for mitochondrial enzymes have been invaluable tools for the study of plant respiration and of the involvement of mitochondrial metabolism in cell redox homeostasis, stress resistance, and cell death, in plants as in animals (Desagher and Martinou, 2000). However, in photosynthetic cells, the crosstalk between mitochondrial and chloroplast metabolism seems to be of crucial importance and needs further investigation.
2.5 Plastid Transformation
N. tabacum was the first higher plant to receive an engineered plastome (Svab et al., 1990) and all basic principles of plastid transformation technology are derived from the work in this species. Tobacco was chosen mainly due to its performance in tissue culture and to the fact that the tobacco plastome was the first to be fully sequenced (Shinozaki et al., 1986; last update Yukawa et al., 2005)
A query to PubMed for plastid or chloroplast transformation results in more than 25 review articles. Some of these are compiled in Table 1. This chapter does not attempt to give a fully comprehensive overview on all data published on tobacco plastid transformation. Rather, it presents aspects that complement the aforementioned reviews, such as significant findings or those, which have not been fully discussed previously. Almost all of the review articles stress the potential advantages of plastid versus nuclear transformants, i.e., high expression level, engineering and co-expression of several genes in polycistronic operons, transgene containment due to maternal inheritance, lack of gene silencing, and unknown position effects and precision of engineering via homologous recombination. The emphasis on potential benefits combined with limited discussion of problems or failures has generated very high expectations with respect to the value of the technology as a platform for recombinant protein expression or generation of agronomically important traits. It should be kept in mind, however, that expectations are not always met. Expression levels of recombinant proteins may indeed be extremely low if the protein of interest is efficiently degraded in the organelle (Leelavathi and Reddy, 2003; Birch-Machin et al., 2004). Also, the lack of post-translational modifications such as glycosylation in plastids severely limits the value of this expression platform where higher order modification of the end product (e.g., a human protein) is of importance for function and/or regulatory aspects.
Authors |
Year |
Title |
|---|---|---|
Maliga et al. |
Toward plastid transformation in flowering plants |
|
Kofer et al. |
PEG-mediated plastid transformation in higher plants |
|
Hager and Bock |
Enslaved bacteria as new hope for plant biotechnologists |
|
Heifetz |
Genetic engineering of the chloroplast |
|
Bock |
Transgenic plastids in basic research and plant biotechnology |
|
Heifetz and Tuttle |
Protein expression in plastids |
|
Maliga |
Progress toward commercialization of plastid transformation technology |
|
Bock and Khan |
Taming plastids for a green future |
|
Maliga |
Plastid transformation in higher plants |
|
Daniell et al. |
Chloroplast-derived vaccine antigens and other therapeutic proteins |
|
Daniell et al. |
Breakthrough in chloroplast genetic engineering of agronomically important crops |
|
Maliga |
New vectors and marker excision systems mark progress in engineering the plastid genome of higher plants |
|
Nugent and Joyce |
Producing human therapeutic proteins in plastids |
|
Dhingra and Daniell |
Chloroplast genetic engineering via organogenesis and somatic embryogenesis |
|
Lutz et al. |
Construction of marker-free transplastomic tobacco using the Cre-loxP site-specific recombination system |
|
Bock |
Plastid biotechnology: prospects for herbicide and insect resistance, metabolic engineering, and molecular farming |
|
Koop et al. |
The genetic transformation of plastids |
2.5.1 Vector design
Plastome transformation is either based on the activity of the organelle's own endogenous recombination capacity or the use of a heterogeneous recombination system, such as the phiC31 phage integrase.
2.5.1.1 Use of the endogenous recombination system
Plant plastid transformation has mainly been achieved through the use of sequences on an engineered transformation vector containing sufficient homology to the target plastome to allow for homologous recombination mediated by the organelle's recombination system. These so-called “homologous flanks” are generally approximately 1kbp in size assuming that shorter flanks would reduce recombination efficiency while significantly longer flanks make cloning more difficult. Expression cassettes in plastid transformation vectors must take into account that regulatory elements, such as promoters, 5′ untranslated regions (UTRs), ribosome binding sites, and 3′ UTRs need to be compatible with the plastid gene expression machinery. A heterologous RNA polymerase can also be used for transcription, if the expression cassette is equipped with a suitable promoter (McBride et al., 1995). Further modifications may include a “downstream box” for enhanced translation efficiency (Kuroda and Maliga, 2001; Herz et al., 2005), fusion, and/or purification tags for enhanced protein stability and facilitation of protein extraction (Leelavathi and Reddy, 2003), and proteolysis recognition sites if authentic starting amino acids are required for a processed protein end product (Staub et al., 2000). A sample plastid transformation vector for the insertion of a dicistronic operon is depicted in Figure 2. A selectable marker gene is certainly also required for the transformation process. If the second cistron is used for this purpose, it is safe to assume that the protein encoded by the first cistron is expressed in the recovered transformants. Alternatively, a selection marker cassette could also be positioned elsewhere on the same transformation vector or on a different transformation vector and used in a co-transformation approach, which works efficiently in plastid transformation (Carrer and Maliga, 1995; Herz et al., 2005). Up to four genes combined in a single operon were successfully introduced into the tobacco plastome (Lossl et al., 2003; Quesada-Vargas et al., 2005), and a principle limitation of the number of cistrons that can be co-introduced into and co-expressed in a plastome cannot be seen.
Note that not all elements depicted in Figure 2 are indispensable. Thus, separate promoters are not required, if transcription is mediated by endogenous transcription start signals (Staub and Maliga, 1995). Such “operon extension vectors” are described in detail by Herz et al. 2005. It is also possible to use incomplete expression cassettes on co-transforming, separate transformation vectors, since complete and functional expression cassettes can be assembled by homologous recombination inside the plant after transformation treatment (Herz et al., 2005).
Elements of a dicistronic tobacco plastid transformation vector
2.5.1.2 Use of the phiC31 phage integrase
It has been speculated that difficulties in transferring the plastid transformation methods developed for tobacco to other species might be related to differences in the efficiencies of the endogenous recombination systems among different species. It is therefore useful to investigate the suitability of heterogeneous systems. Lutz et al. 2004 demonstrated that the integrase of phage phiC31 can be used for integrating foreign DNA into the tobacco plastome if supplied via expression from a nuclear gene and targeted to the plastid or—although less efficiently—if supplied through a transiently expressed plastid vector. While the approach is certainly interesting, its value remains to be demonstrated. Integration using this recombination system requires the presence of a suitable recognition sequence (attB) at the desired integration locus of the target plastome. This, however, must be generated through the endogenous recombination system first, which may be difficult, if indeed the efficiency of the endogenous recombination system was the limiting factor in a particular species.
2.5.2 Target tissues
The larger size of chloroplasts in comparison with proplastids made leaf explants the target tissue of choice for plastid transformation treatments. Indeed, green tissue or protoplasts prepared thereof were the targets for the first successful plastid transformations in tobacco (Svab et al., 1990; Golds et al., 1993) and other species (N. plumbaginifolia: O'neill et al., 1993; Arabidopsis thaliana: Sikdar et al., 1998; S. tuberosum: Sidorov et al., 1999; L. esculentum: Ruf et al., 2001; B. napus: Hou et al., 2003; Lesquerelly fendleri: Skarjinskaia et al., 2003; Petunia hybrida: Zubko et al., 2004; Lactuca sativa: Lelivelt et al., 2005; Populus alba: Okumura et al., 2006). However, nongreen tissues, such as in tobacco, protoplast-derived microcolonies (Huang et al., 2002), albino leaves (Klaus et al., 2003) or cell suspensions (Langbecker et al., 2004), and cell cultures of other species (Oryza sativa: Khan and Maliga, 1999; Daucus carota, G. hirsutum: Kumar et al., 2004a, 2004b; Glycine max: Dufourmantel et al., 2004) have also been used successfully. It is therefore evident that plastid transformation does not require fully developed chloroplasts. This is of significance since in important crops, such as the cereals, regeneration from green tissues is not yet possible.
2.5.3 Methods of gene delivery
There are two methods to deliver transforming DNA into tobacco plastids, which make it possible to recover stably transformed lines, the particle gun-mediated biolistic process and treatment of isolated protoplasts with PEG in the presence of suitable transformation vectors. For detailed protocols the reader is referred to the reviews listed in Table 1 (see e.g., Dhingra and Daniell, 2006 and Lutz et al., 2006a, for particle bombardment and Kofer et al., 1998a for PEG treatment). The mechanism of entry of the transforming DNA is assumed to be by mechanical impact in the case of the biolistic procedure: microprojectiles supposedly penetrate the organelle's envelope thus carrying the DNA inside. It is not known whether or how a chloroplast envelope would reseal after penetration. With PEG-treatment the mechanism of DNA entry into a cell and then through the two envelope membranes is even less clear. The assumption derived from transient expression assays with nuclear reporter constructs is that PEG produces transient discontinuities or “holes”, in the plasma membrane through which DNA can enter into the cell (Paszkowski et al., 1984). Such a process would lead to entry of plasmids into the cytosol, and it remains completely unknown how, subsequently, the DNA could reach the inside of an organelle. If, however, there is transfer of DNA from the cytosol into organelles that have an envelope consisting of two membranes, then it is also conceivable that, through particle bombardment plasmids are primarily delivered into the cytosol and enter the organelle afterward. Experiments that would elucidate the mechanisms of DNA uptake are difficult to conceive. In tobacco, plastid transformation is highly efficient irrespective of the method used for DNA delivery and the precise pathway of DNA entry.
It is important to note that a femtosyringe-based microinjection procedure was used to deliver reporter genes into plastids (Knoblauch et al., 1999; Van Bel et al., 2001), and transient reporter gene expression was clearly achieved; however, stable transformants were not generated. When species, closely related to tobacco, prove recalcitrant to plastid transformation, an interesting approach can be used exploiting the fact that plastids in tobacco can be transformed. Kuchuk et al. 2006 transformed the plastomes of five different recalcitrant solanaceous species after generating cytoplasmic hybrids with tobacco supplying the nuclear genome and the other species donating the cytoplasmic genomes.
2.5.4 Selection systems
Selection systems for higher plant plastid transformation need to fulfil several highly demanding requirements. Selective advantage must be generated on two levels, that of the single plastid and that of the individual cell. It is assumed that the initial transformation event involves a single or few of the high number—up to 10000 per cell in a fully developed leaf (Bendich, 1987)—of plastid chromosomes only. The kinetics of the increase of the transplastome copy number during selection is not known. It has been speculated that the presence of an origin of replication on the transformation vector might allow for multiplication of the vector inside the organelle, which increases the number of transformation events (Dhingra and Daniell, 2006). However, there is no experimental evidence supporting this assumption. Moreover, the origin of replication used by the Daniell laboratory was shown to be dispensable for replication (Muhlbauer et al., 2002). While prolonged presence of vector plasmid molecules has indeed been observed, it is more likely that such molecules are derived from recombination rather than replication events (Staub and Maliga, 1994; Klaus et al., 2004). Replication of the plastome itself, possibly in combination with gene conversion events (Khakhlova and Bock, 2006), therefore, appears to be the cause of increase of transplastome copy number in an organelle. Although molecules are exchanged between individual plastids of a cell (Kohler et al., 1997), there is no evidence that this process also involves plastid DNA. Therefore, the assumption is that plastid DNA replication and plastid division are both necessary processes leading to the increase of numbers of organelles containing transformed DNA copies inside a cell. Selection systems need to favor those plastids that contain the highest proportion of transformed plastomes and/or those cells that contain the highest proportion of plastids with transformed plastomes. At the same time, nontransformed plastids and cells without transformed plastids need to stay viable. Selection pressure, therefore, has to be adjusted carefully.
It is noteworthy that transmittance of different plastome copies to the products of organelle division and transmittance of organelles to the products of cell division are both random processes. Segregation, i.e., the generation of cells, which are homozygous with respect to their plastomes, termed “homoplastomic”, from cells containing different types of plastomes, termed “heteroplastomic”, does not require selective pressure. Segregation is a statistical process (Michaelis, 1966) and cannot be avoided, unless there is counter selection, for example, in the case of the disruption or deletion of an essential gene (Drescher et al., 2000).
Different schemes of selection were used in tobacco plastid transformation. Selection using an antibiotic always comprised the initial step of primary selection, and in some schemes this was followed by secondary selection using a different inhibitor or a pigmentation phenotype. Screenable markers, such as the GFP were also used to assist detection of transformed sectors of tissues in tobacco (Khan and Maliga, 1999) and other species (Sidorov et al., 1999; Skarjinskaia et al., 2003).
2.5.4.1 Primary selection using antibiotic inhibitors
Different selection markers have been used successfully, all of which are based on tolerance toward aminoglycoside antibiotics. Tolerance is either based on mutations of the ribosomal RNA target site (Svab et al., 1990) or on the expression of detoxifying enzymes (Carrer et al., 1993; Svab and Maliga, 1993; Huang et al., 2002). Aminoglycoside antibiotics are inhibitors of protein biosynthesis on prokaryotic/organelle ribosomes and should, therefore, also inhibit mitochondrial protein biosynthesis. Detoxifying enzymes should reduce the concentration of the antibiotic in question in the whole cell and should, therefore, also reduce the effect of the inhibitors on mitochondria. However, in the case of insensitive plastid ribosomal RNA target sites it is unclear why the supposed inhibition of protein biosynthesis in the mitochondria is tolerated by the cell. Possibly, growth and development in vitro, i.e., under heterotrophic conditions, can occur with reduced mitochondrial protein biosynthesis.
Aminoglycoside antibiotics that have been used successfully for the selection of plastid transformants in tobacco are listed in Table 2.
A report that was published under the interesting title “Marker free transgenic plants:…….” received considerable attention. The plant nuclear gene badh encoding BADH, in combination with betaine aldehyde as the selective agent, was described as an alternative selection system and was claimed to be far superior to those listed in Table 2 (Daniell et al., 2001). Certainly, irrespective of its functionality as a selection marker, the badh gene can be expressed in higher plant plastids (Kumar et al., 2004a, 2004b). However, the conclusion presented in Daniell et al. 2001 is curious in that the vectors used for transformation maintained other selectable markers in addition to a badh selection cassette (compare Maliga, 2005). Furthermore, neither the Daniell group nor any other laboratory has since reported successful selection using betaine aldehyde, and a number of laboratories failed to independently reproduce the results. In the absence of reproducibility, attempts to select plastid transformants via the betaine aldehyde system should be avoided. Chloroplast engineering requires a substantial investment of human and laboratory capital and other methods of selection have proven to be effective.
Alternatives to the antibiotic resistance genes listed in Table 2 have not proven reproducibly effective. Herbicides could not be used for selection, although expression of herbicide resistance genes is possible in plastids and can lead to considerable levels of tolerance (Daniell et al., 1998; Iamtham and Day, 2000; Lutz et al., 2001; Ye et al., 2001, 2003). A similar situation was found for other marker genes, which have been successful in selection of nuclear transformants, such as hygromycin phosphotransferase (Dhingra and Maliga, personal communication), sulfadiazin insensitive dihydropteroate synthase, and blasticidin deaminase (U.-H. Koop Lab, personal communication).
2.5.4.2 Secondary selection
Herbicide tolerance can be used to accelerate segregation once a sufficient number of transformed plastid genome copies have accumulated (Iamtham and Day, 2000). As an alternative, pigmentation can help detect sectors containing transformed plastome copies and at the same time considerably accelerate segregation toward homoplastomy. Klaus et al. 2003 used targeted inactivation of pigmentation-related plastid genes to generate lines with pale to white phenotypes. In a second round of transformation, the inactivated genes were reintroduced. Transformation events re-established the wild-type green phenotype, which made it possible to easily detect such transformed regenerants. Regreening could not be achieved by spontaneous mutations. Thus, the appearance of green regenerants clearly indicated true plastid transformation events. In addition, PCR analysis showed that the first regenerated shoots were already homoplastomic for the second transformation, therefore the usual repetitive cycles of regeneration in the presence of selection was no longer required. Evidently, under the culture conditions chosen, the green tissues had a strong selective advantage over the pigment deficient ones.
2.5.5 Strategies for removal of selectable marker
Public concern, whether scientifically justified or not, requests removal of antibiotic resistance marker genes from transgenic plants intended for human consumption or animal feed. Marker removal approaches are of benefit when the number of available selection markers is low and multiple consecutive transformation steps are required for generating a desired end product. Furthermore, expression of marker genes, not required in established transplastomic lines, constitutes an unnecessary metabolic burden on transplastomic plants. In plastid transformation, four different strategies are available and have been successfully applied to tobacco: use of direct repeats included in the transformation vector, use of separate transformation vectors harboring selection marker and gene of interest in a co-transformation approach, use of site-specific recombinases with segregation of different plastomes, and use of a transformation vector architecture, which leads to co-integrate formation and subsequently to automatic marker elimination.
2.5.5.1 Direct repeats for marker removal
Higher plant plastids have a highly active recombination system. This needs to be considered, when designing transformation vectors to avoid undesired rearrangement through direct repeat-mediated loop-out recombination of introduced sequences (Maliga et al., 1993; Zou et al., 2003). On the other hand, direct repeat-mediated loop-out recombination can also be used for marker removal (Iamtham and Day, 2000). Since the timing of loop-out recombination cannot be controlled, and since transformants cannot be distinguished from wild-type lines, this system requires a secondary selection system, e.g., herbicide resistance (Iamtham and Day, 2000). Interestingly, the same approach can be applied for targeted gene inactivation in chloroplasts (Kode et al., 2005, 2006).
2.5.5.2 Co-transformation and segregation
Ye et al. 2003 used two different vectors and a selection scheme, initially based on spectinomycin as the selective inhibitor and subsequently on herbicide selection. The rationale behind this scheme is that initial selection with herbicides is not possible in plastid transformation. However, after enrichment for transplastomic plastome copies, the level of herbicide tolerance might be sufficient to, in a heteroplastomic situation, allow for segregation of lines, which carry the herbicide but not the antibiotic resistance genes. Indeed, 20% of the recovered lines fulfilled this criterion. As with direct repeat-mediated loop-out recombination, the approach requires a secondary marker, i.e., the resulting lines are not “marker-free”, and the expression of the secondary marker might constitute an unnecessary metabolic burden.
2.5.5.3 Use of site-specific recombinases
Hajdukiewicz et al. 2001 and Corneille et al. 2001 independently and simultaneously introduced CRE recombinase-mediated marker removal from transplastomic tobacco. The expression of the CRE protein-encoding gene, derived from the P1 bacteriophage, leads to insertion or excision of sequence elements, provided that recognition elements, loxP sites, are present on the recombination substrate molecules. Marker gene removal thus requires directly repeated loxP elements flanking the marker gene in the plastome. CRE recombinase can be expressed from a nuclear expression cassette, translated in the cytosol and then introduced into the plastid through the organelle's import machinery. Surprisingly, not only the desired excision events are observed, but additional plastome rearrangements that are not necessarily only due to “cryptic” lox sites in the plastome (Corneille et al., 2003) but are either based on short direct repeats or on recombination “hot spots”. CRE recombinase seems to generally increase recombination activity in the plastome. It is not understood why the frequency of plastome rearrangements depends on the way, in which the recombinase is introduced into the transplastomic lines. This can either be by Agrobacterium-mediated stable or transient (Lutz et al., 2006a, 2006b) nuclear transformation or by crossing a transplastomic line with a suitable nuclear transformant. Marker removal through Agroinfiltration-based transient expression (Lutz et al., 2006a, 2006b) is efficient and clearly is preferable since removal of stably integrated expression cassettes from the nuclear genome is not necessary. In addition, plastome rearrangements are not occurring once CRE recombinase is no longer present.
Marker-removal via co-integrate formation. (a) conventional transformation vector and co-integrate resulting from recombination via the 5′ flank; (b) novel transformation vector with the selection marker cassette outside the homologous flanks
2.5.5.4 Automatic marker removal through co-integrate formation
Integration of foreign DNA into the plastome mediated by two homologous flanking regions requires two events of reciprocal recombination. If these recombination events occur more or less simultaneously, the resulting transformed plastome contains the sequences flanked by the homologous regions. Klaus et al. 2004 observed that occurrence of the recombination events at different time points is the rule rather than the exception, and that both homologous flanks participate in the initial recombination event with the same probability. Recombination via—initially—a single flank only leads to the formation of a co-integrate, i.e., the whole vector plasmid will be integrated and the homologous flanks will be duplicated to generate direct repeats. This is shown for integration via the 5′ homologous flank in Figure 3a. Note that the process occurs with the same frequency via the 3′ flank. Because of the presence of direct repeats, which present substrates for loop-out recombination, co-integrates are inherently unstable and either reversal to wild-type plastomes (recombination between elements “2” in Figure 3a) or generation of molecules identical to those formed through two simultaneous recombination events (recombination between elements “5” in Figure 3a) will occur.
Klaus et al. 2004 took advantage of the fact that the vector backbone is initially integrated and is removed by the organelle's recombination system later by positioning the selection marker expression cassette outside the sequences between the homologous flanks (Figure 3a). As long as the presence of a selection inhibitor is maintained there will be a selection against wild-type plastomes and the number of plastomes containing co-integrates will increase. On removal of the selection pressure co-integrates are resolved resulting in plastomes identical to those of the acceptor lines and marker-free plastomes containing the gene of interest. Phenotypical selection using pigmentation or other visible markers assists in detecting the regenerates containing the gene of interest. However, it has also been shown that PCR screening easily identifies the transformed regenerants. Thus, the approach does not depend on the availability of pigment mutant lines. A simple change in vector design leads to automatic marker removal without the need for any other genes or markers.
2.5.6 Chloroplast biology and engineering of important traits
After the first successful demonstration of stable plastid transformation in tobacco (Svab et al., 1990), the methodology has been utilized for understanding basic chloroplast biology and expressing desirable traits. It is noteworthy to mention that advances in the understanding of chloroplast gene expression using plastid transformation in tobacco have led to more efficient vector designs and thus better expression of introduced genes.
2.5.6.1 Understanding chloroplast biology
Chloroplast gene expression is regulated at transcriptional, translational, and post-translational levels. All these stages of gene expression regulation have been investigated in transplastomic tobacco. Understanding of chloroplast gene function and its relation to chloroplast biology was achieved via targeted inactivation of individual genes. Availability of the tobacco chloroplast genome sequence was very beneficial in this exercise, as the sequence and identity of individual genes were already known (Shinozaki et al., 1986; Yukawa et al., 2005). Only the crucial plastid transformation experiments that contributed toward advancing our understanding of chloroplast biology are discussed here.
The chloroplast genetic machinery is prokaryotic in nature and very strong biochemical evidence existed for a functional plastid genome-derived RNA polymerase activity (Hu and Bogorad, 1990; Hu et al., 1991). This fact was not only confirmed via plastid transformation but individual function of the RNA polymerase subunit genes was also ascertained using the transplastomic lines where the subunit genes had been knocked out (Allison et al., 1996; Hajdukiewicz et al., 1997; Serino and Maliga, 1998; De Santis-Maciossek et al., 1999; Krause et al., 2000). Further, the basal plastid promoter and its constituents were identified using a series of promoter deletions in transplastomic tobacco (Allison and Maliga, 1995; Shiina et al., 1998).
The chloroplast genome contains light-regulated photosynthesis-related genes and their expression generally responds positively to light and anterograde signals. Specifically, red and blue light are involved in upregulation of gene expression (Sexton et al., 1990; Grover et al., 1999). The blue light-regulated promoter region of the psbD-C operon was initially investigated in barley and in vitro experiments clearly defined the elements responsible for blue light perception (Christopher et al., 1992). Function of the blue light-regulated psbD-C promoter was confirmed and specific elements characterized using plastid transformation (Allison and Maliga, 1995). It has recently been reported that green light, thought to be a benign part of the light spectrum, negatively regulates plastid gene expression (Dhingra et al., 2006). It would be interesting to identify DNA elements that participate in this unique phenomenon using plastid transformation.
Perhaps one of the most important discoveries that resulted from plastid transformation experiments in tobacco was the identification of second transcription machinery in the plastids (Allison et al., 1996; Hajdukiewicz et al., 1997; Kapoor et al., 1997). The beta subunit of plastid-encoded RNA polymerase was disrupted and that resulted in the detection of the additional polymerase activity (Allison et al., 1996). The information regarding two polymerase plastid transcription machineries is now being incorporated into plastid transformation vector designs.
The chloroplast genome sequence of tobacco revealed the presence of ndh genes based on homology with mitochondrial respiratory chain complex I subunit genes coding for proton pumping NADH:ubiquinone oxidoreductase (Shinozaki et al., 1986; Fearnley and Walker, 1992), but the existence of a functional respiratory chain complex I in chloroplasts was a matter of debate. Individual ndh subunits were disrupted in tobacco and existence of a functional Ndh complex in mature chloroplasts was still demonstrated. It was also shown that the complex is dispensable under normal growth conditions but may be essential under stress (Burrows et al., 1998; Kofer et al., 1998b).
Other targeted inactivation studies include rbcL knockout to facilitate Rubisco engineering (see Section 2.6; Kanevski and Maliga, 1994). The sprA gene was proposed to participate in maturation of 16S rRNA but transplastomic plants with an inactivated sprA were normal (Sugita et al., 1997). In another study attempts were made to disrupt the ycf9 gene but were unsuccessful. Although actual function of ycf9 was not ascertained, it was clear that it had a vital role in plant survival (Maenpaa et al., 2000). Another open reading frame coded by ycf5 was targeted for deletion to study its function. Null mutants of ycf5 were pale green and electron flow around photosystem II (PS II) was found to be affected indicating a role of this gene product in the generation of functional PS II units (Tsuruya et al., 2006). It was revealed that the rps18 gene is indispensable in higher plants, whereas this gene is absent in plastids of nongreen unicellular organisms (Rogalski et al., 2006). Targeted inactivation of psaJ gene had no apparent effect on plant growth but under limiting light conditions it is essential for efficient photosystem I excitation (Schottler et al., 2007). There are other open reading frames (ORFs) in the chloroplast genome of higher plants whose function remains to be understood. Chloroplast transformation approach will continue to expand our understanding of chloroplast biology.
2.5.6.2 Recombinant proteins
Several recombinant proteins have been expressed via plastid transformation in tobacco. Some of the recent reviews provide a list of these proteins with other pertinent details (Daniell et al., 2005c; Grevich and Daniell, 2005). In order to avoid redundancy only recent reports are mentioned here. Four new reports add to the expanding list of biopharmaceutical proteins being expressed via tobacco plastid transformation. Foot-and-mouth disease virus (FMDV) VP1 gene was expressed at 2–3% of total soluble protein in tobacco chloroplasts (Li et al., 2006a). Li et al. 2006b also expressed a partial spike (S) protein of SARS-CoV to generate a vaccine against the severe acute respiratory syndrome (SARS) corona virus in tobacco. Authors indicate this as a first step toward creating an oral vaccine against the SARS virus. Another report details successful expression of anti-Epstein-Barr Virus viral capsid antigen (VCA) using tobacco plastid transformation at 0.04% tsp (Lee et al., 2006). For all these reports immunogenicity remains to be tested. More recently vaccine against amoebiasis has been generated in transplastomic tobacco. The LecA gene used for generating the antigen is expressed at 6.3% tsp and is able to elicit an immunogenic response in mice (Chebolu and Daniell, 2007). These reports present exciting evidence that transplastomic engineering may produce important products for human benefit. However, successful implementation of such technologies will depend on plant cultivation at an agricultural scale. Another recurring theme is the mention of “oral vaccines”, yet these proteins are being expressed in a nonfood and nonfeed crop, tobacco. If oral vaccines are to become a reality, the protein will need to be extracted from tobacco and then administered orally or expressed in other edible crops like tomato.
Other important recombinant proteins can be grouped under the biomaterials, enzymes, and amino acids categories. Two biopolymers have been successfully expressed in transplastomic tobacco. These include p-hydroxybenzoic acid and polyhydroxybutyrate (Lossl et al., 2003; Viitanen et al., 2004). If the production can be increased to industrial levels, plant-based generation of biopolymers will be extremely beneficial in the health care sector. Xylanase (an enzyme) and tryptophan (an amino acid) have also been reported to be well expressed when biosynthesis genes were integrated into the tobacco plastid genome (Zhang et al., 2001; Leelavathi et al., 2003).
2.5.6.3 Agronomic traits
The tobacco plastid genome has been engineered for conferring biotic and abiotic stress tolerance. Disease and insect resistance have been engineered by expressing antimicrobial peptide and the Cry family of genes (see De Cosa et al., 2001; Daniell et al., 2005c; Grevich and Daniell, 2005; Chakrabarti et al., 2006). Drought tolerance was conferred in tobacco by the expression of trehalose phosphate synthase gene (Lee et al., 2003). Effective phytoremediation of mercury was also reported in transplastomic tobacco expressing the bacterial merA and merB genes (Ruiz et al., 2003).
2.5.6.4 Inducible expression
There are several reasons, why inducible expression in plastids is desirable. Metabolic drain during growth and development could be avoided, if an economically feasible preharvest induction was available. Likewise, negative effects of gene product(s) or metabolic changes caused by the function of introduced genes might be a problem, if expression was constitutive. Furthermore, presence of pharmaceutical gene products or metabolites throughout the whole growth phase of a plant is more demanding in terms of biosafety and regulatory control than precisely controlled short-term presence. Finally, for basic research it would be valuable if plastid gene expression could be switched on and off at desired time points.
Expression of plastid genes is mediated by regulated promoters, which supply transcriptional control depending on physiological, developmental, or tissue specificity parameters. Therefore, inducible expression in plastids can only be achieved using heterologous control elements.
The first system that was described used a plastid transgene under the control of the phage T7 promoter in combination with import of the T7 polymerase encoded by a nuclear transgene (McBride et al., 1995). Although using this system-controlled expression is achieved to a certain extent (Magee et al., 2004) and negative effects on fertility observed during constitutive expression of PHB genes were abolished, when these genes were transcribed by an ethanol induced T7 polymerase (Lossl et al., 2005), the system is not optimal. Plastid gene expression is altered in the presence of T7 polymerase even if they do not contain a T7 promoter (Magee and Kavanagh, 2002), and the low level of expression typical for most nuclear inducible systems even in the noninduced state may be sufficient to cause an undesirable phenotype (Magee et al., 2004).
An alternative approach uses constitutive repression of a transgene by the lac repressor and induction by application of isopropyl-β-D-galactopyranoside (IPTG), which leads to an increase of the protein in question by about 20-fold (Mühlbauer and Koop, 2005). While this system is attractive since it uses regulatory elements located in the plastid only and does not require additional nuclear transgenes, it is also not optimal, since, probably due to read-through transcription from a different promoter, there is low level expression in the noninduced state and spraying IPTG in greenhouses or even on open fields does not seem economically feasible. This problem might be at least partially overcome by performing postharvest induction (Mühlbauer and Koop, 2005)
Buhot et al. 2006 reported using the E. coli groE heat shock promoter, a eubacterial promoter, which is not recognized by the nuclear (NEP)- and plastid (PEP)-encoded transcription systems present in plastids. Controlled expression is achieved through a chimeric transcription factor that mediates interaction of NEP and the eubacterial promoter. The system was tested and shown to function via transient expression of the transcription factor, and the performance in stably transformed plants remains to be seen. Like in the case of T7 polymerase/T7 promoter, induction is indirect, i.e., via a protein that is encoded by a nuclear transgene.
Recently, a further approach was described by Tungsuchat et al. 2006. It is based on CRE recombinase-mediated rearrangement of a transplastome. In this case, a gene of interest lacking an AUG translation start codon is linked to the nonexcised start codon of the marker gene through excision of the selection marker gene. The advantage of the system lies in the fact that it is not sensitive to read-through transcription. Control is by generating a translatable open reading frame. Prior to excision there is no detectable gene product (GFP) of the gene of interest, while accumulation of GFP is found up to be 0.3% of the total cellular protein after excision. Again, a transgene expressed from the nucleus is required to trigger plastid expression: primary transplastomic lines harboring an inactive gene of interest were transformed in their nuclear genome using Agrobacterium-mediated gene transfer. It remains to be seen, how the approach can be adapted for practical purposes.
While all the approaches toward inducible plastid gene expression may be valuable for basic research and for lab-scale expression studies, none of these systems is suitable for production-scale or field applications. Further improvements of the described systems and/or development of alternatives are required. Thus, inducible expression remains being a prominent challenge in plastid transformation technology.
2.6 Understanding Rubisco Function in Higher Plants for Improving Photosynthesis
Plants are remarkable factories: efficient green assembly lines that convert inert atmospheric gas and water into complex chemical compounds using solar energy. The foundation of this feat is the capture of atmospheric carbon dioxide through specialized structures and then its integration into higher-order structures starting with Ribulose-1, 5-bisphospate carboxylase/oxygenase (Rubisco). Rubisco is a unique chloroplast stromal enzyme that in higher plants is composed of eight plastid genome-derived large subunits and eight small subunits encoded in the nucleus and imported from the cytosol (Houtz and Portis, 2003). The efficiency of carbon dioxide fixation by Rubisco is negatively impacted by its oxygenase activity (Ogren, 2003). Although an efficient system, Rubisco is an attractive target for engineering added capacity to fix atmospheric carbon. At a time where man seeks to exploit the energy locked in organic bonds present in recent tissues rather than fossil fuels, and when alleged greenhouse gases may negatively shape climate change, research into integration of more atmospheric carbon into usable forms is timely and important. Research endeavors to understand Rubisco action with an aim of improving it has been carried out mainly in tobacco. Most have involved suppression/deletion of individual subunits or expression of both native and heterologous subunits in the nuclear or plastid compartment.
In 1988, the first transgenic Rubisco mutant was reported (Rodermel et al., 1988). This transgenic tobacco mutant was generated to understand coordinated expression of nuclear and organellar genes in the biosynthesis of Rubisco. The mutant is severely depleted in photosynthesis and Rubisco enzyme has been extensively studied to understand the role of the small subunit and the impact of Rubisco depletion on photosynthesis and carbon–nitrogen flux, as well as plant growth and development (Hudson et al., 1992; Jiang and Rodermel, 1995; Fritz et al., 2006).
Most experiments pertaining to Rubisco engineering described so far have attempted to establish an experimental system to assemble a chimeric Rubisco. One of the strategies employed for engineering of Rubisco was to relocate the large subunit to the nuclear genome (Kanevski et al., 1999). Transgenic tobacco plants were generated where the plastid resident large subunit was deleted using plastid transformation. This transgenic mutant plant was used as a host to engineer the large subunit in the nuclear genome. Although expression of the large subunit in the nucleus and its subsequent import into the chloroplast was normal, Rubisco enzyme content and activity were approximately 10% and 3% of the wild-type levels. Thus, the transgenic plants were severely depleted in Rubisco enzyme and its activity (Kanevski et al., 1999). Experimental simulations predict that Rubisco derived from the photosynthetic bacteria Chromatium vinosum should exhibit superior performance compared to higher plant Rubisco (Bainbridge et al., 1995). With this premise, the large subunit gene derived from C. vinosum was engineered in the nuclear genome of Rubisco deficient tobacco mutant SP25. The bacterial large subunit gene was transcribed in the cytosol but no large subunits or Rubisco activity was detectable (Madgwick et al., 2002). From the preceding reports one can discern that integration and allotopic expression of the large subunit of Rubisco is possible but functionality remains a problem. One can only speculate on the reasons for this failure. Post-translational modifications, import or different codon bias of the introduced genes may be responsible for lack of functionality.
Plastid transformation is routinely performed in tobacco (Dhingra and Daniell, 2006). As an alternate approach, plastid transformation was employed to express heterologous or native subunits of Rubisco in the plastid genome of tobacco. The native rbcL gene in tobacco was replaced via homologous recombination with the rbcL gene derived from Synechococcus or Helianthus annus. Chloroplast transgenic tobacco plants expressing the algal rbcL gene lacked photoautotrophic growth and the large subunit protein was completely absent. Plants expressing the Helianthus rbcL gene were compromised in growth, as the carboxylase activity of the hybrid Rubisco was 20% of the wild-type levels (Kanevski et al., 1999). Other reports have attempted to engineer Rubisco genes from algae that exhibit superior enzymatic properties relative to the higher plant enzyme. In these instances, Rubisco is produced from a dicistronic unit comprised of the large and small subunit gene. Expression of rbcLS operons from the rhodophyte Galdieria sulphuraria and the diatom Phaeodactylum tricornutum resulted in abundant foreign Rubisco expression but enzyme assembly remained an issue (Whitney et al., 2001). Perhaps the assembly machinery is specific to the native subunits; thus foreign subunits remain unassembled or incorrectly assembled. A homodimeric form of the Rubisco encoding gene RbcM derived from proteobacterium, Rhodospirillum rubrum was integrated into the chloroplast genome of tobacco replacing the native rbcL gene. This enzyme is unique as it has no small subunit and thus has no assembly issues for activity. Growth of transgenic tobacco plants was supported in carbon dioxide enriched atmosphere and the catalytic properties were similar to the algal enzyme (Whitney and Andrews, 2001b, 2003).
The native tobacco small subunit gene has also been integrated into its own plastid genome. A His-tagged version of the RbcS gene was introduced into the wild-type background. A very low level of plastid-derived small subunit protein was detected (∼1% of total small subunit protein) in the chloroplasts of transgenic plants (Whitney and Andrews, 2001a). This continuum of reports reveals the lack of a system to appropriately express and assemble chimeric Rubisco proteins. One recent report used a creative approach of using the antisense RbcS tobacco plant to engineer the small subunit gene in the plastid genome. Although the introduced RbcS gene was well expressed it was not able to complement the deficiencies generated due to silencing of the small subunit (Zhang et al., 2002). Importantly, this report became a prelude to the successful demonstration of a functional chloroplast-derived Rubisco in a following report. The plastid genome of the RbcS antisense mutant was used as a host to express tobacco RbcS gene with enhanced translation (Dhingra et al., 2004). From this report it seemed that translation was a limiting factor that was overcome by the use of specific 5′ and 3′ untranslated regions that have been previously shown to result in improved expression of chloroplast expressed genes (Eibl et al., 1999). The report by Dhingra et al. 2004 demonstrated that it was feasible to assemble Rubisco entirely in the chloroplast but it remains to be seen if heterologous subunits will be efficiently assembled using the expression enhancements that were described.
3 Conclusions
Twenty-five years ago tobacco was a rapidly regenerating system stemming from a crop with economic impact that is closely related to other high value crops. For these reasons it was held up as the system of choice for pilot transformation studies of both the nucleus and plastid. Upon review of the literature it is clear that these original studies set a stage for the flurry of work soon to follow in Arabidopsis—a laboratory-scale system with easier transformation, a smaller genome, and much faster life cycle. Recent studies in N. benthamiana have brought the genus back to the limelight as an outstanding system for transient protein expression. Overall, it is always important to understand the utility of this system, its advantages and limitations, as it still may be relevant to facets of discovery in plant biology. As a nonfood and nonfeed crop tobacco retains a remarkable potential for use as a biofactory. Ironically, this genus with a notorious health reputation may prove to be indispensable for the production of medically relevant compounds.
- 2-OG
- 2-oxoglutarate
- AAO
- Apoplastic acorbate oxidase
- ABA
- abscisic acid
- ACC
- 1-aminocyclopropane-1-carboxylic acid
- ACP
- acetyl-acyl carrier protein
- ADC
- arginine decarboxylase
- AOX
- alternative oxidase
- APX
- ascorbate peroxidases
- Aea-TMOF
- Aedes aegypti
- BADH
- betaine aldehyde dehydrogenase
- BCATs
- branched-chain amino acid transaminases
- BY2
- Bright Yellow 2
- BiP
- binding protein
- Bt
- Bacillus thuringiensis
- CAT
- chloramphenicol acetyltransferase
- CDH
- choline dehydrogenase
- CMO
- choline monooxygenase
- CMS
- cytoplasmic male sterility
- COD
- choline oxidase
- CP
- coat protein
- CaMV 35S
- cauliflower mosaic virus 35S
- CaN
- Calcineurin
- CpTI
- cowpea trypsin inhibitor
- DHAR
- DHA reductase
- FAD7
- fatty acid desaturase gene
- FMDV
- Foot-and-mouth disease virus
- FeSOD
- Chloroplastic iron superoxide dismutase
- Fed1
- ferredoxin 1
- GABA
- Gamma-aminobutyrate
- GFP
- green fluorescent protein
- GNA
- snowdrop lectin
- GPX
- glutathione peroxidase
- GR
- glutathione reductase
- GSH
- glutathione
- GUS
- β-glucuronidase
- GlyBet
- Glycine betaine
- HR
- hypersensitive response
- HSPs
- Heat shock proteins
- IAA
- β-indoleacetic acid
- IMT1
- myo-inositol O-methyltransferase
- IPTG
- isopropyl-β-D-galactopyranoside
- LBAM
- lightbrown apple moth
- LEA
- late embryogenesis-abundant
- LRR
- leucine-rich repeat
- MDA
- malondialdehyde
- MDA
- monodehydroascorbate
- MDL
- maternally inherited distorted leaf
- MG
- methylglyoxal
- MS
- Murashige and Skoog
- MT-sHSP
- mitochondrial small HSP
- MnSOD
- Mitochondrial manganese superoxide dismutase
- NCS
- nonchromosomal stripe
- ODC
- ornithine decarboxylase
- ORFs
- open reading frames
- P5CS
- pyrroline-5-carboxylate synthetase
- PAL2
- phenylalanine lyase
- PAs
- Polyamines
- PDR
- pathogen-derived resistance
- PEAMT
- phosphoethanolamine N-methyltransferase
- PEG
- polyethylene glycol
- PI
- proteinase inhibitor
- PS II
- photosystem II
- PTGS
- post-transcriptional gene silencing
- PTM
- potato tuber moth
- PhyA
- phytochrome A
- ROS
- reactive oxygen species
- Rf
- restorers of fertility
- SAM
- S-adenosylmethionine
- SARS
- severe acute respiratory syndrome
- SI
- spleen trypsin inhibitor
- SOD
- superoxide dismutase
- TIR
- toll-interleukin
- TMOF
- trypsin modulating and oostatic factor
- TMV
- tobacco mosaic virus
- TPS1
- trehalose-6-phosphate synthetase
- TPS1
- trehalose-6-phosphate synthetase
- UTRs
- untranslated regions
- VCA
- viral capsid antigen
- VIGS
- virus-induced gene silencing
- cDNA
- complementary DNA
- cab
- chlorophyll a/b binding protein
- dsRNA
- double-stranded RNA
- glyI
- glyoxylase I
- glyII
- glyoxylase II
- ipt
- isopentenyl transferase
- mt
- mitochondrial
- mtDNA
- mitochondrial DNA
- mtICDH
- mitochondrial isocitrate dehydrogenase
- phyB
- phytochrome B
- phyC
- phytochrome C
- pta
- Pinellia ternata agglutinin transgene
- rbcS
- ribulose 1-5 bisphosphate carboxlase/oxygenase
- rbcS
- Rubisco small subunit
- samdc
- S-adenosylmethionine decarboxylase
- δ-OAT
- ornithine-δ-aminotransferase



