Corynebacteriales ord. nov.†
Abstract
Co.ry.ne.bac.te.ri'a.les. N.L. neut. n. Corynebacterium type genus of order; suff. -ales ending to denote order; N.L. fem. pl. n. Corynebacteriales the Corynebacterium order.
Actinobacteria / Actinobacteria / Corynebacteriales
Aerobic or facultatively anaerobic, Gram-stain-positive, catalase-positive actinomycetes which may form a branched substrate mycelium that fragments into coccoid- to rod-shaped elements or present as branched filaments, cocci, or as pleomorphic forms. Chemoorganotrophic. Some strains form aerial hyphae. The wall peptidoglycan contains meso-diaminopimelic acid and is of the A1γ type. Arabinose and galactose are major wall sugars. Fatty acid profiles are rich in saturated and unsaturated components and usually contain tuberculostearic acid. Typically contain mycolic acids. The pattern of 16S rRNA signatures consists of nucleotides at positions 127:134 (G–Y), 564 (C), 672:734 (U–G), 833:835 (U–G), 952:1229 (U–A), and 986:1219 (U–A). Forms a distinct monophyletic branch in the 16S rRNA actinobacterial gene tree.
The order encompasses the families Corynebacteriaceae Lehmann and Neumann 1907 emend. Zhi et al. 2009; Dietziaceae Rainey et al. 1997 emend. Zhi et al. 2009; Gordoniaceae Rainey et al. 1997; Mycobacteriaceae Chester 1897 emend. Zhi et al. 2009; Nocardiaceae Castellani and Chalmers 1919 emend. Zhi et al. 2009; Segniliparaceae Butler et al. 2005 emend. Zhi et al. 2009; Tsukamurellaceae Rainey et al. 1997 emend. Zhi et al. 2009; and the genera Hoyosella Jurado et al. 2009; Tomitella Katayama et al. 2010; and Turicella Funke et al. 1994.
Members of the order are found in diverse environments, notably in the soil ecosystem. Some strains are serious pathogens of humans and domesticated animals.
Type genus: Corynebacterium Lehmann and Neumann 1896, 350AL emend. Bernard, Wiebe, Burdz, Reimer, Ng, Singh, Schindle and Pacheco 2010, 877.
Further Descriptive Information
Phylogeny
Until recently, actinomycetes with meso-diaminopimelic acid (meso-A2pm), arabinose, and galactose in the peptidoglycan (wall chemotype IV sensu Lechevalier and Lechevalier (1970a, 1970b) were assigned to two distinct aggregate groups (Goodfellow, 1992; Goodfellow and Lechevalier, 1989; Goodfellow and Minnikin, 1984). Wall chemotype IV actinomycetes that contain mycolic acids (high-molecular-weight 3-hydroxy fatty acids with a long branch in the 2-position) were assigned to the genera Corynebacterium, Dietzia, Gordonia, Mycobacterium, Nocardia, and Tsukamurella in the suborder Corynebacterineae Stackebrandt et al. 1997 and most of their mycolateless counterparts to the suborder Pseudonocardineae (Warwick et al., 1994) Stackebrandt et al. 1997. The group of mycolic acid-containing actinomycetes encompassed a few species which appear to have lost the capacity to synthesize mycolates, including Turicella otitidis, the type species of the monospecific genus Turicella (Funke et al., 1993, 1994).
Mycolic acid-containing strains were found to have many phenotypic properties in common (Goodfellow and Cross, 1984; Goodfellow and Wayne, 1982) and formed a recognizable suprageneric group (Mordarski et al., 1980; Stackebrandt and Woese, 1981; Stackebrandt et al., 1980). In addition, representative strains were seen to be closely related using serological techniques (Magnusson, 1976; Pier, 1984), notably immunological procedures which showed the presence of common precipitinogens among corynebacteria, gordoniae, mycobacteria, nocardiae, and rhodococci (Lind et al., 1980; Lind and Ridell, 1976).
The suborders Corynebacterineae and Pseudonocardineae have been recast in this volume into the orders Corynebacteriales and Pseudonocardiales, respectively. The order Corynebacteriales contains the established mycolic acid-containing genera (Goodfellow and Maldonado, 2006; Stackebrandt et al., 1980) and the recently described taxa Millisia Soddell et al. 2006, Segniliparius Butler et al. 2005, Skermania Chun et al. 1997, Smaragdicoccus Adachi et al. 2007, Tomitella Katayama et al. 2010, and Williamsia Kämpfer et al. 1999. It is becoming increasingly apparent that the order provides a home for a range of mycolateless wall chemotype IV taxa including the genera Amycolicococcus Wang et al. 2010 and Hoyosella Jurado et al. 2009. Corynebacteria that lack mycolic acids have been assigned to several species including Corynebacterium amycolatum Collins et al. 1988a, Corynebacterium atypicum Hall et al. 2003, Corynebacterium capsum Collins et al. 2004, Corynebacterium ciconiae Fernández-Garayzábal et al. 2004, Corynebacterium kroppenstedtii Collins et al. 1998, which form distinct phyletic lines in the 16S rRNA Corynebacterium tree. Members of the species Corynebacterium amycolatum encompass strains previously were misidentified as Corynebacterium minutissimum, Corynebacterium striatium, and Corynebacterium xerosis (Funke et al., 1996; Wauters et al., 1996; Zinkernagel et al., 1996).
Initially, the genera Corynebacterium, Mycobacterium, and Nocardia were assigned to the families Corynebacteraceae Lehmann and Neumann 1907, Mycobacteraceae Chester 1897, and Nocardiaceae Castellani and Chalmers 1919, respectively, based on morphological and staining properties. The composition of these taxa varied over time as classifications were generated using different combinations of phenotypic properties, especially chemotaxonomic and morphological features (Bergey et al., 1939; Goodfellow and Magee, 1998; Lechevalier and Lechevalier, 1970a, 1970b; McClung, 1974). In contrast, mycolic acid-containing genera are now assigned to families based on 16S rRNA gene sequence similarity values and taxon specific 16S rRNA signature nucleotides (Stackebrandt et al., 1980; Zhi et al., 2009). In general, the taxonomic integrity of these families is underpinned by the discontinuous distribution of some chemical markers, as shown in Figure 1.
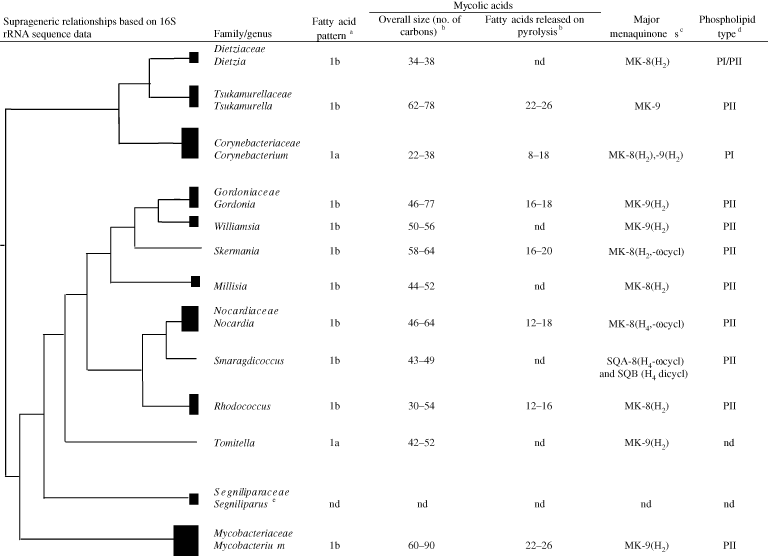
The current assignment of mycolic acid-containing genera to families is a marked improvement on earlier taxonomies of the group, but even so present classifications need to be seen as part of a progression towards better classifications in the future. It is already clear that the current classification needs to be reassessed with the recognition of novel taxa, as exemplified by the assignment of the mycolateless genus Amycolicococcus to the family Mycobacteriaceae (Wang et al., 2010). In addition, further comparative taxonomic studies are needed to establish family assignments for the genera Hoyosella (Jurado et al., 2009), Tomitella (Katayama et al., 2010), and Turicella (Funke et al., 1994).
Chemotaxonomy
Chemical characterization of actinomycete cells provides invaluable data for the delineation of suprageneric groups such as the orders Corynebacteriales and Pseudonocardiales, and for the recognition of genera encompassed by them (Goodfellow and Maldonado, 2006; Katayama et al., 2010; Minnikin and Goodfellow, 1980; Minnikin et al., 1978; Wang et al., 2010). The most useful chemical markers for the classification of wall chemotype IV actinomycetes have been obtained from analyses of cellular fatty acids, menaquinones, mycolic acids, polar lipids, wall peptidoglycans, and DNA base composition (Collins, 1994; Embley and Wait, 1994; Goodfellow and Magee, 1998; Kroppenstedt, 1985; Minnikin and Goodfellow, 1976; Schleifer and Kandler, 1972; Schleifer and Seidl, 1985; Suzuki et al., 1994; Uchida et al., 1999). Some of these analyses have provided qualitative information, as exemplified by the detection of mycolic acid and polar lipid patterns, whereas others have yielded quantitative data, as in the case of cellular fatty acid and menaquinone analyses. The detection of variations in the structures, chain lengths, and degree of unsaturation of mycolic acids have proved to be especially useful in the delineation of mycolic acid-containing genera (Goodfellow et al., 1998, 1998; Goodfellow and Maldonado, 2006; Goodfellow and Magee, 1998; Katayama et al., 2010; Minnikin et al., 1980, 1984a, 1984b, 1984c). It is now known that mycolic acids are involved in the formation of an outer cellular membrane that has a bilayer structure (Hoffmann et al., 2008; Zuber et al., 2008).
The chemical profiles of genera assigned to the families which encompass mycolic acid-containing actinomycetes are shown in Figure 1. The genera classified in the families Gordoniaceae, Mycobacteriaceae, Nocardiaceae, and Tsukamurellaceae contain N-glycolated muramic acid (Uchida and Aida, 1977, 1979a, 1979b), a phospholipid pattern which includes diphosphatidylglycerol, phosphatidylethanolamine (taxonomically significant nitrogenous phospholipid), phosphatidylinositol, and phosphatidylinositol mannosides (phospholipid type II sensu Lechevalier et al., (1977, 1981) and a fatty acid profile rich in straight-chain, unsaturated, and tuberculostearic acids (Kroppenstedt, 1985), but can be distinguished on the basis of menaquinone composition and the overall chain length of mycolic acids. The genera assigned to the families Corynebacteriaceae and Dietziaceae can be distinguished on this basis but are united in having a peptidoglycan containing N-acetylated muramic acid. In turn, members of the genus Segniliparus of the family Segniliparaceae are characterized by the presence of multiple chemical functional groups of high-molecular-weight, non-polar mycolic acids (Butler et al., 2005). Tomitella strains cannot be readily assigned to any of the mycolic acid-containing families but have a chemotaxonomic profile that distinguishes them from genera assigned to these families (Figure 1). Additional chemotaxonomic properties of the mycolic acid-containing genera are shown in Table 1.
| Characteristic | Corynebacterium | Dietzia | Gordonia | Millisia | Mycobacterium | Nocardia | Rhodococcus | Segniliparus | Skermania | Smaragdicoccus | Tomitella | Tsukamurella | Williamsia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell morphology | Pleomorphic rods, often club-shaped; commonly in angular and palisade arrangement | Short rods and cocci | Rods and cocci or moderately branching hyphae | Rudimentary right angled branching | Rods, occasionally branched filaments which fragment into rods and coccoid elements | Mycelium which fragments into rods and cocci | Rods to extensive substrate mycelium which fragments into irregular rods and cocci | Rods | Mycelium resembling a pine tree | Coccoid cells | Irregular rods that exhibit snapping division; cells turn to short coccoid rods after prolonged culture | Rods occur singly, in pairs, or in masses; coccobacillary forms occur | Thin irregular rods or cocci occur singly or in small clusters |
| Aerial hyphae | Absent | Absent | Absent | Present | Usually absent | Present | Absent | Absent | Present but not visible to the naked eye | Absent | Absent | Absent | Present |
| Time to growth of visible colonies (d) | 1–2 | 1–3 | 1–3 | 1–3 | 2–40 | 1–5 | 1–3 | 3–4 | 9–21 | 7 | nd | 1–3 | 1–4 |
| Acid-fastness | Sometimes weakly acid-fast | Not acid-fast | Partially acid–alcohol-fast | Acid-alcohol-fast | Strongly acid-fast | Partially acid fast | Partially acid-fast at some stage of the growth cycle | Acid–alcohol-fast | Not acid fast | nd | nd | Partially acid–alcohol-fast | nd |
| Strictly aerobic | − | + | + | + | + | + | + | + | − | + | + | + | + |
| Fatty acid compositionc | S, Ud | S, U, T | S, U, T | S, U, T | S, U, T | S, U, T | S, U, T | S, U, T | S, U, T | S, U | S, U | S, U, T | S, U, T |
| Major menaquinone(s)e | MK-8(H2) | MK-8(H2) | MK-9(H2) | MK-8(H2) | MK-9(H2) | MK-8(H4,ω-cycl) | MK-8(H2) | nd | MK-8(H4,ω-cycl) | SQA-8(H4 ωcycl) and SQB (H4 dicycl) | MK-9(H2) | MK-9 | MK-9(H2) |
| Muramic acid type | Acetylated | Acetylated | Glycolated | Glycolated | Glycolated | Glycolated | Glycolated | nd | Glycolated | Glycolated | Glycolated | Glycolated | Glycolated |
| Mycolic acid patternf | Single spot | Single spot | Single spot | Single spot | Multiple spots | Single spot | Single spot | Multiple spots | Single spot | nd | nd | Two spots | Single spot |
| Mycolic acids: | |||||||||||||
| Overall size (number of carbons) | 22–38 | 34–38 | 46–77 | 44–52 | 60–90 | 46–64 | 30–54 | nd | 58–64 | 43–49 | 42–52 | 62–78 | 50–56 |
| No. double bonds | 0–2 | 0–1 | 1–6 | nd | 1–4 | 0–3 | 0–4 | nd | 2–6 | nd | nd | 1–7 | nd |
| Fatty acids released on pyrolysis | 8–18 | nd | 16–18 | nd | 22–26 | 12–18 | 12–16 | nd | 16–20 | nd | nd | 22–26 | nd |
| Phosphatidylethanal-amine present in polar lipid patterns | −g | +/− | + | + | + | + | + | nd | + | + | + | + | + |
| DNA G+C content (mol%) | 51–67 | 66–73 | 63–69 | 64.7 | 57–73 | 63–72 | 63–73 | 68–72 | 67.5 | 63.7 | 69.3–71.6 | 68–78 | 64–65 |
- a The wall chemotype of Segniliparus has yet to be established.
- b Symbols: +, positive; −, negative; and nd, not determined. Data taken from Butler et al., (2005); Soddell et al., (2006); and Adachi et al. (2007).
- c Abbreviations: S, straight chain; U, unsaturated; T, tuberculostearic acid (10-methyloctadecanoic acid).
- d Corynebacterium bovis, Corynebacterium minutissimum, Corynebacterium urealyticum and Corynebacterium variabile contain tuberculostearic acid (Kämpfer et al., 1999; Lechevalier et al., 1977).
- e Examples of abbreviations: MK-9 (H2), menaquinone with two of the nine isoprene units hydrogenated; SQA and SQB, smaradiquinones A and B.
- f Number of mycolic acid spots produced from whole organism methanolysates (Minnikin et al., 1975, 1980,). In mycobacterial mycolic acids, double bonds may be converted to cyclopropane rings; methyl branches and other oxygen functions may be present (Dobson et al., 1985).
- g Present in Corynebacterium bovis and Corynebacterium urealyticum (Kämpfer et al., 1999).
The chemotaxonomic profiles of wall chemotype IV genera which lack mycolic acids are shown in Table 2. All of the genera contain unsaturated menaquinones, N-acetylated muramic acid moieties, and a combination of chemical markers which distinguish them from mycolic acid-containing taxa. It is interesting that the type strain of Hoyosella altamirensis has a DNA G+C content of 49.3 mol%, the lowest recorded value among all of the taxa classified in the order Corynebacteriales (Jurado et al., 2009). Little is known about the chemotaxonomic properties of mycolateless members of the genus Corynebacterium; these organisms are rich in saturated and unsaturated fatty acids though only Corynebacterium kroppenstedtii contains tuberculostearic acid (Collins et al., 1998; Fernández-Garayzábal et al., 2004; Hall et al., 2003). Ultrarapid pyrosequencing of Corynebacterium kroppenstedtii DSM 44385T indicates that its inability to synthesize mycolic acids may be due to gene loss, including a condensase gene cluster and a mycolate reductase gene (Tauch et al., 2008). Corynebacterium amycolatum synthesizes unsaturated menaquinones with nine isoprene units and can thereby be distinguished from Corynebacterium kroppenstedii which has corresponding menaquinones, albeit with eight isoprene units like Hoyosella altamirensis (Jurado et al., 2009).
| Characteristic | Amycolicococcus | Hoyosella | Turicella |
|---|---|---|---|
| Micromorphology | Cocci | Cocci found singly, in pairs, or in small groups | Single cells, V-shaped, or in palisades |
| Aerial hyphae | Absent | Absent | Absent |
| Major polar lipidsc | DPG, PC, PE, PG, PI, GluNu | DPG, PE, PG, PI | nd |
| Muramic acid moieties | Acetylated | Acetylated | Acetylated |
| Predominant menaquinones | MK-7, 8 | MK-8 | MK-10, 11 |
| Presence of tuberculostearic acid | + | + | + |
| DNA G+C content (mol%) | 66.0 | 49.3 | 65–72 |
- a Symbols: +, present; nd, not determined.
- b Data taken from Funke et al. (1994), Jurado et al. (2009), and Wang et al. (2010).
- c Abbreviations: DPG, diphosphatidylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol and GluNu, unknown glucosamine containing phospholipid.
Lipoglycans found in the walls of some actinomycetes have been assigned to a number of structural archetypes (Sutcliffe, 1995). The most extensively studied lipoglycans are the mycobacterial lipoarabinomannans (Bricken et al., 2004; Chatterjee and Khoo, 1998; Nigou et al., 2003). However, lipoarabinomannans (LAM) and LAM-like/lipomannan components have been extracted and characterized from other mycolic acid-containing taxa including Corynebacterium diphtheriae (Moreira et al., 2008), Dietzia maris (Sutcliffe, 2000), Gordonia rubripertincta (Flaherty and Sutcliffe, 1999), Rhodococcus equi (Garton et al., 2002), Rhodococcus ruber (Gibson et al., 2003a), and Tsukamurella paurometabola (Gibson et al., 2004), and mycolateless Amycolatopsis sulfurea (Gibson et al., 2003b), Lechevalieria aerocolonigenes (Gibson et al., 2005), and Turicella otitidis (Gilleron et al., 2005). Consequently, it is clear that the distribution of the LAM family of lipoglycans is not limited to mycolic acid-containing genera.
The cell walls of some mycolic acid-containing actinomycetes contain one or more cell-wall channels (Lichtinger et al., 2000, 2001, 2004; Riess et al., 1998; Trias et al., 1992). These channels allow the uptake of hydrophilic solutes through the thick mycolic acid layers which form an effective permeability barrier on the outer surface of cells (Brennan and Nikaido, 1995; Jarlier and Nikaido, 1994) that is analogous to the outer membrane of Gram-stain-negative bacteria. There is evidence that the cell wall of Corynebacterium amycolatum contains sufficient lipids to form a permeability barrier on the outer wall surface, thereby accounting for the presence of channel-forming proteins in this organism (Dörner et al., 2009). There are grounds for believing that Micromonospora purpurea and Streptomyces griseus contain cell-wall channels with properties similar to those of mycolic acid-containing genera (Kim et al., 2002).
Detection of Chemotaxonomic Markers
Members of genera and families classified in the order Corynebacteriales can be distinguished from one another and from corresponding taxa in the phylum Actinobacteria by 16S rRNA similarity values and by taxon-specific 16S rRNA oligonucleotide sequences (Stackebrandt et al., 1980; Zhi et al., 2009). However, actinobacterial phylogenies need to be supported by additional evidence, as phylogenetic relationships are not always sufficiently robust to allow groups to be delineated with confidence (Labeda et al., 2011) while major differences can be found in actinobacterial trees based on different algorithms (e.g. Ludwig and Klenk, 2005; Zhi et al., 2009).
Chemical markers have provided an effective way of evaluating actinobacterial phylogenies as they are discontinuously distributed across taxa, as exemplified in Figure 1 and Table 1 and Table 2. Standard chemotaxonomic procedures are available for the detection of wall diamino acids (Hancock, 1994; Staneck and Roberts, 1974), fatty acids (Kroppenstedt, 1985; Suzuki and Komagata, 1983) including mycolic acids (Minnikin et al., 1975, 1980), menaquinones (Collins, 1994; Kroppenstedt, Collins, 1982, Collins, 1985; Minnikin et al., 1984a), muramic acid residues (Uchida and Aida, 1977, 1979a, 1979b; Uchida et al., 1999), and polar lipids (Minnikin et al., 1977, 1984a; Tindall, 1990).
Examination of whole-organism hydrolysates for the presence of meso-A2pm, arabinose, and galactose, and the detection of mycolic acids in whole-cell methanolysates are the first steps in the chemotaxonomic procedure. The thin-layer-chromatographic (TLC) procedures of Staneck and Roberts (1974) provide an easy way of detecting the diagnostic amino acid and sugar markers, while mycolic acids can be detected using the acid methanolysis protocol described by Minnikin et al. (1975); modification of these procedures are described in detail by Goodfellow (1996).
The detection of meso-A2pm, arabinose, galactose, and mycolic acids serves to distinguish Corynebacteriales strains from corresponding taxa such as the orders Actinopolysporiales, Calenulosporiales, Micrococciniales, Micromonosporiniales, Pseudonocardiales, Streptomycetiales, and Streptosporangiales. The mycolateless wall chemotype IV genera Amycolicicoccus, Hoyosella, and Turicella can be distinguished from corresponding actinomycetes classified in the order Pseudonocardiales as they contain unsaturated menaquinones (Table 2); a variety of chromatographic procedures can be used to establish menaquinone profiles (Collins, 1994; Minnikin et al., 1985). The menaquinone composition of most corynebacteria which lack mycolic acids has still to be established.
Mycolic acids show structural variations which range from relatively simple mixtures of saturated and unsaturated components in corynebacteria to the highly complex mixtures characteristic of mycobacteria (Goodfellow and Magee, 1998; Minnikin, 1982, 1993; Minnikin and Goodfellow, 1980). Mycobacterial mycolates have 60–90 carbon atoms with up to 4 double bonds; those of the remaining genera vary in chain length and the number of double bonds (Table 2). Qualitative TLC analyses of mycolic acids after Minnikin et al. (1975) show that the methanolysates of mycobacteria, with the exception of Mycobacterium brumae, Mycobacterium fallax, and Mycobacterium triviate (Dobson et al., 1985; Luquin et al., 1993), give a multispot pattern, tsukamurellae two spots, and most of the remaining taxa a single spot (Table 1); the different Rf values of the mycolic acid spots reflect differences in chain lengths and structures (Embley and Wait, 1994; Minnikin, 1988, 1993; Minnikin et al., 1980; Yassin et al., 1997). Segniliparus strains also produce a multispot pattern (Butler et al., 2005). Mycolic esters can be positively identified on thin-layer chromatograms by their characteristic immobility when plates are subsequently washed in methanol water (5:2, v/v) (Minnikin et al., 1975). Mycobacterial species can be assigned to one of several groups based on the presence or absence of different types of mycolic acids (Goodfellow and Magee, 1998; Minnikin, 1988, 1993; Minnikin et al., 1984a, 1984c, 1985); the latter can be detected more precisely by two dimensional TLC using appropriate protocols (Dobson et al., 1985; Embley and Wait, 1994; Minnikin, 1988; Minnikin et al., 1980).
Several precipitation methods have been recommended to distinguish between mycobacterial mycolic acids and those from related taxa. Mycobacterial mycolic acids can be precipitated from ether solution by the addition of an equal (Kanetsuna and Bartoli, 1972) or double volume (Hecht and Causey, 1976) of ethanol. In contrast, mycolic acids from corynebacteria, gordoniae, and rhodococci are not precipitated in these procedures though Hecht and Causey (1976) detected their presence in supernatants by TLC. A more sensitive and reliable precipitation procedure was introduced by Hamid et al. (1993) to separate mycobacteria from other mycolic acid-containing genera. This method is based on the solubility of mycolic acid methyl esters in acetonitrile/toluene (1:2, v/v) and the insolubility of those from mycobacteria in acetonitrile/toluene (3:2, v/v).
Once the presence of mycolic acids has been detected, their esters can be extracted and studied further to yield additional taxonomic data, notably on the overall size of mycolic acids, their degree of unsaturation, and the length of alkyl side chains (Table 1). Methods used for such purposes include pyrolysis gas chromatography (Collins et al., 1982b; Kroppenstedt, 1985), mass spectrometry (Collins et al., 1982a), gas chromatography-mass spectrometry of trimethylsilyl ether and tetrabutyldimethyl derivatives of mycolic acids (Athalye et al., 1984; Pommier and Michel, 1985; Tomiyasu et al., 1981; Yano et al., 1978), and high performance liquid chromatography (HPLC) of para-bromophenyl and N-methyl-N-nitro-p-toluene-sulfonamide derivatives of mycolic acids (Butler et al., 2005; Butler et al., 1996; Lévy-Trébault et al., 1986). Reverse-phase HPLC of para-bromophenyl esters of mycolic-acids provides a particularly rapid way of distinguishing between mycolic acid-containing genera (Butler et al., 1986); appropriate columns, detectors, and solvent gradients have been described by Butler and his colleagues (Butler et al., 1986, 1987; Butler and Kilburn, 1988; De Briel et al., 1992). In addition, different classes of mycobacterial mycolic acids can be separated by HPLC (Butler et al., 2005; Kaneda et al., 1995; Ramos, 1994; Steck et al., 1978). This separation is based upon chain lengths, the degree of unsaturation, and other functional groups in mycolic acids (Svensson et al., 1982).
10-Methyloctadecanoic (tuberculostearic) acid, phosphatidylethanolamine, and the acyl types of muramyl residues in wall peptidoglycans are important markers in the classification and identification of genera which belong to the order Corynebacteriales (Table 1 and Table 2). In addition, phosphatidyle-thanolamine has not been detected in some Dietzia species (Li et al., 2008). The genus Corynebacterium stands apart from other mycolic acid-containing genera as nearly all of its constituent species lack both phosphatidylethanolamine and tuberculostearic acid, though Millisia, Smaragdicoccus, and Tomitella strains do not contain this latter component. The genera Corynebacterium and Dietzia can be distinguished from the other mycolic acid-containing genera as they contain N-acetyl, as opposed to N-glycolyl residues in the glycan moiety of the peptidoglycan. The genera Amycolicococcus, Hoyosella, and Tomitella contain tuberculostearic acid and N-acetylated muramic acid residues, though differences are seen in the polar lipid patterns of the former two taxa. Standard procedures are available for the detection of tuberculostearic acid (Kroppenstedt, 1985), phosphatidylethanolamine (Minnikin et al., 1985), and the acyl residues of wall peptidoglycans (Uchida et al., 1999). A small-scale integrated procedure can be used for the detection of wall and lipid markers (O'Donnell et al., 1985).
Most of the genera assigned to the order Corynebacteriales can be separated solely on the basis of their predominant menaquinones (Table 1 and Table 2). The presence of fully unsaturated menaquinones with nine isoprene units (MK-9) serves to distinguish tsukamurellae from all other mycolic acid-containing genera (Collins et al., 1982b; Nam et al., 2004) and from the unsaturated menaquinones characteristic of the genera Amycolicoccus, Hoyosella, and Turicella (Funke et al., 1994; Jurado et al., 2009; Wang et al., 2010). Nocardiae and skermaniae can be distinguished from all of the other taxa as they contain predominant proportions of hexahydrogenated menaquinones with eight isoprene units where the last two are cyclized (Black-all et al., 1989; Chun et al., 1997; Collins et al., 1987; Howarth et al., 1986). In turn, smaragdicocci contain two novel cyclic forms of menaquinones, smaragdiquinone A-8 (H4, ω-cycl) and smaragdiquinone B-8 (H4, ω-cycl) (Adachi et al., 2007). Corynebacteria, dietziae, millisiae, and rhodococci all contain major amounts of dihydrogenated menaquinones with eight isoprene units as the predominant components; gordoniae, mycobacteria, and williamsiae have major proportions of the corresponding component with nine isoprene units (Collins et al., 1977, 1985; Goodfellow and Maldonado, 2006). Detailed analytical procedures for establishing menaquinone profiles have been described (Collins, 1994; Kroppenstedt, 1982, 1985), including the integrated method introduced for the analysis of bacterial quinones and polar lipids (Minnikin et al., 1985).



