3 ‘Omics’ Approaches Towards Understanding Plant Phosphorus Acquisition and Use
Abstract
Low availability of inorganic phosphate (Pi) induces dramatic changes in developmental and metabolic programs as well as in transcriptomic, proteomic and metabolomics profiles. Technical advances in ‘bottom-up’ omics technologies have allowed novel and integrative insights into acclimative responses of plants, most of which are readily applicable to the development of Pi-efficient crops. In this chapter, recent omics-based progress in the genome-wide analysis of transcriptional and post-transcriptional gene regulation, Pi starvation-induced histone modifications, and metabolic alterations that lead to remodelling of membrane lipids and rerouting of the carbohydrate flux is reviewed. Inferred from publicly available data sets, suites are further defined of commonly identified Pi-responsive genes and proteins as core Pi starvation-inducible (PSI) genes (the ‘phosphatome’) and proteins in the model species Arabidopsis thaliana.
3.1 Introduction
Phosphorus (P), taken up as inorganic phosphate (Pi) by plants, is involved in multiple crucial cellular processes, including protein activation, energy transfer, signalling and photosynthesis. Phosphate is an essential component of fundamental macromolecules such as nucleic acids, ATP and phospholipids, and is thus indispensable for life. Due to its tendency to form complexes with soil components, in most natural and agricultural soil systems the bioavailability of Pi is far below the plant's demand (Chapter Phosphorus: Back to the Roots). In agricultural settings, Pi deficiency is a major cause of severe yield losses and poor quality of edible plant parts. Low Pi availability is often corrected by the application of large quantities of fertilizers, but this is unsustainable and associated with environmental pollution and substantial costs. An understanding of how plants acclimatise to low Pi availability is therefore critical for the development of a Pi-efficient germplasm.
- Morphological changes, comprising a reduction in primary root growth, an increase in the density and length of lateral roots, and the formation of longer and denser root hairs, collectively leading to improved topsoil foraging.
- Increased excretion of organic acids and Pi-releasing enzymes such as RNases and purple acid phosphatases (PAPs), resulting in a larger rhizospheric Pi pool for root uptake.
- Dynamic regulation of the expression and activity of high-affinity Pitransporters, controlling the influx of extracellular Pi.
- The reprogramming of lipid and carbohydrate metabolism to improve Piuse efficiency (PUE).
To orchestrate these responses, plants have evolved systems to sense the Pi-status both locally and systemically, and to regulate the activity of Pi-starvation-inducible (PSI) genes and post-translational modifications (PTMs) of key proteins according to the plant's demand (Chapters Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications and The Role of Post‐Translational Enzyme Modifications in the Metabolic Adaptations of Phosphorus‐Deprived Plants). While several components of the Pi-signalling pathway have been identified (chiefly by genetic approaches), a sensor for Pi is yet to be discovered. Detailed information on Pi sensing and signal transduction, and the importance of protein PTMs to the Pi starvation response (PSR) are provided in Chapters Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications and The Role of Post‐Translational Enzyme Modifications in the Metabolic Adaptations of Phosphorus‐Deprived Plants.
Over the past decade, ‘omics’ approaches which are mainly focused at the RNA and protein levels have been used extensively to investigate and correlate global gene and protein expression changes under Pi-limiting conditions. Large suites of genes (>1000) are regulated by Pi. These studies have identified several novel players and regulators of the PSR (Chapter Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications) (also reviewed by Chiou & Lin, 2011; López-Arredondo et al., 2014). In spite of the much smaller number of studies using proteome profiling of plant responses to Pi deficiency, several hallmark processes that allow plants to acclimatise to low Pi availability have been uncovered by means of proteomic approaches. Moreover, several Pi-responsive proteins are not regulated at the transcriptional level, indicating that these two approaches are complementary and both are required to acquire a comprehensive, integrative picture of how plants acclimatise to Pi limitation.
3.2 Towards a Transcriptomics-Derived ‘Phosphatome’
The genome-wide interrogation of changes in gene expression upon Pi deficiency can be divided into four types: (i) early studies using custom-made microarrays with limited probe sets (e.g. Wu et al., 2003); (ii) studies relying on commercial microarrays such as the Affymetrix ATH1 Genome Array that can probe approximately 24 000 genes in the model plant Arabidopsis thaliana (e.g. Misson et al., 2005; Morcuende et al., 2007; Lin et al., 2010); (iii) next-generation sequencing-based techniques (RNA-seq), which provide digital information on gene expression without the limitation of predefined probes (e.g. Lan et al., 2012; Secco et al., 2014), and (iv) small RNA-seq aimed at identifying miRNAs and other small RNAs that accumulate differentially upon Pi starvation (Hsieh et al., 2009). Transcriptome profiling studies have been carried out in Arabidopsis (Wu et al., 2003; Misson et al., 2005; Morcuende et al., 2007; Lin et al., 2011; Lan et al., 2012; Woo et al., 2012), rice (Li et al., 2009; Li et al., 2010; Oono et al., 2011; Cai et al., 2012; Oono et al., 2013a; Secco et al., 2013), maize (Li et al., 2012; Lin et al., 2013) and other plant species such as white lupin (Lupinus albus L.), wheat (Triticum aestivum L.) and bean (Phaseolus vulgaris L.) (Hernandez et al., 2007; Aparicio-Fabre et al., 2013; Oono et al., 2013b; Secco et al., 2014).
Although transcriptomic studies have uncovered a surprisingly large number of PSI genes, most of these genes are not shared among the different studies; this can be attributed to stochastic fluctuations, variations in growth conditions, sample handling, subtle differences in the experimental design, or combinations of these factors. To identify genes that are robustly affected by Pi across a wider range of conditions, publicly available transcriptomic data were surveyed to define such a population of commonly identified Pi-responsive genes. Since the majority of transcriptomic studies focused on the reference species Arabidopsis, the core PSI genes are depicted here for Arabidopsis. Three microarray studies (Misson et al., 2005; Morcuende et al., 2007; Woo et al., 2012) and one RNA-seq data set (Lan et al., 2012) have been chosen for this attempt, since all studies were conducted either with whole seedlings or roots and the plants used in these studies were of similar age. The overlap of differentially expressed genes between these surveys (the ‘phosphatome’) was identified by comparing PSI genes of the four data sets considering genes that displayed a fold-change in gene expression that was greater than or equal to two with P ≤ 0.05. This procedure yielded a subset of 95 PSI genes, herein referred to as core PSI genes (Figure 3.1; Table 3.1).
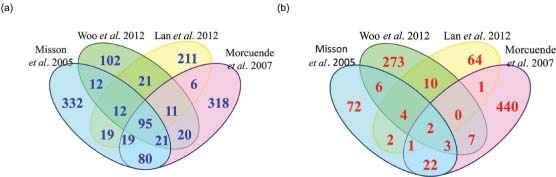
| Treatments Tissues Locus | Biol. process | Misson et al. 2005 | Morcuende et al. 2007 | Woo et al. 2012 | Lan et al. 2012 | |||
|---|---|---|---|---|---|---|---|---|
| -Pi 3, 6, 12 h Seedlings | -Pi 1-2 d | low Pi media | -Pi 2-3 d | -Pi 10 d | -Pi 3 d | |||
| Roots | Seedlings | Roots | Roots | |||||
| Fold-changes (-Pi/+Pi) | ||||||||
| Galactolipid biosynthesis, lipid metabolism | ||||||||
| At5g20410 a | MGD2, MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE 2 | Galactolipid biosynthetic process | 10.3 | 14.3 | 11.2 | 20.5 | 12.7 | 9.1 |
| At2g11810 | MGD3, MONOGALACTOSYL DIACYLGLYCEROL SYNTHASE 3 | Galactolipid biosynthetic process | 7.0 | 26.0 | 48.0 | 61.4 | 95.8 | 69.5 |
| At1g08310 | Alpha/beta-Hydrolases superfamily protein | Galactolipid biosynthetic process | 11.4 | 38.5 | 10.0 | 13.9 | ||
| At3g44510 | Alpha/beta-Hydrolases superfamily protein | Galactolipid biosynthetic process | 4.2 | 4.9 | 7.6 | 3.9 | 4.4 | |
| At2g42690 | Alpha/beta-Hydrolases superfamily protein | Galactolipid biosynthetic process | 5.7 | 2.1 | 4.8 | 3.7 | ||
| At1g17710 | PEPC1, PHOSPHOETHANOLAMINE/PHOSPHOCHOLINE PHOSPHATASE 1 | Galactolipid biosynthetic process | 21.3 | 58.1 | 54.2 | 98.4 | 70.0 | 1785.6 |
| At3g05630 | PHOSPHOLIPASE D ZETA 2, PLDZETA2 | Galactolipid biosynthetic process | 9.7 | 21.6 | 19.1 | 21.8 | 18.9 | 21.8 |
| At1g73010 | PS2, PHOSPHATE STARVATION-INDUCED GENE 2 | Galactolipid biosynthetic process | 17.6 | 38.3 | 36.2 | 174.10 | 63.5 | 72.5 |
| At1g30500 | NUCLEAR FACTOR Y, SUBUNIT A7”, NF-YA7 | Galactolipid biosynthetic process | 2.8 | 5.0 | 4.3 | 3.5 | ||
| At1g19200 | Protein of unknown function | Galactolipid biosynthetic process | 37.5 | 21.1 | 10.2 | 11.5 | 6.1 | |
| At1g67600 | Acid phosphatase/vanadium-dependent haloperoxidase-related protein | Galactolipid biosynthetic process | 17.8 | 16.1 | 9.7 | 10.6 | 15.4 | |
| At3g03790 | Ankyrin repeat family protein / regulator of chromosome condensation (RCC1) family protein | Galactolipid biosynthetic process | 5.8 | 5.1 | 7.9 | 3.3 | 2.5 | |
| At3g04530 | PHOSPHOENOLPYRUVATE CARBOXYLASE KINASE 2, PPCK2 | Galactolipid biosynthetic process | 3.9 | 2.3 | 12.0 | 4.5 | 4.3 | |
| At4g00550 | DIGALACTOSYL DIACYLGLYCEROL DEFICIENT 2, DGD2 | Galactolipid biosynthetic process | 4.8 | 3.8 | 2.8 | 2.6 | ||
| At3g56040 | UDP-GLUCOSE PYROPHOSPHORYLASE 3, UGP3 | Galactolipid biosynthetic process | 4.1 | 7.1 | 7.4 | 7.5 | 5.0 | |
| At4g23000 | Calcineurin-like metallo-phosphoesterase superfamily protein | Galactolipid biosynthetic process | 3.2 | 4.4 | 5.6 | 5.3 | 10.2 | |
| At4g33030 | SQD1, SULFOQUINOVOSYLDIACYLGLYCEROL 1 | Galactolipid biosynthetic process | 2.6 | 3.0 | 10.0 | 6.9 | 9.5 | 8.1 |
| At5g01220 | SQD2, SULFOQUINOVOSYLDIACYLGLYCEROL 2 | Glycolipid biosynthetic process | 5.0 | 15.8 | 12.4 | 19.8 | 17.3 | 10.5 |
| At4g00500 | Alpha/beta-Hydrolases superfamily protein | Lipid metabolic process | 2.3 | 3.5 | 5.7 | 2.9 | 2.6 | |
| At3g02040 | GDPD1, GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE 1 | Lipid metabolic process | 11.4 | 12.5 | 33.3 | 31.1 | 18.2 | 32.6 |
| At1g74210 | GDPD5, GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE 5 | Lipid metabolic process | 2.8 | 2.8 | 2.6 | 2.1 | ||
| At3g03310 | LCAT3, LECITHIN:CHOLESTEROL ACYLTRANSFERASE 3 | Lipid metabolic process | 2.8 | 3.5 | 4.0 | 2.2 | 2.2 | |
| At5g41080 | GDPD2, GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE 2 | Lipid metabolic process | 3.1 | 3.9 | 3.3 | 3.1 | 2.9 | 3.9 |
| Phosphorylation/Dephosphorylation | ||||||||
| At3g17790 | PAP17, PURPLE ACID PHOSPHATASE 17 | Pi scavenging and remobilisation | 5.5 | 5.6 | 12.1 | 12.6 | 65.2 | 29.4 |
| At2g01880 | PAP7, PURPLE ACID PHOSPHATASE 7 | n.d. b | 4.2 | 6.9 | 2.1 | 3.3 | ||
| At2g27190 | PAP12, PURPLE ACID PHOSPHATASE 1, PURPLE ACID PHOSPHATASE 12 | Pi scavenging from extracellular organic-P | 2.5 | 3.6 | 4.2 | 6.1 | 4.4 | 4.3 |
| At3g52820 | PAP22, PURPLE ACID PHOSPHATASE 22 | Galactolipid biosynthetic process | 9.5 | 14.7 | 4.5 | 4.1 | ||
| At3g53620 | PYROPHOSPHORYLASE 4, PPA4 | Metabolic process | 2.4 | 4.2 | 2.5 | 6.9 | 2.6 | 3.0 |
| At4g01480 | PPA5, PYROPHOSPHORYLASE 5 | Metabolic process | 3.3 | 2.4 | 2.1 | 2.1 | ||
| At1g05000 | ATPFA-DSP1, PFA-DSP1, PLANT AND FUNGI ATYPICAL DUAL-SPECIFICITY PHOSPHATASE 1 | Intracellular protein kinase cascade | 3.4 | 3.1 | 8.3 | 6.8 | 4.5 | |
| At4g03960 | PLANT AND FUNGI ATYPICAL DUAL-SPECIFICITY PHOSPHATASE 4 | Intracellular protein kinase cascade | 2.7 | 3.6 | 7.0 | 16.9 | 8.6 | 8.7 |
| At5g67080 | MAPKKK19, MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE 19 | Protein phosphorylation, | 4.2 | 13.5 | 14.4 | 26.6 | 6.7 | 5.2 |
| At4g17615 | CALCINEURIN B-LIKE PROTEIN 1, CBL1 | MAPK cascade | 3.7 | 4.6 | 3.2 | 2.7 | 2.1 | |
| At4g33770 | INOSITOL 1,3,4-TRISPHOSPHATE 5/6 KINASE 2, ITPK2 | Inositol trisphosphate metabolic process | 2.9 | 4.7 | 5.8 | 3.5 | 2.8 | |
| At3g02870 | L-galactose-1-phosphate phosphatase | L-ascorbic acid biosynthetic process | 2.6 | 2.5 | 5.4 | 3.5 | 4.3 | 4.6 |
| At3g10420 | SEEDLING PLASTID DEVELOPMENT 1, SPD1 | Mo-molybdopterin cofactor biosynthetic process | 4.0 | 2.7 | 2.6 | 2.2 | ||
| At1g13750 | Purple acid phosphatases superfamily protein | Indoleacetic acid biosynthetic process | 3.2 | 5.3 | 4.0 | 8.9 | 3.2 | 4.8 |
| At5g57610 | Protein kinase superfamily protein | Protein phosphorylation | 2.3 | 2.4 | 3.0 | 3.1 | 2.1 | |
| Transport | ||||||||
| At1g20860 | PHOSPHATE TRANSPORTER 1;8, PHT1;8 | Phosphate ion transport | 8.8 | 3.2 | 17.9 | ∞ | ||
| At5g43370 | PHOSPHATE TRANSPORTER 2, PHT1;2, PHT2 | Phosphate ion transport | 2.4 | 3.5 | 3.7 | 2.2 | 96.5 | |
| At1g76430 | PHOSPHATE TRANSPORTER 1;9, PHT1;9 | Phosphate ion transport | 4.3 | 5.5 | 7.0 | 7.7 | 20.0 | |
| At2g38940 | PHOSPHATE TRANSPORTER 1;4, PHT1;4 | Phosphate ion transport | 2.5 | 10.5 | 20.4 | 35.5 | 12.6 | 12.4 |
| At2g32830 | PHOSPHATE TRANSPORTER 5, PHT1;5, PHO1;H1 | Phosphate ion transport | 9.7 | 12.4 | 7.9 | 4.3 | 15.6 | |
| At1g68740 | Phosphate ion transport | 2.6 | 6.5 | 9.6 | 4.5 | 2.3 | ||
| At3g52190 | PHF1, PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 | Endoplasmic reticulum to Golgi vesicle-mediated transport | 2.7 | 4.3 | 4.5 | 4.1 | 5.3 | |
| At3g47420 | G3PP1, GLYCEROL-3-PHOSPHATE PERMEASE 1, PS3 | Carbohydrate transport | 2.7 | 3.6 | 9.2 | 16.6 | 12.2 | 12.8 |
| At1g22150 | SULFATE TRANSPORTER 1;3, SULTR1;3 | Sulphate transport | 31.6 | 13.2 | 3.1 | 7.0 | 10.6 | |
| At1g73220 | OCT1, ORGANIC CATION/CARNITINE TRANSPORTER1 | Transmembrane transport | ∞ | 23.2 | 59.9 | 71.5 | 196.7 | |
| At1g48370 | YELLOW STRIPE LIKE 8, YSL8 | Oligopeptide transport | 3.5 | 3.7 | 4.0 | 2.4 | 2.4 | |
| At5g51050 | APC2, ATP/PHOSPHATE CARRIER 2 | ATP transport | 3.2 | 3.1 | 3.1 | 3.1 | ||
| At2g37890 | Mitochondrial substrate carrier family protein | Mitochondrial transport | 2.8 | 4.5 | 3.0 | 2.5 | ||
| Metabolism | ||||||||
| At5g63680 | Pyruvate kinase family protein | Glycolysis | 2.7 | 2.6 | 2.7 | 2.3 | 2.2 | |
| At1g22170 | Phosphoglyceratemutase family protein | Glycolysis | 3.5 | 2.4 | 3.1 | 2.7 | 3.1 | |
| At2g17280 | Phosphoglyceratemutase family protein | Metabolic process | 3.4 | 3.0 | 5.6 | 2.2 | 2.0 | |
| At1g08650 | PHOSPHOENOLPYRUVATE CARBOXYLASE KINASE 1, PPCK1 | Protein phosphorylation | 2.7 | 6.8 | 6.1 | 11.4 | 2.3 | 2.6 |
| At1g64590 | NAD(P)-binding Rossmann-fold superfamily protein | Metabolic process | 4.6 | 3.5 | 15.2 | 4.6 | 2.4 | |
| At2g38740 | Haloacid dehalogenase-like hydrolase (HAD) superfamily protein | Metabolic process | 2.3 | 3.9 | 7.4 | 2.5 | 2.2 | |
| At1g67810 | SUFE2, SULFUR E2 | Iron-sulphur cluster assembly | 3.4 | 6.7 | 2.6 | 18.0 | 3.8 | 3.1 |
| At1g03020 | Thioredoxin superfamily protein | Cell redox homeostasis | 3.7 | ∞ | 5.2 | 2.4 | ||
| At1g73600 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein | Metabolic process | 0.26 | 0.17 | 0.11 | 0.18 | 0.23 | |
| At5g04950 | NICOTIANAMINE SYNTHASE 1 | Cellular response to iron ion | 0.33 | 0.27 | 0.29 | 0.49 | ||
| Unknown | ||||||||
| At3g43110 | Unknown protein | Galactolipid biosynthetic process | 4.0 | 13.6 | 8.1 | 13.5 | 7.6 | 8.4 |
| At5g20790 | Unknown protein | n.d. | 15.2 | 23.6 | 50.1 | 164.3 | 292.6 | 90.3 |
| At5g17340 | Putative membrane lipoprotein | n.d. | 24.4 | 21.6 | 13.6 | 99.5 | 96.3 | |
| At1g17830 | Protein of unknown function | n.d. | 4.6 | 5.4 | 6.0 | 4.5 | 2.2 | |
| At1g24575 | Unknown protein | n.d. | 2.7 | 5.9 | 15.3 | 3.5 | 2.5 | |
| At5g24600 | Protein of unknown function | n.d. | 2.5 | 4.8 | 3.2 | 3.7 | 4.3 | |
| At1g23140 | Unknown protein | n.d. | 3.6 | 2.5 | 22.4 | 3.2 | 5.3 | |
| At3g03150 | Unknown protein | n.d. | 2.1 | 2.1 | 2.2 | 3.0 | ||
| At1g70900 | Unknown protein | n.d. | 2.8 | 3.5 | 4.1 | 2.8 | 2.6 | |
| At3g07350 | Unknown protein | n.d. | 4.2 | 7.6 | 12.4 | 10.1 | 4.4 | |
| At3g50350 | Unknown protein | n.d. | 2.6 | 3.0 | 4.5 | 3.7 | 3.4 | |
| At3g61410 | Unknown protein | n.d. | 6.2 | 6.4 | 2.2 | 3.8 | ||
| Transcription | ||||||||
| At5g20150 | SPX DOMAIN GENE 1, SPX1 | Negative regulation of transcription | 5.8 | 8.9 | 27.3 | 55.9 | 40.6 | 76.3 |
| At2g26660 | SPX DOMAIN GENE 2, SPX2 | Negative regulation of transcription | 3.1 | 4.8 | 5.2 | 4.4 | 4.8 | |
| At2g45130 | SPX DOMAIN GENE 3, SPX3 | Negative regulation of transcription | 19.5 | ∞ | 128.1 | 61.4 | 52.2 | 244.7 |
| At1g68670 | Myb-like transcription factor family protein | Regulation of transcription | 2.6 | 3.6 | 4.3 | 2.9 | 2.3 | |
| At1g71130 | ETHYLENE RESPONSE FACTOR 70, ERF070 | Regulation of transcription | 3.6 | 6.1 | 4.8 | 6.1 | 4.0 | 4.7 |
| At3g05690 | NUCLEAR FACTOR Y, SUBUNIT A2”, HAP2B, | Regulation of transcription | 3.2 | 5.0 | 2.4 | 2.1 | ||
| Miscellaneous | ||||||||
| At1g72070 | Chaperone DnaJ-domain superfamily protein | Protein folding | 2.7 | 5.7 | 5.3 | 6.6 | ||
| At1g72890 | Disease resistance protein (TIR-NBS class) | Signal transduction | 5.1 | 4.8 | 6.0 | 2.4 | 4.2 | |
| At2g43535 | Defensin-like (DEFL) family protein | Defence response | 4.1 | 3.0 | 4.1 | 7.8 | 5.7 | |
| At3g19970 | Alpha/beta-Hydrolases superfamily protein | Defence response by callose deposition | 2.9 | 2.3 | 6.1 | 6.9 | 4.1 | 4.4 |
| At4g15740 | Calcium-dependent lipid-binding (CaLB domain) family protein | n.d. | 4.0 | 3.0 | 2.8 | 3.9 | ||
| At4g21470 | FMN/FHY, RIBOFLAVIN KINASE/FMN HYDROLASE | Riboflavin biosynthetic process | 4.3 | 3.6 | 3.3 | 2.7 | ||
| At4g31240 | NRX2, NUCLEOREDOXIN 2 | |||||||
| Oxidation-reduction process | 3.2 | 5.7 | 7.4 | 6.2 | 6.5 | |||
| At4g35750 | SEC14 cytosolic factor family protein / phosphoglyceride transfer family protein | n.d. | 2.3 | 2.7 | 2.4 | 2.5 | 2.5 | |
| At5g02200 | FAR-RED-ELONGATED HYPOCOTYL1-LIKE, FHL | Maintenance of protein location in nucleus | 2.9 | 3.7 | 6.4 | 5.3 | 5.0 | |
| At5g09570 | Cox19-like CHCH family protein | Negative regulation of transcription | 5.9 | 10.7 | 9.3 | 6.4 | 6.2 | |
| At5g13820 | TBP1, TELOMERIC DNA BINDING PROTEIN 1 | Telomere maintenance | 2.7 | 3.0 | 2.7 | 2.1 | ||
| At5g19430 | RING/U-box superfamily protein | Photoperiodism | 3.3 | 3.4 | 4.3 | 4.5 | 2.8 | |
| At5g39720 | AIG2L, AVIRULENCE INDUCED GENE 2 LIKE PROTEIN | n.d. | 32.9 | 8.8 | 5.6 | 9.0 | ||
| At5g47740 | Adenine nucleotide alpha hydrolases-like superfamily protein | n.d. | 8.3 | 9.0 | 4.3 | 4.3 | ||
| At5g63130 | Octicosapeptide/Phox/Bem1p family protein | Ethylene biosynthetic process | 3.6 | 6.0 | 12.3 | 5.7 | 3.4 | |
| At2g04460 | Transposable element gene | n.d. | 3.7 | 8.9 | 52.8 | 27.9 | 71.9 | 1723.0 |
| Protein ubiquitination | ||||||||
| At5g61550 | U-box domain-containing protein kinase family protein | Protein phosphorylation, protein ubiquitination | 4.3 | 10.4 | 8.2 | 3.5 | ||
| At4g25160 | U-box domain-containing protein kinase family protein | Protein phosphorylation, protein ubiquitination | 8.0 | 6.0 | 13.2 | 6.1 | ||
- For genes with multiple gene ontology (GO) annotations (biological process), GO annotations were selected based on coexpression patterns or by avoiding obvious categories (e.g. response to phosphate starvation).
- a Genes and the corresponding fold-changes indicated in bold letters were induced by ≥10-fold in at least two independent studies.
- b n.d.; not determined.
Within this core, genes related to lipid metabolism and galactolipid biosynthesis represent the largest group, emphasizing the importance of this process for Pi recycling. High induction was also observed for the SPX (SYG/PHO81/XPR1) domain-containing transcription factors SPX1, SPX2 and SPX3. Similarly, the transcription factor ERF070, which recently was shown to be crucial for root development under Pi starvation, was induced early after the onset of Pi deficiency. Another group that was strongly upregulated among the PSI core genes comprises Pi transporters of the PHT family and the PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 (PHF1) (Chapter Phosphate Transporters). PHF1 is a plant-specific SEC12-related protein that is required by transporters of the PHT1 family to exit the endoplasmic reticulum and for correct targeting (Gonzáles et al., 2005; Bayle et al., 2011). In addition, several genes encoding transporters with less well-explored functions in the PSR, such as the putative glycerol-3-phosphate transporter G3PP1, the sulphate transporter SULTR1;3, and the organic cation transporter OCT1, were strongly induced in all studies. The acquisition of Pi is supported by the upregulation of several intracellular and secreted PAPs (Chapter The Role of Intracellular and Secreted Purple Acid Phosphatases in Plant Phosphorus Scavenging and Recycling), pyrophosphorylases and other phosphatases of various types within the PSI core genes. In addition, several genes encoding regulatory proteins, such as protein kinases associated with signal transduction or the control of cellular metabolism, have been identified in the phosphatome, which suggests that these genes might represent key regulators of the PSR. For example, the gene encoding MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE19 (MAPKKK19) was highly and rapidly induced in all studies. Surprisingly, only a few genes encoding proteins involved in primary carbohydrate metabolism are represented in the phosphatome, despite the impairment of glycolysis by decreased levels of adenylates and Pi. An exception is the phosphoenolpyruvate (PEP) carboxylase kinase (PPCK), which phosphorylates and thereby activates PEP carboxylase during Pi starvation (Gregory et al., 2009; Shane et al., 2013). PEP carboxylase catalyses conversion of the glycolytic intermediate PEP to oxaloacetate (OAA) and Pi, thereby bypassing ADP-limited pyruvate kinase during the conversion of PEP to pyruvate in glycolysis, and providing pyruvate to the tricarboxylic acid (TCA) cycle. Notably, several genes in the PSI core encode proteins of unknown functions. Of particular interest are two highly similar proteins, encoded by gene loci At3g43110 and At5g20790, which are tightly coexpressed with several genes involved in galactolipid biosynthesis, as well as with SPX-domain transcription factors. The unknown protein At5g17340, a putative membrane lipid protein, was also highly induced in all of the studies. Only two PSI core genes – the nicotianamine synthase NAS1 and the S-adenosyl-L-methionine-dependent methyltransferases superfamily protein At1g73600 – showed decreased expression upon Pi starvation. At1g73600 has similarities to phosphoethanolamine N-methyltransferase (PEAMT) that catalyses a rate-limiting step in the de-novo synthesis of phosphatidylcholine, a major membrane phospholipid. NAS1 has been associated with the distribution of iron, the concentration of which is markedly increased upon Pi deficiency (Misson et al., 2005).
Genes that show similar expression patterns under diverse conditions often have correlative functions (Eisen et al., 1998). Coexpression networks can thus aid in understanding large data sets derived from high-throughput approaches, prioritizing genes for follow-up research and linking proteins with unknown functions to relevant biological processes (‘guilt by association’). Putative functional modules (clusters) of the PSI core genes were generated based on pair-wise coexpression analysis as described previously (Lin et al., 2010) with a Pearson correlation coefficient of R > 0.7, a threshold frequently used to produce coexpression networks of relatively high stringency. This procedure yielded a coexpression network comprising 61 PSI core genes, subdivided into three highly coexpressed gene subclusters (Figure 3.2). Among the genes (nodes) with the highest connectivity (largest amount of edges) were genes related to lipid metabolism, including glycerophosphodiester phosphodiesterase 1 (GDPD1), phosphoethanolamine/phosphocholine phosphatase 1 (PEPC1), and monogalactosyl diacylglycerol synthase 3 (MGD3), and the transcription factor SPX3. SPX domain transcription factors are involved in the control of the PSR in Arabidopsis, rice and bean, and their function appears to be highly conserved in plants (Chapter Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications) (Duan et al., 2008; Wang et al., 2009; Yao et al., 2014). GDPD1 is localised in plastids and catalyses the hydrolysis of glycerophosphodiesters to form glycerol-3-phosphate (G3P), a substrate for PAPs. Under Pi-deficient conditions, G3P is also required for galactolipid biosynthesis. Alternatively, G3P can be dephosphorylated by PAPs to release glycerol and free Pi (Cheng et al., 2011). PEPC1 is also involved in the liberation of Pi from phospholipids via the formation of phosphocholine and phosphoethanolamine (May et al., 2012). MGD3 encodes the major enzyme for galactolipid biosynthesis under Pi starvation by supplying MGDG as a precursor for DGDG synthesis. It thus appears that a tight coregulation of these genes provides the basis for the recycling of membrane phospholipids to maintain Pi-dependent processes (Chapter Membrane Remodelling in Phosphorus‐Deficient Plants).
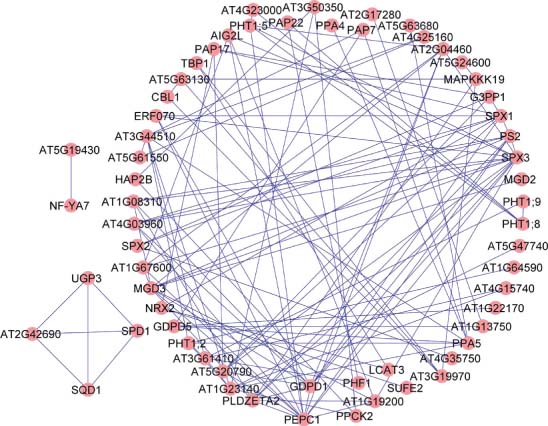
The formation of extra root hairs, caused by restricted cell elongation and additional root hair cell fate assignment, is a hallmark of Pi-deficient plants (Ma et al., 2001; Müller and Schmidt, 2004; Savage et al., 2013). Unexpectedly, none of the PSI core genes was associated with root-hair development. Three genes without a well-defined function – the U-box domain-containing protein kinase family proteins At4g25160 and At5g61550, and the unknown protein At3g50350 – are closely coexpressed with several root-hair-specific genes (Won et al., 2009) under Pi-replete conditions (available at: http://atted.jp). The two U-box proteins have also been defined as ‘core’ root-hair genes (Bruex et al., 2012), and their expression was strongly enriched in root-hair cells (Lan et al., 2013). Based on this information, a putative function of these proteins in the induction of the root-hair phenotype typical of Pi-deficient plants may be inferred. Another RING/U-box superfamily protein, At5g19430, is coexpressed with genes involved in galactolipid synthesis. The expression of At5g19430 is also tightly linked to the heterotrimeric transcription factors NFYA7 and NFYA2, which are conserved in eukaryotic cells and part of the phosphatome. It has been suggested that NFYA7 plays a role in the adaptation to nutrient stress by adjusting growth rates and cell elongation through modifications in carbohydrate metabolism. It may thus be speculated that NFYA transcription factors represent a novel class of transcriptional regulators of the PSR.
A large proportion of the genes comprising the phosphatome has not yet been linked to the acclimatisation to Pi starvation, and awaits experimental characterisation. Notably, a very high induction (>1700-fold) was observed for the transposable element gene At2g04460, encoding a putative copia-like polyprotein named AtCopeg1 (Copia evolved gene 1; Duan et al., 2008). AtCopeg1 is the only gene of the AtCopia95 family that was expressed. Interestingly, AtCopeg1 is specifically upregulated by Pi deficiency, probably driven by the GNATATNC sequence in the AtCopeg1 promoter that is recognised by the transcription factor PHR1 (Rubio et al., 2001; Duan et al., 2008). AtCopeg1 contains an intron and lost the features of a retrotransposon, which is indicative of the acquisition of a novel function during evolution. While the role of AtCopeg1in the PSR of Arabidopsis cannot be deduced at present, it is speculated that At2g04460-derived RNA plays a role in the post-transcriptional or translational regulation of PSI genes. Notably, in an RNA-seq-based survey of small RNAs, the long terminal repeat of another Copia95 retrotranposon in the intergenic region between At5g27990 and At5g28000was differentially expressed under Pi-deficient conditions, and produced a cluster of small RNAs. Similar to AtCopeg1, the most abundant small RNA from the long terminal repeat, smRPi1LTR, accumulated specifically in Pi-deficient roots (Hsieh et al., 2009). Inferred from these robustly regulated, but almost completely uncharacterised genes, it appears that the catalogue of putative key players that control the PSR is far from complete.
3.3 Pi Deficiency-Induced Alterations in the Proteome
Although transcriptional studies have yielded a comprehensive inventory of Pi-responsive genes, surveys at the transcript level do not allow for direct estimates of the abundance of the encoded proteins, which are the ultimate mediators of biological function. Therefore, the determination and quantification of proteins is mandatory for a systems perspective of the control of cellular Pi metabolism. Moreover, a fairly large part of the components that collectively orchestrate the acclimation to Pi deficiency depends on post-transcriptional processes, such as protein degradation, allosteric regulation, and protein PTMs (Chapter The Role of Post‐Translational Enzyme Modifications in the Metabolic Adaptations of Phosphorus‐Deprived Plants).
As an important model for dicotyledonous plants, Arabidopsis has been used intensively to investigate global changes in the proteome in response to Pi deficiency, comprising the identification of intracellular (Chevalier & Rossignol, 2011, Lan et al., 2012), secreted (Tran & Plaxton, 2008), membrane-bound (Huang et al., 2013), and nuclear-enriched proteins (Iglesias et al., 2013). Furthermore, a number of mutants with compromised response to Pi deficiency and Arabidopsis accessions that differ in their responses to Pi shortage are available, allowing for comparative proteomics analysis. In the most comprehensive analysis of Pi-deficient Arabidopsis roots performed to date, 356 proteins were defined as differentially expressed in roots of Col-0 plants by iTRAQ (isobaric tags for relative and absolute quantitation) proteomics (Lan et al., 2012). In this study, the majority of proteins that accumulated differentially were related to Pi transport, lipid metabolism, cellular defence against reactive oxygen species (ROS) and abiotic stress. In addition, the abundance of several ribosomal and histone-related proteins was strongly affected by Pi starvation.
The Arabidopsis accessions Be-0 and Ll-0 displayed contrasting alterations of their root system architecture in response to Pi deficiency (Chevalier & Rossignol, 2011). While Ll-0 roots showed the increase in lateral roots that is typical of Pi-deficient plants, in Be-0 the number of laterals root was decreased when grown on Pi-depleted media (Ren et al., 2011). Subsets of 13 and 24 proteins accumulated differentially in Ll-0 and Be-0 under Pi stress, respectively. Besides some proteins that were similarly modulated in both accessions, such as alcohol dehydrogenase, malic enzyme, and aconitase (ACO), it was striking that some proteins showed opposite accumulation patterns when plants were subjected to Pi deficiency. For example, most ACO isoforms were highly induced in Be-0 and showed a lower abundance in Ll-0. ACO catalyses the interconversion of isocitrate and citrate in the TCA cycle, and participates in the regulation of iron concentrations in plants (Shlizerman et al., 2007); the role of ACO in Pi deficiency-induced alterations of the root architecture is still unknown.
Analysis of the secretome (culture filtrate proteome) of Pi-sufficient and Pi-deficient Arabidopsis suspension cell cultures identified 24 secreted proteins as Pi-responsive (Tran & Plaxton, 2008). Eighteen proteins showed an increased abundance under Pi-deficient conditions, comprising one RNase that was involved in scavenging Pi from extracellular nucleic acids, and enzymes involved in cell-wall modification, proteolysis, pathogen responses, and ROS metabolism.
Nuclear proteins respond rapidly to changes in Pi supply; thus, proteomic analysis of nuclear-enriched fractions represents a novel strategy to identify new regulators of the Pi-signalling pathway. Using difference gel electrophoresis (DIGE), Iglesias et al. (2013) compared nuclear proteomic profiles of Arabidopsis roots under Pi-replete and Pi-depleted conditions. Subsets of 14 and 16 proteins showed either increased or decreased accumulation upon Pi deprivation, including proteins involved in chromatin remodelling, DNA replication, and mRNA splicing. Several regulatory nuclear proteins were identified in this study, including HOG1, an S-adenosyl-L-homocysteine hydrolase required for DNA methylation-dependent gene silencing, the DNA-helicase RIN1, the splicing factor RSP31, and the histone chaperone NAP1;2, which may represent novel regulators of the PSR (see Section 7).
The ubiquitin-conjugating enzyme UBC24/PHOSPHATE2 (PHO2) is an important regulator for Pi acquisition and translocation in plants; homozygous pho2 mutants overaccumulate Pi in leaves under Pi-replete conditions (Chapters Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications and Phosphate Transporters) (Bari et al., 2006). In order to reveal a potential involvement of membrane proteins in the PHO2-dependent regulatory pathway, a quantitative iTRAQ-based proteomic comparison between membranes of wild-type and pho2 plants was conducted that yielded 7491 proteins, approximately half of which were predicted or validated as being located in membranes (Huang et al., 2013). The microRNA399-mediated regulation of PHO2 is of critical importance for regulation of the acquisition and translocation of Pi in plants. Combined with genetic analyses, it was confirmed that PHO2 modulates Pi acquisition by regulating the abundance of polypeptides derived from Pi transporters of the PHT1 family in the secretory pathway destined for the plasma membrane. It was further shown that decreased ubiquitination of PHT1s can direct these proteins to the plasma membrane without being degraded in the secretory pathway (Chapter Phosphate Transporters). Such control allows for a rapid redistribution of the protein in response to changing nutrient or other conditions without the involvement of de-novo protein synthesis.
Both maize and rice are important staple crops of constantly increasing demand that are dependent on high-input, monoculture-based agro-systems to secure high yields. Almost 50% of the rice soils are currently deficient in Pi (Ismail et al., 2007), necessitating the application of large amounts of fertilizers to avoid significant growth inhibition. Improving the foraging capacity by both transgenic approaches and breeding programs based on existing germplasm can help to translate high-input systems into more natural, low-input systems (Tian and Doerner, 2013). Such attempts will greatly benefit from a thorough understanding of the mechanisms underlying P-acquisition efficiency (PAE) and PUE. Variations in the protein abundance of rice in response to Pi deficiency were related to nucleotide monomer synthesis, glycolysis, and defence responses (Fukuda et al., 2007; Kim et al., 2011). Several glycolysis- and TCA-related proteins were also upregulated upon Pi starvation, consistent with transcriptomic profile changes of Pi-deficient rice plants (Wasaki et al., 2003). These results indicate that the increased demand for carbon skeleton supply for organic acid synthesis is matched by enhanced carbohydrate metabolism.
A comparison of the proteome profiles from roots of the near-isogenic maize line NIL6-4 that carries a major P uptake QTL (Pup1) on chromosome 12 and the parental line Nipponbare identified 17 proteins that were differentially expressed between the two genotypes under Pi-deficient conditions (Torabi et al., 2009). NIL6-4 maintained relatively rapid root growth rates under low Pi supply when compared with Nipponbare, and showed a more pronounced Pi-deficiency-induced decrease in the abundance of isocitrate dehydrogenase and ACO. The decreased activity of isocitrate dehydrogenase and ACO presumably resulted in citrate accumulation; the release of citrate, in turn, supports Pi solubilisation and, consequently, Pi acquisition. The upregulation of oxidative stress defence enzymes was also more pronounced in NIL6-4 when compared with the parental line, which was indicative of a more efficient ROS detoxification in NIL6-4 during its adaptation to Pi deficiency.
Li et al. (2008) analysed proteomic changes in the roots of the maize wild-type Qi-319 and the low-P tolerant mutant 99038 in response to Pi starvation. The 106 Pi-deficiency-responsive proteins in cultivar Qi-319 were involved in phytohormone biosynthesis, carbon and energy metabolisms, signal transduction, cell cycle, cellular organisation and defence, with a clear bias towards carbon metabolism and the regulation of cell proliferation. Proteins involved in citrate secretion, sugar metabolism and root-cell proliferation were regarded as the main reasons for a higher tolerance to low Pi levels in the mutant 99038 (Li et al., 2008).
3.4 Core PSR Proteins
While proteomic surveys revealed important metabolic pathways that are reprogrammed during Pi starvation, it appears that these changes differ substantially among species. In addition, the metabolic processes were affected differentially depending on the severity of Pi stress, rendering a generalisation very difficult, particularly with regards to the direction of the changes in protein abundance. The core responses to Pi starvation as inferred from proteomic analysis are summarised in Figure 3.3. It is important to note that most of the proteins in this group are highly abundant and some important players – in particular proteins with regulatory function and low abundance – are excluded from the group of core PSR proteins because of technical constraints that render robust detection difficult. In addition, many proteins involved in secondary metabolism, such as those involved in the production of anthocyanin, a hallmark of Pi-deficient plants, escaped from this group for similar reasons. Advances in mass spectrometry will help to complete this catalogue in the near future.
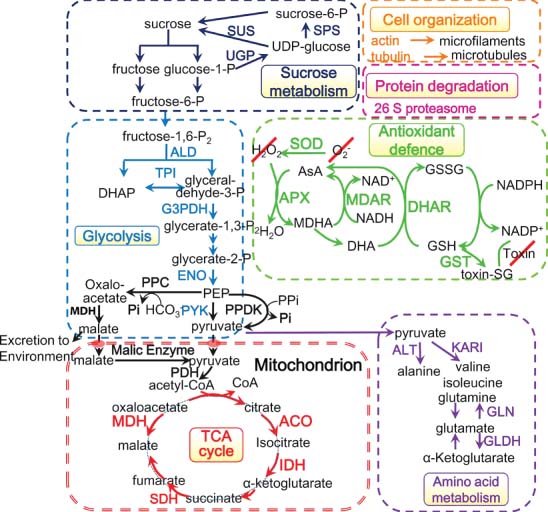
Genetic and genomic studies showed that sucrose is a global regulator of Pi metabolism and transport in Arabidopsis. Enhanced levels of sucrose can directly affect Pi signalling as well as the transport, mobilisation and allocation of Pi (Lei et al., 2011). Consistent with this, sucrose synthase (SUS) in maize (Li et al., 2007; Li et al., 2008), and sucrose-phosphate synthase (SPS) in Arabidopsis (Lan et al., 2012) and Brassica napus (rapeseed) (Yao et al., 2011) were significantly increased upon Pi deficiency. Proteomics analyses further revealed a large subset of proteins related to glycolysis and organic acid metabolism with altered abundance in response to Pi starvation, in particular enzymes associated with the TCA cycle. An increased accumulation and exudation of organic acids decreases the pH of the rhizosphere and enhances Pi availability in soils with high pH (Chapters Phosphorus: Back to the Roots and Metabolic Adaptations of the Non‐Mycotrophic Proteaceae to Soils with Low Phosphorus Availability) (Gahoonia et al., 2000). Under Pi-deficient conditions, the abundance of glyceraldehyde-3-phosphate dehydrogenase (G3PDH), ACO and malate dehydrogenase (MDH) was upregulated in rice (Fukuda et al., 2007); in maize, G3PDH, pyruvate orthophosphate dikinase (PPDK), enolase (ENO), triosephosphate isomerase (TPI), and MDH showed increased abundance (Li et al., 2007), indicating that alterations in the activity of enzymes involved in glycolysis and the TCA cycle constitute a response that is largely conserved among species.
Amino acids are one of the preferred storage metabolites in Pi-deficient plants when carbohydrate supply is limited. Several proteins related to amino acid metabolism are commonly changed under Pi deficiency. Among these, glutamine synthetase (GLN) is of great importance for root growth and for the acclimatisation to Pi deficiency. GLN catalyses the conversion of glutamate into glutamine, an important metabolite in N-assimilation, photorespiration, and amino acid metabolism. The abundance of GLN was significantly decreased under Pi stress in rice (Torabi et al., 2009; Kim et al., 2011) and maize (Li et al., 2007), resulting in suppressed respiration and decreased N uptake. By contrast, GLN levels were increased in the secretome of Arabidopsis, indicative of possible alternative ‘moonlighting’ roles for secreted GLN (Tran & Plaxton, 2008). A sharp increase in the concentrations of glutamine in both shoots and roots was also observed in severely Pi-deficient barley (Hordeum vulgare L.) plants (Huang et al., 2008). Rice mutants lacking OsGS1;1, a cytosolic GLN, showed a reduction in leaf blade elongation, plant height, panicle size, and grain filling (Tabuchi et al., 2005), underlining the importance of GLN for growth.
Oxidative stress is a significant and common reaction associated with various stresses, accompanied with the rapid accumulation of ROS (O2 − and H2O2), lipid peroxides, a decrease in thiol concentration and reduced levels of glutathione (GSH) and ascorbate. In all proteomic studies, more than one antioxidant enzyme was changed upon Pi starvation in rice (Torabi et al., 2009), maize (Li et al., 2007), Arabidopsis (Tran & Plaxton, 2008; Chevalier & Rossignol, 2011), B. napus (Yao et al., 2011) and ramie (Deng et al., 2014), including glutathione S-transferase (GST), ascorbate peroxidase (APX), superoxide dismutase (SOD), and monodehydroascorbate reductase (MDAR). Changes in the steady-state levels of these enzymes can efficiently recalibrate the cellular redox state when perturbed by environmental constrains.
As anticipated, cultivars with high PUE showed generally larger root systems with more lateral roots, longer primary roots, and higher root-hair frequency (Li et al., 2007; Chevalier & Rossignol, 2011). Consistent with these phenotypes, the abundance of proteins involved in cell-wall biosynthesis, cytoskeletal organisation and auxin metabolism changed upon Pi deficiency. Several actin and tubulin proteins increased significantly under conditions of Pi deficiency in rice (Fukuda et al., 2007), maize (Li et al., 2007), Arabidopsis (Col-0; Iglesias et al., 2013) and ramie (Deng et al., 2014), but decreased in the Arabidopsis accession Be-0 that, in contrast to most other Arabidopsis accessions, respond to Pi starvation with decreased formation of lateral roots (Chevalier & Rossignol, 2011). These findings support a close linkage of these classes of proteins, alterations in root architecture and foraging capacity.
Post-translational mechanisms, in particular the modification of proteins by PTMs such as ubiquitination and phosphorylation, are important regulators of Pi signalling and the plant PSR (Chapters Sensing, Signalling, and Control of Phosphate Starvation in Plants: Molecular Players and Applications and The Role of Post‐Translational Enzyme Modifications in the Metabolic Adaptations of Phosphorus‐Deprived Plants) (Alexova & Millar, 2013; Rojas-Triana et al., 2013). The 26S proteasome-processed polyu-biquitinated proteins provide an important mechanism for the removal of misfolded or damaged proteins. The abundance of proteins associated with the 26S proteasome was significantly increased upon Pi starvation in maize (Li et al., 2007) and Arabidopsis (Chevalier & Rossignol, 2011), supporting the importance of ubiquitin-mediated protein turnover for control of the PSR. In addition to lysine 48-linked polyubiquitin chains that target proteins to degradation via the 26S proteasome, monoubiquitination and lysine 63 polyubiquitination emerge as important regulatory mechanisms for the adaptation to nutrient deficiency (Li & Schmidt, 2010; Shane et al., 2013; Pan & Schmidt, 2013). Proteomic studies that focus on how stresses such as P-deprivation influence the plant ‘ubiquitome’ should thus consider both poly- and monoubiquitination. Knowledge of Pi-specific changes in the ubiquitome would strongly contribute to the present understanding of control of the PSR.
3.5 Membrane Lipid Remodelling: Insights from the Transcriptome, the Proteome, and the Lipidome
Membrane phospholipids constitute about 20% of the total amount of P in the leaves of Pi-replete plants, and thus represent a large pool of P that can be mobilised when internal Pi levels are insufficient to match the demand of the plant (Veneklaas et al., 2012). As discussed in detail in Chapter Membrane Remodelling in Phosphorus‐Deficient Plants, phospholipids can be replaced by the galactolipid digalactosyldiacylglycerol (DGDG) and by the sulfolipid sulfoquinovosyldiacylglycerol (SQDG), a process that has been referred to as membrane lipid remodelling (Nakamura, 2013). As inferred from transcriptional analyses, membrane lipid remodelling is one of the hallmark processes during Pi starvation (Nakamura, 2013; Zhang et al., 2014). The transition from phospholipids to non-phospholipids in vascular plants was initially observed in the Arabidopsis mutant pho1, which is defective in Pi loading into the xylem (Chapter Phosphate Transporters). In pho1 plants, the Pi concentration in leaves was reduced to 5% of the wild-type level, associated with a decrease in phospholipids and an increase in DGDG and SQDG (Essigmann et al., 1998; Poirier et al., 1991). These lipids are usually found in chloroplast membranes, and play important roles in chloroplast function and biogenesis. In Pi-deficient plants, DGDG is also present in extraplastidic membranes (Andersson et al., 2005; Jouhet et al., 2004; Hartel et al., 2000; Dormann et al., 1999). The remodelling pathway includes two steps: (i) the hydrolysis of phospholipids to yield diacylglycerol (DAG); and (ii) the biosynthesis of galactolipid and sulfolipids using DAG as a substrate (Nakamura, 2013). Phospholipase C (PLC), phospholipase D (PLD) and phosphatidic acid phosphatase are involved in converting phospholipids to DAG (Nakamura et al., 2005). The Arabidopsis genome harbours six orthologues of prokaryotic NPC (non-specific PLC), two of which (NPC4 and NPC5) were induced under Pi-deficient conditions (Gaude et al., 2008; Nakamura et al., 2005). In Arabidopsis, the phospholipases D zeta 1 and 2, (PLDζ1 and PLDζ2) that hydrolyse major phospholipids such as phosphatidylcholine and phosphatidylethanolamine, and the phosphatidate phosphohydrolases 1 and 2 (PAH1 and PAH2) that hydrolyse phosphatidic acid to produce DAG (a precursor for galactolipids) are strongly upregulated by Pi deficiency. Homozygous pldz2 mutants and pah1pah2 double mutants are impaired in DGDG accumulation (Cruz-Ramirez et al., 2006; Nakamura et al., 2009). In Arabidopsis, genes involved in MGDG, DGDG and SQDG synthesis, as well as genes functioning in phospholipid degradation, were massively upregulated under Pi-deficient conditions, both at the transcript and protein level (Lan et al., 2012). Three MGDG synthase genes have been identified in Arabidopsis (MGD1–MGD3). MGD1 is abundantly expressed in photosynthetic tissues and plays important roles in photosynthetic membrane biogenesis and embryogenesis (Kobayashi et al., 2007; Awai et al., 2001; Jarvis et al., 2000). By contrast, MGD2 and MGD3 are expressed in flowers and roots, respectively, and are responsible for the production of MGDG as a substrate for DGDG synthesis (Kobayashi et al., 2009). DGD1 and DGD2 account for the increase in DGDG upon Pi starvation (Kelly et al., 2003). SQD2 catalyses the final step in sulfolipid biosynthesis, but appeared also to be required for the production of a novel lipid, glucuronosyldiacylglycerol, under Pi-deficient conditions (Okazaki et al., 2013). Notably, SQD2, but not SQD1, is required for plant survival under Pi-deficient conditions (Yu et al., 2002; Okazaki et al., 2013). Transcriptomic analysis showed that membrane lipid remodelling is commonly found across species. For Arabidopsis, Misson et al. (2005) reported that 7% of the Pi-responsive genes (44 genes) were involved in lipid biosynthetic pathways. MGD2 and MGD3 were induced four- to ten-fold during short-term Pi deprivation; DGD1 and DGD2 were upregulated during medium- and long-term Pi deficiency, respectively (Misson et al., 2005). These changes were rapidly reversed after the addition of Pi (Morcuende et al., 2007). During later stages of Pi deficiency, genes associated with membrane lipid remodelling, including SQD1, SQD2, DGD1, DGD2 and lipid transfer proteins, were upregulated in rice (Oono et al., 2011). The induction of lipid metabolism-related genes may be associated with changes in the fluidity of the membranes, which in turn controls several plant cellular processes. In addition, auxin is thought to be involved in the regulation of membrane lipid remodelling. The transcript levels of MGD2 and MGD3 are regulated by auxin signalling, and the Pi-deficiency-induced accumulation of DGDG and SQDG is suppressed in the auxin signalling mutants slr-1 and arf7arf19 (Kobayashi et al., 2006; Narise et al., 2010). The involvement of hormones in membrane lipid remodelling is currently underexplored and deserves further studies.
Changes in lipid metabolism and signalling that occur during Pi starvation cannot be completely explained by transcriptomic or proteomic analysis. In addition to detailed studies of key genes and the proteins they encode, the large-scale profiling of cellular lipid composition (lipidomics) provides a direct means of studying the changes in membrane lipids upon Pi starvation. Traditionally, lipid analysis depends on analytical techniques with low resolution and sensitivity, such as thin-layer chromatography, and although this method has certain limitations it is still commonly used based on its relative ease of use and low costs (Loizides-Mangold, 2013). With advances in mass spectrometry (MS), however, it has become feasible to approach the entire lipidome of cells or organelles. When the term ‘lipidome’ first appeared in 2001, it referred to the complete lipid composition within a cell, tissue or organism (Kishimoto et al., 2001). Subsequently, Rilfors & Lindblom (2002) introduced the term ‘functional lipidomics’, which aims at elucidating the role played by membrane lipids. In 2003, lipidomics was defined as a global analysis of cellular lipidomes by a comprehensive MS approach (Han & Gross, 2003).
Various MS-based methods have been successfully used to identify and quantify lipids directly (‘shotgun’ lipidomics) or coupled with different chromatographic systems, such as liquid chromatography-MS or gas chromatography-MS (Lam & Shui, 2013). With the development of soft ionisation techniques such as electrospray ionisation (ESI) and matrix-assisted laser desorption/ionisation (ESI) MS, the progress of lipidomics has leapt considerably (Han et al., 2012). Alterations in individual lipid classes were investigated using ESI-MS analysis in algal thalli cultured in artificial seawater. An increase in DGDG and SQDG ion intensity, accompanied by a decrease in phosphatidylglycerol and phosphatidylethanolamine ion intensity, was observed upon Pi starvation, which is consistent with what has been deduced from changes in gene activity (Kumari et al., 2014). Targeted lipidomics are used to study single lipids or a specific subclass, while nontargeted lipidomics are designed to cover lipids as broadly as possible, an approach that also allows for discovering novel lipids (Lam & Shui, 2013). Hydrophilic interaction liquid chromatography mass spectrometry (HILIC-MS) offers unique advantages for the MS detection of highly polar compounds when compared to reversed-phase chromatography. This approach was used to identify glucuronosyldiacylglycerol, a novel lipid that accumulate specifically under Pi-deficient conditions. This lipid was also identified in rice and suggested to play an essential role in protecting plants against Pi-deficient conditions (Okazaki et al., 2013). Lipidomics has been widely used to analyse lipid signalling pathways and metabolism in various plants under different conditions. For example, the combination of biochemical and lipidomic tools revealed that Arabidopsis PAH1 and PAH2 are responsible for galactolipid synthesis (Nakamura et al., 2009).
Today, lipidomics is emerging as a powerful technology to explore the changes in lipid composition more directly, and for identifying novel lipids. However, the identification of all classes of lipids using a single extraction method remains a major challenge. The concentration of lipids is highly dynamic and responsive to environmental stimuli. In addition, the chemical constitution of lipids differs among plant organs. Using multiple methods and collecting data at different time points after the onset of Pi deficiency will set the stage to gain insights into the dynamics of changes in membrane-lipid composition. The combination of lipidomics with other omics strategies, as well as explorations of the biological functions of the lipids via biochemical and molecular approaches, will provide a comprehensive picture of regulatory mechanisms that control membrane remodelling and lipid signalling under Pi-deficient conditions.
3.6 Genome-Wide Histone Modifications in Pi-Deficient Plants
Transcriptional activation in response to environmental signals requires a coordinated interplay of the general transcriptional machinery, trans-acting factors, and proteins that control cotranscriptional and post-initiation processes such as pre-mRNA splicing and mRNA processing. Typically, PTMs of core histones tune the accessibility of the DNA to the transcriptional machinery, and thus constitute a major epigenetic mechanism regulating gene expression and other DNA-templated processes. The complex pattern and, in the case of histone tail methylation, the extent of these PTMs (e.g. mono-, di- or trimethylation) that ultimately dictates the transcriptional readout, has been referred to as ‘histone code’. However, histone modifications are not categorical, and additional information encrypted in the context of other PTMs at the same or neighbouring histones and provided by interacting proteins is required for their correct interpretation. Thus, histone marks rather resemble the more nuanced grammar of a ‘histone language’ than a rigid code (Lee et al., 2010). The recruitment of protein effectors or readers of these PTMs, and the dynamic interplay of the various PTMs, further complicates a straight-forward prediction of the functional outcome. Environmental factors have a strong impact on histone modifications in yeast and mammalian cells, but the effect of external cues on the epigenetic landscape in plants is still understudied. The first evidence for a chromatin-level regulation of PSI genes derived from studying the phenotypes of mutants defective in the expression of the nuclear actin-related protein ARP6, a component of the chromatin remodeller SWR1 (Clapier & Cairns, 2009). SRW1 is a one megadalton complex involved in the exchange of the histone H2A.Z-H2B dimer for a canonical H2A/H2B dimer, resulting in the formation of a variant nucleosome containing two H2A.Z-H2H dimers and, consequently, an alteration in chromatin structure. H2A.Z is the only conserved variant isoform of histone H2A, and plays disparate roles in chromosomal processes such as DNA repair, telomere silencing and genome stability in protozoa, fungi, and animals. The function of H2A.Z in plants remains enigmatic. H2A.Z is critical to chromatin condensation by promoting intramolecular folding and inhibiting the intermolecular association of nucleosomes that impedes the formation of highly condensed chromatin, thereby creating unique chromatin domains (Fan et al., 2002). Generally, the deposition of H2A.Z is biased towards nucleosomes proximal to regulatory DNA sequences such as promoters or enhancers (Raisner et al., 2005; Tolstorukov et al., 2009). The eviction of H2A-H2B, coupled to the deposition of the H2A.Z variant, is important for the regulation of transcriptional competency by establishing a nucleosome-depleted region around the promoter, thereby allowing the transcriptional machinery to access the DNA. In Arabidopsis, AtARP6 is required for the correct deposition of H2A.Z; compromised H2A.Z deposition at a number of PSI loci in arp6 mutants resulted in the constitutive activation of these genes, phenotypically visible by the formation of dense and long root hairs, resembling Pi-deficient plants (Smith et al., 2010). A puzzling result was that, in contrast to yeast in which loss of H2A.Z resulted in decreased expression of PHO genes (Santisteban et al., 2000; Lindstrom et al., 2006), the compromised deposition of H2A.Z in Arabidopsis caused a de-repression of PSI genes. It thus appears that H2A.Z is required for the control of PSI gene expression, most likely by regulating the binding of activators and repressors to the promoters of a subset of PSI genes. Interestingly, a protein associated with H2A.Z deposition, the histone chaperone NUCLEOSOME ASSEMBLY PROTEIN1 (NAP1), is required for the control of PSI gene expression (Iglesias et al., 2013). Histone chaperones play a critical role in the deposition of histones and histone variants, and are crucial for nucleosome assembly and disassembly – as occur during transcription and other DNA-associated processes – to prevent the ectopic aggregation of oppositionally charged DNA and histones. Some histone chaperons, including NAP1, also function as nucleocytoplasmic shuttling proteins of newly synthesised histones from the cytosol to the nucleus. NAP1 proteins are encoded by a single gene in yeast and small gene families in mammals and plants, and are conserved among eukaryotes (Ohkuni et al., 2003; Dong et al., 2003; Dong et al., 2005). Triple mutants defective in the expression of three NAP1 family members (AtNAP1;1, AtNAP1;2, and AtNAP1;3) in Arabidopsis show dramatically decreased PSI gene expression and reduced Pi concentrations, indicating that NAP1 is critical for the regulation of cellular Pi concentrations (Iglesias et al., 2013). Phosphate deficiency causes a redistribution of AtNAP1;2 from the nucleus to the cytosol, which suggests that this change in localisation is related to the function of NAP1;2 under Pi-deficient conditions. A possible scenario that explains these findings involves the association of NAP1;2 with H2A.Z, aiding the nuclear import of H2A.Z-H2B dimers. Canonical H2A-H2B dimers are required to control the expression of PSI genes, probably by allowing access to the relevant trans-acting elements. In yeast, Nap1 has a chaperone function for both H2A-H2B and H2A.Z, and together with another nuclear chaperone for histone H2A.Z, Chz1, delivers substrate to SWR1 (Luk et al., 2007). Notably, in yeast Nap1 – but not Htz1 – is associated with unincorporated H2A.Z in the cytosol (Straube et al., 2010), supporting a role of NAP1 in nuclear import of H2A.Z. Extending this scenario to Arabidopsis, it appears plausible that AtNAP1 serves to transport H2A.Z-H2B dimers from the cytosol to the nucleus, where they are required to modulate the expression of PSI genes. The question remains why, in Arabidopsis, compromised H2A.Z deposition results in constitutively activated PSR genes. While answering these questions requires more experimentation, different, but not mutually exclusive, possibilities can be envisaged. First, it remains unclear as to which and how many PSI genes are activated, and also whether a subset of Pi starvation-responsive genes is repressed in arp6 mutants. It is possible that a more open chromatin structure provided by H2A.Z at the promoter region is required to bind transcriptional repressors, fine-tuning the activity of these genes. In another scenario, the NAP1 level is reduced in the nucleus upon Pi-starvation to decrease the amount of H2AZ, allowing PSI genes to be activated. A decreased NAP1 activity results, however, in a reduced activity of PSI genes (Iglesias et al., 2013), making a shuttle function of NAP1 more plausible.
Post-translational histone modifications require a reader to interpret the information, which is then translated into transcriptional activity. ALFIN-LIKE6 (AL6), a member of the Alfin1-like plant homeodomain (PHD) protein family, has been identified in a forward genetic screen for mutants with Pi-deficiency-specific root-hair defects (Chandrika et al., 2013). Alfin proteins are plant-specific, but highly similar to the broadly distributed ING (inhibitor of growth) proteins and bromodomain PHD finger transcription factors (Pena et al., 2006; Shi et al., 2007; Aasland et al., 1995; Bienz, 2006). AL6 is a bona fide histone reader that can bind to di- and, more readily, trimethylated H3K4 through its PHD domain (Li et al., 2009). In eukaryotes, the di- and trimethylation of histone H3 at lysine 4 (H3K4me3/2) is located near the transcription start site of actively transcribed genes (Shilatifard, 2008). Direct targets of AL6 have not yet been identified, but several Pi-responsive genes showed decreased expression in homozygous al6 mutants, including the cell fate-determining transcription factor ETC1 (Chandrika et al., 2013). Notably, AL6 also affected other hallmark responses to Pi deficiency such as primary root growth, lateral root number, anthocyanin accumulation and Pi concentration, and transcript levels of some key genes in membrane lipid metabolism. The expression of AL6 is not responsive to Pi levels, suggesting that alterations at the histone level constitute the underlying mechanism for AL6. Also, trimethylation of H3K4 per se does not appear to influence transcription (Pavri et al., 2006; Sims & Reinberg, 2006). Rather, H3K4me3 provides a binding platform for transcriptional activators and for factors that mediate post-initiation processes such as transcript elongation, pre-mRNA splicing and mRNA maturation (Sims & Reinberg, 2006). Although, to date, no information on histone modifications in response to Pi deficiency is available, the massive changes in gene expression suggest major alterations in histone PTMs. However, the complex syntax of the histone language may take much more effort to be completely deciphered. NAP1 and AL6 are new ‘actors’ in the control of PSI genes, and most probably mark only the beginning of the unfolding landscape of epigenetic regulation that is almost completely unexplored at present. Interestingly, H3K4me3 in gene bodies is required for epigenetic memory of recent transcriptional activity (Ding et al., 2012), adding a further layer of possible impact of histone PTMs on the adaptation to low Pi availability.
3.7 Conclusions and Outlook
Omics profiling carried out on model and non-model plant species has uncovered diverse physiological and biochemical processes that are triggered by Pi starvation. The integration of different omics approaches, while still in its infancy, is a promising route to obtain a comprehensive picture of the PSR, but the interwoven network of Pi signalling and metabolic pathways is far from being completely understood. Multiple layers of transcriptional and post-transcriptional regulation, ranging from chromatin structure to protein PTMs, act in concert to orchestrate the plant PSR. The identification of a histone reader (AL6) as an important regulator of the PSR allows speculation as to whether Pi deficiency is associated with genome-wide changes in histone methylation, an aspect that lacks experimental support so far. Abiotic stresses trigger multiple histone PTMs (Yuan et al., 2013), suggesting that the regulation of gene activity under Pi-deficient conditions may depend on the interpretation of a Pi-specific histone language. The exploration of the roles of the writers, erasers, and readers of the histone code, and the dynamic crosstalk of histone modifications in the regulation of PSI genes and other abiotic stresses, may set the stage for the identification of mechanisms that improve PAE or PUE. Moreover, Pi-specific expression of transposons suggests that reversible RNA-directed DNA methylation might be involved in the regulation of gene expression under Pi-deficient conditions. Also, as emphasised in Chapter The Role of Post‐Translational Enzyme Modifications in the Metabolic Adaptations of Phosphorus‐Deprived Plants, changes in protein polyubiquitination versus monoubiquitination, phosphorylation, and other crucial PTMs such as glycosylation upon Pi deficiency have not yet been surveyed genome-wide. The role of the ubiquitin-specific protease UBC14, identified in a forward genetic screen aimed at identifying mutants with Pi-specific defects in root-hair formation, underpins the importance of ubiquitin-related processes for the PSR (Li et al., 2009). The mutation was caused by a synonymous substitution in the third position of a conserved proline fourfold redundancy genetic code (CCC to CCT) in the twelfth exon of UBP14, causing a subtle decrease in UBP14 protein abundance (Li et al., 2009). While the function of UBP14 in root-hair elongation under Pi-deficient conditions remains to be elucidated, it will be interesting to investigate how frequent such silent mutations (i.e. a change in codon usage without affecting the amino acid sequence) are on a genome-wide basis and if they influence the phenotypic readout, particularly under abiotic stress conditions. Differences in codon usage, leading to small changes in the translational fitness of specific mRNAs and ultimately to a change in protein abundance, may thus contribute to the different developmental patterns and phenotypic plasticity towards environmental cues observed among Arabidopsis accessions.
Whereas, upregulated proteins mostly corresponded well to changes in the abundance of their cognate transcripts, the abundance of transcribed mRNAs derived from genes that are downregulated in response to Pi starvation is not always linked to the level of its encoded protein (Lan et al., 2012), a phenomenon that has been also observed with other systems (Lee et al., 2011). The lack of changes in protein abundance encoded by these genes might be due to post-translational control of the protein's activity to avoid a de novo cycle of synthesis after the stress is relieved. Phosphate deficiency induces the production of intron-containing, nonfunctional transcripts that are targeted by the nonsense-mediated decay RNA surveillance pathway, probably as a means for rapidly tuning the amount of functional transcripts and to prioritise the translation of stress-relevant proteins (Li et al., 2013). Translatome profiling and in-vivo translation studies at the mRNA (ribosome profiling; Ingolia et al., 2012) or protein level (puromycin-associated nascent chain proteomics; Aviner et al., 2014) may help to explain the discordance between transcript and protein level and to identify critical regulatory nodes. In addition, a large number of unknown functional proteins have been uncovered by omics approaches, prioritizing some putative novel players for follow-up research.
Acknowledgements
The authors acknowledge input provided by Drs Han Wang (ISSAS, Nanjing), Lu Zheng (ISSAS, Nanjing), William C. Plaxton (Queen's University, Kingston), Hans Lambers (University of Western Australia) and Yuki Nakamura (IPMB, Taipei) during the writing of this chapter. Ping Lan is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDB15030103, the Natural Science Foundation of China (31370280), and by the Chinese Academy of Sciences through its One Hundred Talents Program. Wenfeng Li is supported by the Jiangsu Specially-Appointed Professor program and by the Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Nanjing Institute of Soil Science, Chinese Academy of Science (Y412201446). Research in the Schmidt laboratory is funded by MoST and Academia Sinica.



