Biodegradation of Polyethers (Polyethylene Glycol, Polypropylene Glycol, Polytetramethylene glycol, and Others)
Abstract
- Introduction
- Historical Outline
- Chemical Structures
- Biodegradation of PEG
- Aerobic Biodegradation of PEG
- Microorganisms Capable of Assimilating PEG
- Metabolism of PEG and Enzymes Relevant to the Metabolism
- Anaerobic Biodegradation and Metabolism of PEG
- Anaerobic Bacteria Capable of Assimilating PEG
- PEG Acetaldehyde Lyase
- Extracellular One-electron Oxidation of PEG
- Aerobic Biodegradation of PEG
- Biodegradation of PPG
- Biodegradation of PTMG
- Biodegradation of Miscellaneous Polyethers
- Physiology
- Production
- Outlook and Perspectives
- Patents
- Acknowledgments
1 Introduction
Poly(alkylene glycol)s have a long history as specialty polymers used as raw materials in the synthesis of detergents or polyurethanes. They are either water-soluble or oily liquids, which eventually find their way into either environmental or wastewater systems. Biodegradability is necessary for materials entering these water streams because they can neither be recycled nor incinerated. Poly(alkylene glycol)s include polyethylene glycol (PEG), polypropylene glycol (PPG, polymer of 1,2-propylene oxide), polytetramethylene glycol (PTMG), and polybutylene glycol (PBG, polymer of 1,2-butylene oxide).
Among these compounds, PEG is manufactured in large quantities and used as a commodity chemical in various industrial areas. The most common hydrophilic moieties contained in the nonionic surfactants are the ethylene oxide polymers. The majority of PEGs produced are used to make nonionic surfactants, a very important group of industrial products with applications ranging from domestic detergents to agrochemicals, food emulsifiers, and other industrial preparations. Ultimately, these will make a significant contribution to both domestic and industrial wastewater systems, and consequently much attention has been paid during the past 40 years to their biodegradability. Because of their low toxicity and lack of skin irritation, PEGs are widely used in the pharmaceutical industry in the preparation of ointments, suppositories, tablets, and solvents for injection, as well as in cosmetics such as creams, lotions, powders, cakes, and lipsticks. PEGs are also used as intermediates in the preparation of resins such as alkyd resin and polyurethane resin, and as components in the manufacture of lubricants, antifreeze agents, wetting agents, printing inks, adhesives, shoe polish, softening agents, sizing agents, and plasticizers. In addition, PEG has been used as resin gels that are used to immobilize enzymes or microbial cells, and for the chemical modification of enzymes.
Chemically unsubstituted PPG is used in solvents for drugs and as the ingredients of paints, lubricants, inks and cosmetics, though most are transformed to polyurethanes or surface-active agents.
PBG is an oily material used for sizing and cleaning agents and dispersants, whilst PTMG is used exclusively as a constituent of polyurethane. The oligomers (up to octamers) are water-soluble and can to a large degree be washed out as impurities from polymers with water, although the solubility of PTMG in water is much lower than that of PEG and PPG. Wastewaters containing these materials are produced not only by the synthetic processes involved, but also from domestic and industrial sources.
In addition to poly(alkylene glycol)s, a number of different types of polyethers are also available. Because of their resistance to biodegradation (aliphatic ether bonds are chemically very stable and cannot be hydrolyzed; the exception is sugar linkages, which form part of an ether linkage and are readily hydrolyzed by glycosidases), these materials are retained in natural environments as pollutants. To date, no reports have been made on the accumulation of polyethers in nature, which suggests that they are completely metabolized by microorganisms. Nonetheless, there remains a need to know the metabolic fate not only of the polyethers but also of their metabolites. In this way, it may in future become possible to use microorganisms and/or enzymes not only to assimilate polyethers, but also to remove them as pollutants from both environmental and industrial situations.
In principle, the properties of polyethers impose major constraints on their enzymatic degradation. For example, the polyether molecule is large, and unless extracellular enzymes can be used for degradation, the organism's membranes act as a barrier against substrate uptake. The active site of the enzyme must, by necessity, also be large. When dissolved in water, PEG adopts a random coil formation (Cox, 1978), with the alcohol groups at the termini distributed within the macromolecular space, and it is this conformation which causes problems when an enzymatic attack is targeted at the termini.
The biodegradation of polyethers, and in particular that of PEG and PPG, is summarized in this chapter.
2 Historical Outline
PEG, the first example of poly(alkylene glycol)s, was first produced commercially under the brand name of Carbowax; it has an average number molecular weight (Mn) ranging from 200 to 2000 Da. During the 1950s, the synthesis of polyethylene oxide (PEO) with a far greater Mn ranging from a few hundreds of thousands to a few million daltons became feasible. The majority of PEGs are used in the synthesis of nonionic surfactants or polyurethanes, and PEG with a low Mn (e.g., Carbowax) is mainly used in these products. By contrast, polyurethanes are mostly used in solid forms, and so cause no pollution problems in water streams.
Unfortunately, detergents cause serious environmental pollution in water streams, especially when used in industrial, agricultural, and domestic applications; consequently, methods to render these compounds biodegradable have attracted attention worldwide. Minimally or nonbiodegradable detergents have gradually been replaced by more biodegradable alternatives. Most nonionic detergents include PEG as their major component, and aliphatic nonionic detergents are known to be readily biodegraded; hence, many reports on their biodegradability have been published during the past 40 years. In 1962, Fincher and Payne were the first to report the biodegradation of PEG with a molecular weight of up to 400 daltons. Subsequently, because of their low toxicity and minimal skin irritation, high stability and excellent physical characteristics, PEGs have been applied to a wide variety of industrial uses, as well as agricultural, medical or pharmaceutical, sanitary, and domestic products. At present, PEGs of Mn up to 20,000 Da are known to be biodegradable (Kawai, 1995, 2002). The biodegradation of higher Mn PEGs may be more difficult as the degradation mechanisms involved–whether aerobic or anaerobic–require that at least one terminal hydroxyl group must be free. When the molecular size is increased, the ratio of terminal hydroxyl groups per molecule is reduced, and this leads to difficulties in the enzyme attacking the termini. An increase in Mn however rapidly increases the viscosity of the polymer, and this hinders the free contact of the terminal residues with the microorganism's metabolizing enzymes. Nonetheless, most PEGs which enter water streams have a Mn <20,000 Da, and so cause no serious environmental pollution.
In addition to PEG, several other polyethers have been produced commercially, including PPG, PBG, PTMG, polyglycidol, and polyglycerine. Although a greater quantity of PPG is produced than PEG, most of it is used in the production of polyurethanes and so only small amounts are lost as waste and enter the water streams. A number of other polyethers have been designed for use as builders; these are chelating agents that remove metals from the water to improve detergent performance, and include polyglyoxylic ether, poly(disodium epoxysuccinate), and poly(sodium glycidate). Whilst polyisobutylene oxide is an exceptional photodegradable plastic, other similar compounds are either liquid or waxy in nature.
As biodegradable polymers continue to attract an increasing amount of attention, polyethers such as PEG will be used as the major “biodegradable” component of copolymers, and also blended with other biodegradable plastics in order to improve their physico-chemical characteristics. The variety of applications for the polyethers, together with their excellent physico-chemical properties, suggest that these materials will play a central role in the long-term use of polyethers and their derivatives.
3 Chemical Structures
Poly(alkylene glycol)s have a common structural formula: HO[R-O]nH (R=CH2CH2 for PEG; CH3CHCH2 for PPG; (CH2)4 for PTMG; and C2H5(CHCH2) for PBG), where n represents an average range of units. These materials are synthesized from alkylene oxides, using ring-opening polymerization. This reaction usually results in the formation of a mixture of homologous compounds that differ in molecular weight; the mixture is subsequently fractionated into different ranges of molecular weight and a Mn value is assigned to each fraction. A number following the abbreviation of a polyether usually indicates the Mn: for example, PEG 400 and 20,000 indicate PEGs with Mns of ∼400 and ∼20,000 Da, respectively. On occasion, an abbreviation and actual molecular size are different; for example, commercially available PEG 6000 has a molecular size of ∼7000–8000 Da. Ambiguity is often found in the molecular weights of commercially available reagent-grade PEG or PPG, as to whether their molecular weight indicates Mn or Mw.
The physical properties of PEGs vary from viscous liquids to waxy solids according to their Mn values, although all PEGs – from oligomers up to those with a Mn of a few million – are totally and highly water-soluble. Commercially available PPGs are divided into two groups – the diol and triol types – due to the straight- or branched-chain structure of the polymer. When a chain of 1,2-propylene glycol is expanded from ethylene glycol (EG), a straight chain is obtained (diol type); however, when glycerol is used in place of EG, a branched polymer chain is created (triol type). The water solubility of PPGs is lost when the Mn is increased to more than ∼700 (triol type) or ∼1000 (diol type). Therefore, copolymers comprised of PEG and PPG blocks are used as detergents, where PEG is a hydrophilic constituent, and PPG is a hydrophobic constituent.
Another type of copolymer in which ethylene oxide and propylene oxide are randomly copolymerized is used as a water-soluble, flame-resisting pressure liquid. PBG is an oily polymer due to the presence of pendant ethyl groups, whilst PTMG is generally used as a waxy material, from which water-soluble oligomeric impurities have been removed. The structures of poly(alkylene glycol)s and other miscellaneous polyethers are summarized in Figure 1.
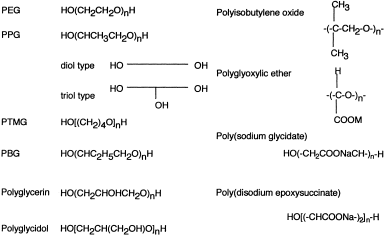
Chemical structures of polyethers.
Yamagishi et al. (1973) reported that polyisobutylene oxide may be a promising photodegradable plastic. Unlike other polyethers, this material is highly crystalline, and the exposure of a 25 µm-thick film under ultraviolet light at 60°C leads rapidly to the formation of acetone, formic acid, and acetic acid as principal degradation products; all of these are readily integrated into the ecological cycle. Monsanto has developed polymerized glyoxylic ethers which are polyacetals and stable in alkali, but not in acid (US Patents). When used as detergents, these materials are stable, but they are hydrolyzed to the biodegradable monomer as the pH falls to ∼7 in sewer systems. Matsumura et al. (1987) synthesized polycarboxylate oligomers containing ether linkages, and tested their building performances in detergents and biodegradability, among which poly(sodium glycidate) and poly(disodium epoxysuccinate) were biodegraded by 25% and 40%, respectively in a five-day biochemical oxygen demand (BOD) test. Polyglycidol is a type of poly(alkylene glycol) which is synthesized from glycidol, where methylhydroxyl groups are substituted for the methyl groups of PPG. Polyglycerine is synthesized by the condensation of glycerine with NaOH at 250°C, and is mainly used as fatty acid esters.
4 Biodegradation of PEG
4.1 Aerobic Biodegradation of PEG
4.1.1 Microorganisms Capable of Assimilating PEG
Following several earlier reports on the biodegradability of glycols, in 1962 Fincher and Payne first described an isolated bacterium from a soil enrichment containing trimer that could grow in media containing EG, DEG, TriEG, TetraEG, or PEG 400 as sole sources of carbon and energy. Since then, many reports have been made on aerobic PEG-utilizing bacteria which assimilated PEGs with a variety of molecular sizes (Kawai, 1995, 2002) (Table 1).
Researcher |
Year |
PEG compound |
Microorganism |
|---|---|---|---|
Fincher and Payne |
1962 |
diEG-PEG 400 |
Soil bacterium |
Borstlap and Kortland |
1967 |
PEG 400 and 1000 |
Activated sludge |
Patterson et al. |
1970 |
<PEG 400 |
Air-dried activated sludge |
Sturm |
1973 |
<PEG 1000 |
Acclimated sewage sludge |
Pitter |
1973 |
EG-PEG 3500a |
Sludges and pure culture |
Ohmata et al. |
1974 |
triEG-PEG 400 |
Soil bacterium (possibly |
Pseudomonas) |
|||
Harada and Nagashima |
1975 |
triEG or PEG 400 |
Sludge or soil bacterium |
(Alcaligenes) |
|||
Ogata et al. |
1975 |
PEG up to 20,000 |
Pure cultures and symbiotic |
Kawai et al. |
1977 |
mixed culturesb |
|
Haines and Alexander |
1975 |
PEG up to 20,000 |
Pseudomonas aeruginosa |
Cox and Conway |
1976 |
PEG up to 4000 |
Adapted activated sludge |
Jones and Watson |
1976 |
EG-PEG 400 |
Possibly an Actinetobacter |
Watson and Jones |
1977 |
EG-PEG 1500 |
Actinetobacter |
Pseudomonas |
|||
Flavobacterium |
|||
Suzuki and Kusunoki |
1977 |
PEG 200–2000 |
Acclimated activated sludge |
Hosoya et al. |
1978 |
PEG 400 and 6000 |
Soil bacteria |
Jenkins and Cook |
1979 |
EG-PEG 200 |
Bacteria |
PEG 200–4000 |
|||
Thélu et al. |
1980 |
diEG-PEG 400 |
Pseudomonas |
Pearce and Heydeman |
1980 |
EG-PEG 1500 |
Actinetobacter |
(different utilization |
Pseudomonas |
||
range) |
Aeromonas |
||
Schöberl |
1983/1985 |
diEG and triEG |
Pseudomonas fluorescens |
1986 |
tetraEG/PEG600 |
Alcaligenes glycovorans |
|
Steber and Wierich |
1985 |
PEG 400 |
Activated sludge |
Obradors and Aguilar |
1991 |
PEG up to 10,000 |
Pseudomonas stutzeri |
Kawai and Takeuchi |
1996 |
PEG 4000 and PEG |
Sphingomonads |
up to 20,000 |
|||
Kohlweyer et al. |
2000 |
PEG 4000 and 8000 |
Pseudonocardia sp. |
- a The growth might be caused by lower molecular PEG included in a sample.
- b A representative mixed culture was composed of Sphingomonas terrae and Rhizobium sp.
PEGs with a Mn >1000 Da had long been considered to be bioresistant, but PEGs of Mn up to ∼20,000 Da have been shown to be biodegradable until now. Several investigating groups have reported the recalcitrance of PEG with Mn >4000 Da, however. As with the biodegradability of PEGs of various Mn, Ogata et al. (1975) isolated various PEG-utilizing cultures by enrichment on PEGs. These were classified into five groups according to their PEG-utilizing abilities: PEG 400, PEG 600, PEG 1000, PEG 4000, and PEG 20,000. PEG 400 or 600-utilizing bacteria were enriched with PEG 400, whilst PEG 1000-utilizing bacteria were enriched with PEG 1000. However, PEG 4000-utilizing bacteria were enriched with PEG 2000, and PEG 20,000-utilizing bacteria were enriched with PEG 6000. Various ubiquitous bacteria utilized low PEGs up to 4000, whilst high PEGs from 4000 to 20,000 were assimilated by limited numbers of species, including: Pseudomonas aeruginosa (up to 20,000; Haines and Alexander, 1975); soil bacteria (up to 6000; Hosoya et al., 1978); Pseudomonas stutzeri (up to 13,500; Obradors and Aguilar, 1991); and Sphingomonas species (up to 20,000). PEG 6000 and higher were completely metabolized by consortia of Sphingomonas terrae and concomitant associates (Rhizobium, Agrobacterium, and Methylobacterium sp.) (Takeuchi et al., 1993), and more recently by a pure culture of Sphingomonas sp. (Kawai and Takeuchi, 1996). Sphingomonads include sphingolipids in their outer membranes, instead of lipopolysaccharides as are found in most Gram-negative bacteria. Various lipophilic xenobiotic-assimilating bacteria were included in this genus: poly(chlorophenol-, polycyclic aromatic hydrocarbons-, γ-hexachlorocyclohexane-, 2,4-dichlorophenoxyacetic acid-, dibenzo-p-dioxin, and diphenyl ether-utilizing bacteria (Nohynek et al., 1996; Kawai, 1999). Although a barrier for the macromolecules might possibly exist for the assimilation of polyethers, the characteristic membrane structure of this genus is possibly correlated with the uptake of large PEG by membranes. This was partly supported by the findings that Rhodopseudomonas acidophila M402 is able to oxidize PEGs up to a certain size limit by using its alcohol dehydrogenase, but it cannot grow on these compounds (Yamanaka, 1991); and that cell-free extracts of PEG 400-, 1000- or 4000-utilizing bacteria could dehydrogenate higher PEGs (6000 and 20,000), which could not be utilized as the sole carbon and energy sources (Kawai and Yamanaka, 1989). Most recently, a Gram-positive actinomycete, Pseudonocardia sp. strain K1 was originally isolated as a tetrahydrofuran degrader but subsequently found to grow also on PEG 4000 and 8000 (Kohlweyer et al., 2000). As this is the only Gram-positive bacterium known to grow on PEG, it provides another interesting focus as to whether the actinomycete has the same metabolic pathway and has the uptake system of large PEG into cells, as is found in Sphingomonads.
Although pure cultures might be able to degrade high-molecular weight PEGs in their own right, symbiosis remains an important and ubiquitous phenomenon in natural environments, and seems to be involved in the biodegradation of recalcitrant materials. The mechanism for symbiotic biodegradation of PEG by S. terrae and Rhizobium sp. (Kawai and Yamanaka, 1986) was elucidated as being due to removal of a toxic metabolite, glyoxylic acid (GOA) being formed during the biodegradation process, as shown in Figure 2.
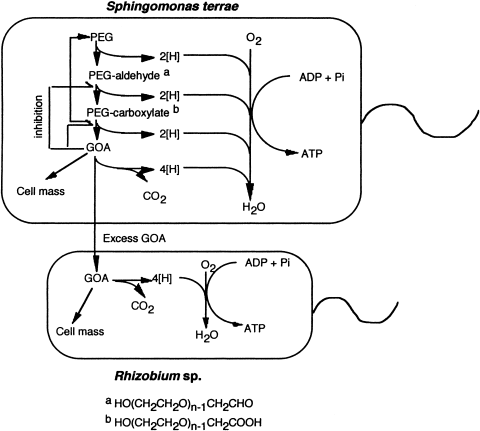
Mechanism of symbiotic degradation of PEG by S. terrae and Rhizobium sp. GOA, glyoxylic acid.
4.1.2 Metabolism of PEG and Enzymes Relevant to the Metabolism
4.1.2.1 Metabolic Route for PEG
Early studies on the aerobic degradation of PEG indicated that its metabolism must proceed via oxidation (Kawai, 1987):
-
PEG dehydrogenation was indicated in many reports. Payne (1963) found that cell-free extracts of a bacterium grown on tetraEG dehydrogenated PEGs ranging from DEG to PEG 6000, and that the oxygen uptake rates were approximately the same with samples from DEG to PEG 300, though virtually no uptake was observed with either PEG 1000 or 6000. Dehydrogenations of PEGs linked with electron acceptors such as NAD, flavins, and ferricyanide were observed with enzyme preparations obtained from lower PEG-grown bacteria, which acted on lower PEGs from dimer up to 600, but not on PEGs with Mn >1000. These enzymes had substrate specificities towards PEGs close to the Mn range of material on which bacteria could grow. The involvement of a PEG-oxidizing enzyme in the metabolism of PEG was suggested for various PEG (400–20,000)-utilizing bacteria (Kawai et al., 1984). These dehydrogenases are induced by PEGs, which can be a sole carbon and energy source, respectively, except that the enzyme of PEG 4000-utilizing Sphingomonas macrogoltabidus No. 203 has a constitutive PEG-dehydrogenase (Yamanaka and Kawai, 1989).
-
Oxidation products were obtained from reaction mixtures or culture filtrates of PEG-utilizing bacteria on various PEGs and their derivatives. Harada and Nagashima (1975) isolated metabolites from a six-day culture of Alcaligenes sp. incubated with EG monoethyl ether or monomethyl ether, and identified them as ethoxy and methoxy acetic acid. Patterson et al. (1970) detected acidic metabolic intermediates from the degradation of alcohol ethoxylate. Hosoya et al. (1978) obtained dicarboxylic acids of EG dimer and trimer from a culture filtrate of bacteria grown on the trimer, and suggested that PEG might be degraded oxidatively by splitting a C2 unit from a terminal alcohol which is first oxidized to a carboxyl. Kawai et al. (1978) detected mono- and dicarboxylic acids of tetraEG and depolymerized oligomers (trimer to monomer) in a reaction mixture with tetraEG. In addition, tetraEG-aldehyde or valeraldehyde was characterized as a reaction product of tetramer or pentanol incubated with a crude PEG-DH (cell-free extracts of a PEG 20,000-utilizing symbiotic culture E-1) (Kawai et al., 1983).
The results of these studies clearly indicated the metabolic pathway for PEG in aerobes (Figure 3).
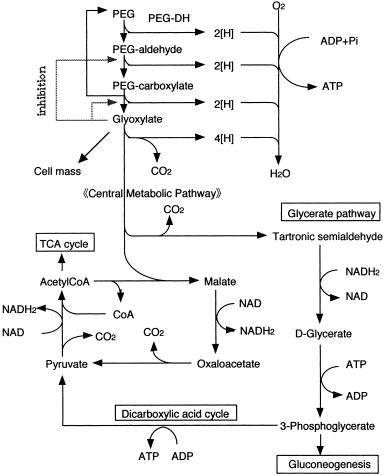
Aerobic metabolism of PEG.
PEG is successively oxidized to an aldehyde and a monocarboxylic acid, and this is followed by the cleavage of the ether bond, resulting in PEG molecules that are shortened by one glycol unit. The simultaneous oxidation of two terminal alcohol groups of the molecule is also possible. Depolymerization might proceed via the same reaction observed with the monocarboxylic acid. This sequence is repeated and eventually yields depolymerized PEG. The resultant GOA may then enter into central metabolic routes by known pathways, e.g., the oxidative dicarboxylic acid cycle, tricarboxylic acid (TCA) cycle, and the glycerate pathway. Originally, it was postulated that three enzymes are required for this: (1) An alcohol dehydrogenase/oxidase; (2) an aldehyde dehydrogenase/oxidase, which converts the terminal alcohol groups of PEG into carboxylic acid groups; and (3) an ether-bond-splitting enzyme yielding glyoxylate as the final product (see Figure 3). The degradability of PEG with different terminal structures was also examined, using PEG 20,000-utilizing symbiotic mixed culture E-1 (S. terrae and Rhizobium sp.) (Kawai, 1993). It is rational that PEG-diglycolic acid was utilized, because this is a metabolite of PEG. Monoalkyl (but not dialkyl) PEG was utilized, suggesting that the degradation of PEG is exogenously started from a terminal alcohol group. This inference was supported by the observation that depolymerized products were not detected during degradation of PEG by the mixed culture. In other words, the endogenous breakdown of a polymer molecule rapidly yields depolymerized products to some extent, but the exogenous breakdown does not, because of a gradual, step-by-step depolymerization. All these data support the metabolic route shown in Figure 3. The culture neither grows on PPG nor PTMG. Thus, biodegradation proceeds exogenously from a terminal group and depends strictly on the chemical structures of the monomer units.
Haines and Alexander (1975) reported that PEG 20,000 might be hydrolyzed by an extracellular enzyme of Pseudomonas aeruginosa to yield oligomers as metabolic products, though these results have not been reproduced and the original strain was lost. Pearce and Heydeman (1980) suggested a nonoxidative removal of EG units as acetaldehyde by a membrane-bound, novel oxygen-sensitive enzyme, diEG lyase. Of the cofactors tested, only cyanocobalamin and adenosylcobalamin stimulated the reaction, but this varied from one preparation to another. These authors assayed enzyme activity by vapor-phase chromatography of the incubation mixture. However, the measurement of compounds in the presence of unidentified metabolizable materials is risky. Schöberl (1983) first suggested that PEG is catabolized by a C1 step, liberating formate, which is metabolized by a serine pathway. However, he later (1985) corrected the C1 hypothesis and reported that dicarboxy products were obtained from dimer ∼ tetramer, suggesting that an ether bond was hydrolyzed to liberate glycolic acid. Thélu et al. (1980) reported that cell-free extracts from Pseudomonas sp. grown on PEG 400 dehydrogenated 2-ethoxyacetic acid or dimethylethyl derivatives of diEG and tetraEG as well as PEG, and suggested the transient formation of a double bond in a terminal glycol unit, followed by hydration, according to: R-O-CH2CH2OH→[R-O-CH=CHOH]→R-O-CHOHCH2OH.
Microbial diversity might in the future demonstrate the presence of another pathway, but to date no acceptable evidence has been proposed (Duine and Kawai, 1998).
4.1.2.2 Enzymes Relevant to the Metabolism
Ether-alcohol dehydrogenases showed a weak or appreciable activity on low PEGs up to 600 (Harada and Sawada, 1977; Hino et al., 1981). Significant membrane-bound PEG dehydrogenase (PEG-DH) activities were found with diverse PEG-utilizing bacteria, among which PEG-DHs were purified from the symbiotic mixed culture E-1 (PEG 20,000-assimilating, inducible) and from S. macrogoltabidus No. 203 (PEG 4000-assimilating, constitutive) (Kawai et al., 1980; Yamanaka and Kawai, 1989). Both enzymes were solubilized with surfactants, and stabilized with 10% glycerol or ethylene glycol. The purified enzymes showed quite similar substrate specificities toward PEGs up to 20,000, although S. macrogoltabidus No. 203 cannot grow on PEGs 6000–20,000. Sequencing of PEG-DH (accession No. AB050784) revealed that the enzyme belongs to the group of GMC flavoproteins, with one molecule of FAD being bound to the monomer protein of the homodimeric protein enzyme. Alignments of amino acid sequences of three PEG-DHs of S. terrae, S. macrogoltabidus 203 and 103 showed that they differ from each other in only one amino acid (unpublished data). It has already been shown that PEG-DH has an interesting feature with respect to the kinetic resolution of racemic alcohols (Geerlof et al., 1994). The application of these enzymes (organisms) can be envisaged in the field of bioconversions as well as analytical chemistry.
Quinohemoprotein alcohol dehydrogenases (QH-ADHs) can be subdivided into soluble (type I) and membrane-bound (type II) forms. Type II QH-ADH has so far only been found in Gluconobacter and Acetobacter bacteria (Ameyama and Adachi, 1982), and no information exists as to whether they are able to oxidize PEG. However, certain type I QH-ADHs purified from other bacteria which cannot grow on PEG act as ADHs for PEG (Duine and Kawai, 1998) (Table 2): ADH from Comamonas testosteroni (Groen et al., 1986; de Jon et al., 1995); vanillyl alcohol dehydrogenase from R. acidophila strain M402 (Yamanaka and Tsuyuki, 1983; Yasuda et al., 1996); and tetrahydrofurfuryl alcohol dehydrogenase from Ralstonia eutropha (formerly Alcaligenes eutrophus; Zarnt et al., 1997). The N-terminal part of the enzyme from C. testosteroni shows high similarity with other pyrroloquinolinequinone (PQQ)-containing enzymes, in particular with those of methanol dehydrogenases (Stoorvogel et al., 1996), whereas the C-terminal part does not but contains the heme c. Based on this and the three-dimensional structure of methanol dehydrogenase, modeling studies have shown that QH-ADH from C. testosteroni has a large active site, which explains the enzyme's ability to oxidize PEG and secondary alcohols and to act as polyvinyl alcohol dehydrogenase (Jongejan et al., 1998). However, as it has not yet been found in a PEG-utilizing organism, it is possible that QH-ADH might be an alcohol dehydrogenase by design, but a “PEG dehydrogenase” by accident.
Substrate |
Relative activity (%) at varying substrate concentrations |
|||
|---|---|---|---|---|
RA enzyme |
CT enzyme |
|||
5 mM |
0.5 mM |
0.05 mM |
5 mM |
|
TetraEG |
100a |
30 |
0 |
100b |
PEG 400 |
113 |
– |
– |
83 |
PEG 1000 |
87 |
– |
– |
58 |
PEG 4000 |
45 |
– |
– |
63 |
PEG 6000 |
78 |
– |
– |
69 |
Solketal |
29 |
– |
– |
– |
Glycidol |
26 |
– |
– |
– |
Ethanol |
72 |
– |
– |
– |
n-Propanol |
143 |
– |
– |
– |
n-Butanol |
125 |
– |
– |
82 |
n-Hexanol |
16 |
100 |
82 |
– |
n-Octanol |
17 |
41 |
4 |
– |
2-Propanol |
0 |
0 |
0 |
– |
2-Hexanol |
28 |
2 |
0 |
– |
2-Octanol |
24 |
16 |
1 |
– |
- a Enzyme activity: 8.0 U mg−1.
- b Enzyme activity: 6.3 U mg−1. Reproduced from Yasuda et al. (1996).
PEG is known to cause a fatal toxic syndrome when absorbed, which might be due to the formation of mono- and dicarboxylated PEG (Herold et al., 1982). The presence of organic acids of PEG in the blood of poisoned patients and in an animal model suggested that PEG is metabolized in vivo. Oxidation of PEG homologues (n=1∼8) was catalyzed by equine liver alcohol dehydrogenase (Herold et al., 1989); this finding suggested that sequential oxidations by ADH and aldehyde dehydrogenase were possible causes of the syndrome. Thus, PEG may be oxidized by various ADHs, the origins of which are diverse.
Studies on PEG degradation by Sphingomonads revealed an enzyme which was able to oxidize PEG-carboxylic acid under formation of GOA (Kawai, 1985). Since diglycolic acid (DGA) was also a good substrate, the enzyme was called DGA dehydrogenase (DGA-DH). In the presence of lauryl maltoside, the purified enzyme was seen to be clearly distinct from PEG-DH, as neither PEG nor any other alcohol (including diols) or aldehyde was a substrate (Enokibara and Kawai, 1997). Apparently, the presence of a terminal carboxylic acid group is crucial for oxidation to occur at the α-position in the molecule. However, the presence of an ether bond is not essential, as GOA and glycolic acid are also substrates for DGA-DH. Therefore, the oxidation mechanism might be similar to that of the peroxisomal, flavoprotein glycolate oxidase (EC 1.1.3.l) or to that of flavoprotein α-hydroxyl acid dehydrogenases, proceeding via hydride transfer or via deprotonation, followed by electron transfer or covalent catalysis which occurs in the adduct formed from the (carbanion) substrate molecule and the cofactor (Lindqvist, 1992). As no O2 consumption has been detected in the conversion of PEG-dicarboxylic acid by DGA-DH in vitro, the process must be accompanied by an attack of a H2O molecule, such that rearrangement of the oxidized ether bond can take place under formation of a terminal aldehyde group (in GOA) and a terminal alcohol group (in the PEGn–1). Elucidation of the mechanism and identification of the cofactors involved in this reaction sequence must await the purification of substantial quantities of DGA-DH, however.
It has been reported that DGA-utilizing Rhodococcus sp. 432 contains a flavoprotein DGA oxidase (Yamanaka and Kawai, 1991) which lacks a periplasmic space. As ferricyanide and dichlorophenolindophenol (DCIP) were much better electron acceptors than O2, the enzyme might be a dehydrogenase oxidizing DGA at the cytoplasmic side of the membrane, and be able to use O2 as electron acceptor in vitro. As the enzyme uses glycolic acid as well as GOA (but not PEG), similarity with glycolate oxidase might also be possible. Although the different properties of the DGA oxidase seem to exclude structural similarity with DGA-DH, further studies are required to prove this.
The same uncertainty concerning similarity to DGA-DH exists with respect to the DCIP-linked dehydrogenase from a Pseudomonas stutzeri strain, which oxidizes PEG directly under formation of GOA (Obradors and Aguilar, 1991). Based on results from polyacrylamide gel electrophoresis (PAGE) and activity staining, the authors claimed that this involves one single enzyme oxidizing PEG as well as DGA, and purification of the enzyme did not require solubilization with a detergent. Although this suggests significant differences from the Sphingomonad system (which consists of two distinct enzymes, PEG-DH and DGA-DH, that each require detergent solubilization), in the absence of any further information on the molecular properties of the enzyme, no conclusion can be drawn at present.
The whole-cell or cell-free extracts of Pseudomonas sp. strain SC25A that can grow on PEG dodecyl ethers yielded dodecanol as the first step of the metabolism. However, the scission mechanism is still to be elucidated (Tidswell et al., 1996).
Until recently, Gram-negative bacteria have been exclusively isolated as PEG-utilizing microorganisms. A Gram-positive actinomycete, originally isolated as tetrahydrofuran-degrader, was found to grow on either PEG 4000 or 8000 (Kohlweyer et al., 2000). Whether the actinomycete has the same metabolic pathway and the same uptake system of the large PEG as found in Sphingomonads remains to be clarified. As suggested for anaerobic bacteria (see below), PEG appears to be directly transported into the cytoplasmic space, where it might regulate the metabolic genes. In contrast, the metabolic genes of aerobic bacteria–which seem to possess a barrier against large molecules–must be controlled not by the large PEG molecule itself, but by another signal transduced from PEG. The real regulation elements for PEG operons either in aerobes or in anaerobes remain to be identified.
4.2 Anaerobic Biodegradation and Metabolism of PEG
4.2.1 Anaerobic Bacteria Capable of Assimilating PEG
As for the anaerobic biodegradation of PEGs, an early study by Mills and Stack (1954) succeeded in a reduction in chemical oxygen demand (COD) using two-stage continuous anaerobic sludge reactors fed with industrial waste supplemented with either DEG, triEG or PEG 400 over 40 days. Some 30 years later, three groups almost simultaneously reported the anaerobic growth of isolates or consortia on PEGs. According to the report of Schink and Stieb (1983), PEG with a molecular weight of 20,000 was anaerobically degraded in an enrichment culture inoculated with mud of limnic and marine origins. Three strains of rod-shaped, Gram-negative, nonspore-forming strictly anaerobic bacteria were isolated, which were proposed as a new species, Pelobacter venetianus sp. nov. Dwyer and Tiedje (1983) obtained methanogenic consortia from sewage sludge, which can degrade EG, DEG and PEG with Mns of 400, 1000, and 20,000. The enrichments were shown to best metabolize glycols close to the Mn of the substrate on which they were enriched, as was found with aerobic bacteria. Fincher and Payne (1962) first isolated a PEG-utilizing bacterium, TEG-5, which was identified as Alcaligenes faecalis var. denitrificans. This organism grew aerobically, but not fermentatively, at the expense of several free ether glycols, as well as ethoxylates attached to alkyl and benzyl derivatives. However, it degraded both free ether glycols (EG and the other short-chained ether glycols and PEG 200 and 300) and several nonionic detergents while growing anaerobically as a denitrifier (nitrate-reducing), with 55% degradation of DEG being obtained (Grant and Payne, 1983).
4.2.2 PEG Acetaldehyde Lyase
Anaerobic bacteria must contain a different metabolic system for polymers from that in aerobic bacteria (Schink et al., 1992). Extracts from PEG-degrading, anaerobic bacteria revealed a diol dehydratase and a PEG-degrading enzyme yielding acetaldehyde as product (Schramm and Schink, 1991; Frings et al., 1992). Unfortunately, both enzymes appeared to be very sensitive to oxygen, and this most likely prohibited the authors from purifying either enzyme. As the PEG-degrading activity was stimulated by the addition of certain corrinoids, it was postulated that ether bond splitting in these bacteria occurred with a PEG acetaldehyde lyase that was analogous to a diol dehydratase (Toraya et al., 1979; Toraya and Fukui, 1982; Masuda et al., 1999). In order to explain the formation of acetaldehyde and PEGn-1 as products, these authors assumed that the terminal OH group of PEG shifts to the C2-position (an intramolecular rearrangement which is in fact a mutase reaction) so that an unstable hemiacetal group is formed which rearranges to the products (Figure 4). It was suggested that at least one unmasked terminal hydroxyl group was necessary for the formation of the hemiacetal intermediate by transhydroxylation. Pearce and Heydeman (1980) first suggested this concept for the aerobic degradation of PEG. Although this seems a plausible reaction, and the concept was further supported by growth experiments with Pelobacter venetianus (Schink and Stieb, 1983; Strass and Schink, 1986), confirmation must wait until purification and characterization of the enzyme. In addition, the enzymes are cytoplasm-located; hence, it must be explained how the PEG is transported to this compartment. Two porins were identified in P. venetianus described above, but were not suited for the transport of high-molecular mass PEGs across the outer cell membrane (Schmid et al., 1991). Frings and Schink (1994) found that Acetobacterium sp. (strain LuPhet1) fermented phenoxyethanol stoichiometrically to phenol and acetate, but phenoxyacetic acid was not degraded. Cell-free extracts cleaved the ether linkage to yield acetaldehyde as a reaction product, which clearly suggested that the compound is degraded by the same strategy as in the anaerobic degradation of PEG. Very recently, Schink suggested that his enzyme for splitting an ether bond might differ from that analogous to diol hydrase which was assumed earlier (personal communication), though detailed studies are required before any firm conclusions can be drawn. Dwyer and Tiedje (1986) also suggested that acetaldehyde was a direct metabolite of PEG as they had detected the presence of a DCIP-dependent PEG-DH. Schink et al. considered the level of PEG-DH activity to be too low for a primary metabolic enzyme, but that the activity might be effective on impurities such as EG and acetaldehyde. These results coincide with those obtained with aerobic bacteria – that degradation commences from the terminus of a long molecular chain and that therefore at least one free alcohol group is necessary – though both aerobic and anaerobic mechanisms appear to be different, and both ether-cleaving mechanisms require further investigation.
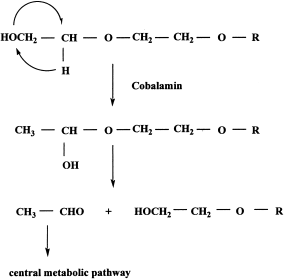
Proposed mechanism for anaerobic degradation of PEG by PEG lyase.
The incubation of stratal waters from a petroleum reservoir containing OP-10 nonionic surfactant showed that, under aerobic conditions at 32°C and pH 6.6, only 31% of OP-10 was biodecomposed in 7 days, whilst under identical anaerobic conditions, 79–85% was decomposed (Gvozdyat et al., 1983).
4.3 Extracellular One-electron Oxidation of PEG
The brown-rot basidiomycete Gloeophyllum trabeum is known to cause decay in wooden structures by degrading lignocellulose. Kerem et al. (1998) found that the extracellular system produced by this fungus depolymerized [14C]PEG 4000. The extracellular system functioned as free radical oxidants to cleave PEG rapidly by the endo route, i.e., by C–C bond cleavage: a radical oxidant abstract hydrogens from the PEG's internal methylene groups, just as the Fenton reagent (Fe2+/H2O2) depolymerizes PEG in vitro. The application of Fenton reagent to the depolymerization of PEG has been suggested previously (Kawai, 1993; Cho and Okamoto, 1994). The degradation of PEG by the fungus required 2,5-dimethoxyhydroquinone (DMHQ) and Fe3+, and was inhibited by catalase (Kerem et al., 1999): DMHQ was formed from 1,5-dimethoxy benzoquinone by the mycelial reductase. Direct nonenzymatic reaction between DMHQ and Fe3+ produced Fe2+ and H2O2. Thus, the degradation mechanism of PEG by the fungus was proved to be a biological Fenton mechanism. It was suggested that brown-rot fungi contribute not only to the degradation of lignocellulose, but also to that of recalcitrant organopollutants; that is, other polyethers as well as PEG might be depolymerized by the same system.
Hydrogen peroxide can be produced by various oxidation systems. Iron is one of most abundant metals in the Earth's crust, and is reduced to Fe2+ in the presence of natural reductants or under anaerobic conditions. Thus, although a biological Fenton reaction might be possible in terrestrial environments, the metabolism of polyethers is still thought to play the major role in polymer degradation, especially in aquatic environments, where Fe2+ and H2O2 concentrations available might be insufficient for the reaction to proceed.
5 Biodegradation of PPG
As the use of PPG is expected to increase in the future, studies of its biodegradation are equally important as those for PEG. The susceptibility of PPG to biological degradation has not been well characterized, although several groups have reported microbial assimilation of the monomer, 1,2-propylene glycol, which is supplied by the petrochemical industry at low cost. Fincher and Payne (1962) noted that a PEG-utilizing isolate could assimilate 1,2-propylene glycol and dimer as a sole carbon and energy source. Meanwhile, our PEG-utilizing isolates (Kawai, 1987), or those isolated by Watson and Jones (1977), did not grow on dimer or PPG. Neither did the anaerobic PEG-utilizing bacteria isolated by Schink and Stieb (1983) and Dwyer and Tiedje (1986) degrade PPG.
PPG-utilizing bacteria were isolated by an enrichment culture containing PPG 2000 or 4000 from soils or activated sludges acclimatized to PPG 2000 or 4000 for a few months under aerobic conditions (Kawai et al., 1977). As the culture medium became turbid after vigorous shaking, the cells were collected by centrifugation and resuspended in distilled water; the turbidity of the cell suspension was then measured using its optical density at 610 nm. In a preliminary study, Tween 20 was used to emulsify a PPG medium homogeneously, but it was found later that sonication would emulsify PPG uniformly (unpublished data). Strain No. 7 was the most favorable; this was identified as Corynebacterium sp., but later re-identified as Stenotrophomonas maltophilia based on 16S rRNA homology (Tachibana et al., 2002). The strain grew on various PPGs (diol and triol types, Mn 670∼4000), monomer and dimer, but did not assimilate PEGs (Table 3). The strain also grew on a few PEG-PPG copolymers, which contained a larger amount of PPG than PEG, where from the weight ratio of PPG and PEG (approximately 10:1), perhaps either two or one of the terminal hydroxyl groups of PPG was not blocked by PEG and could be available to the organism. In contrast to this, the block copolymers Epan 485 and 785–both of which contained a greater amount of PEG than PPG – were utilized by a PEG 20,000-utilizing consortium E-1, which cannot utilize PPG (Kawai, 1992). Epan 450 and 750 seemed to be toxic for either PPG- or PEG-utilizing bacteria.
Substrate |
Growth [OD610] |
|||
|---|---|---|---|---|
PPG diol type |
670 |
2.23 |
||
1000 |
2.04 |
|||
2000 |
2.45 |
|||
Triol type |
1000 |
2.04 |
||
3000 |
2.48 |
|||
4000 |
1.82 |
|||
1,2-Propylene glycol |
2.18 |
|||
Dipropylene glycol |
2.40 |
|||
PEG |
400 |
0.27 |
||
1000 |
0.30 |
|||
2000 |
0.28 |
|||
6000 |
0.30 |
|||
Epana |
410 |
1.32 |
||
450 |
0.25 |
|||
485 |
0.28 |
|||
710 |
1.06 |
|||
750 |
0.29 |
|||
785 |
0.27 |
|||
Ethylene glycol |
0.30 |
|||
Methanol |
0.59 |
|||
Ethanol |
0.99 |
|||
Propanol |
1.94 |
|||
iso-Propanol |
0.90 |
|||
Glucose |
2.51 |
|||
Glycerol |
1.75 |
|||
None |
0.30 |
Epan: HO(CH2CH2O)a[CH(CH3)CH2O]b(CH2CH2O)cH |
||||
|---|---|---|---|---|
Mn |
PPG content |
PEG content |
||
Epan |
410 |
1330 |
1200 |
130 |
450 |
2400 |
1200 |
1200 |
|
485 |
8000 |
1200 |
6800 |
|
710 |
2220 |
2000 |
220 |
|
750 |
4000 |
2000 |
2000 |
|
785 |
13,000 |
2000 |
11,330 |
|
The aerobic metabolism of PPG by the strain was studied using dimer – dipropylene glycol (DPG) – as a model substrate for biodegradation, since PPG contains molecules of different molecular weights (Kawai et al., 1985). As commercially obtained PPG is randomly polymerized from optically active 1,2-propylene oxide, the resultant polymer must include atactic structures, and DPG must include (in theory) several structural and optical isomers, as shown in Figure 5. These isomers were separated by gas chromatography on a PEG 20M column (0.25 mm×25 m). R,R- and S,S-isomers were eluted together as a single peak, and R,S- and S,R-isomers as another single peak (Figure 6). The area ratios of peaks II to III and IV to V were almost equal by either total ion monitoring or selective ion monitoring on GC/MS analysis. From their mass spectra, relative quantities of five peaks and retention times, peak I was assigned to be structural isomer A, II and III to be B, and IV and V to be C (Figure 7); in peak I, optical isomers could not be separated; peaks II and III are diastereomers (the R,R-S,S and R,S-S,R complexes); peaks IV and V are also diastereomers (the R,R-S,S complex and the meso form). The ratio of structural isomers A, B, and C was 36.3, 48.5, and 15.2%, respectively.
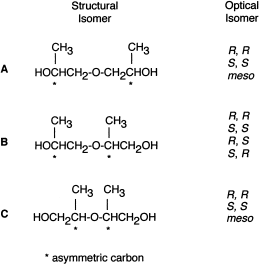
Presumed structural and optical isomers in chemically synthesized DPG. (Modified from Kawai et al., 1985.)
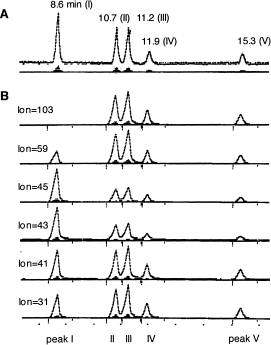
Isolation and identification of isomers in DPG by GC/MS. (A) Total ion monitoring; (B) selective ion monitoring. (Modified from Kawai et al., 1985.)
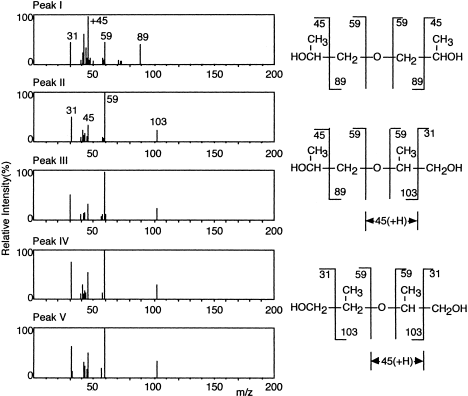
Mass spectra of isomers contained in DPG. (Modified from Kawai et al., 1985.)
When the intact cells of the strain No. 7 were incubated with DPG, degradation of DPG depended on the shaking conditions, as in a nonshaken culture DPG was barely degraded. Taken together with the results of a culture filtrate or cell-free extract, this suggested that DPG was not metabolized by a hydrolytic reaction, but by an oxidative reaction. With vigorous shaking on a reciprocal shaker, over 90% of DPG was consumed within 23 h, but traces of metabolites were accumulated in the reaction mixtures. With moderate shaking, the degradation rate was slower, but considerable amounts of metabolic products were accumulated (M1 and M2). Hence, the reaction was carried out at 30°C for 20–50 h with moderate shaking (60–70 rpm). Metabolites (M1 and M2) were characterized by GC/MS analysis using a capillary column (0.25 mm×50 m) (Figure 8). M1 corresponded to 1,2-propylene glycol. M2 was further separated into two peaks, M-2 and M-2′. From the mass spectra of the two peaks, M-2 and M-2′ seemed to correspond to OC(CH3)CH2OCH2CH(CH3)OH and OC(CH3)CH2OCH(CH3)CH2OH, respectively. The fourth small peak, M3 was found on capillary GC, and considered to be OC(CH3)CH2OCH2CO(CH3), based on its elution position and mass spectrum. The residual isomers in the reaction supernatant (30-h incubation) were analyzed. Isomer A (peak I) was degraded by 51.4%, while the diastereomers of isomer B (peaks II and III) were equally degraded by 40.7%, and those of isomer C (peaks IV and V) were also equally degraded by 18.5%. This result supports the assumption that compounds corresponding to peaks II and III, and IV and V have the same structure, respectively. These results indicated that secondary alcohol groups were preferentially oxidized, with the bacterium utilizing all structural and optical isomers included in the dimer, as well as isotactic and atactic structures. Generally speaking, the stereospecificity of microorganisms and their enzymes is strictly controlled, but in this case the bacterium either has several stereospecific enzymes for optical isomers, or a nonstereospecific enzyme.
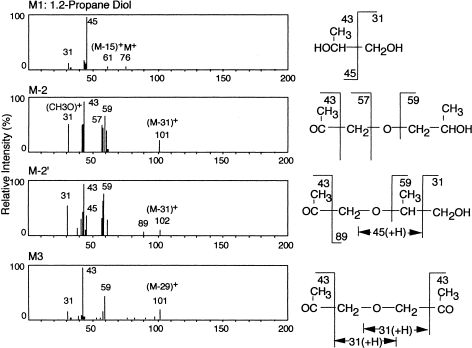
Mass spectra of metabolic products from DPG. (Modified from Kawai et al., 1985.)
PPG was not degraded by either a culture filtrate or a cell-free extract, but could be degraded by intact cells and/or cell debris. Hence, PPG was not metabolized by extracellular enzymes or a hydrolase, but possibly by intracellular enzymes including membrane-bound enzymes. The intracellular metabolism of PPG was supported by the finding that bacterial cells entrapped in polyacrylamide gels degraded PPG efficiently (Kawai, 1987). The PPG-degrading activity of the cell-free extracts prepared from DPG-grown cells was investigated, but because of clouding due to the PPG attached to the cells the activity of the cell-free extract prepared from PPG-grown cells could not be measured. DCIP- and phenazine methosulfate (PMS)-dependent dehydrogenase (PPG-DH) activities were detected with cell-free extracts, and these must be linked with a respiratory chain of the organism. The effects of side or main chain structures on the growth of PPG-utilizing strain were examined (Table 4). As the bacterium grew well on PBG 400 and 2000, a methyl group in PPG was replaceable by an ethyl group in PBG. The microorganism grew on polyglycerines to some extent, but not on polyglycidols, PEG, PTMG or polyvinyl alcohol. These results indicated that the bacterium recognized an ether oxygen adjacent to two or three carbon chains and a hydrophobic side group such as a methyl or ethyl group.
Substrate [0.5%] |
Growth [OD610] |
pH |
|---|---|---|
None |
0.12 |
7.0 |
PEG 400 |
NG |
7.0 |
PPG 2000 |
1.34 |
5.8 |
PBO 400 |
1.27 |
5.0 |
2000 |
0.92 |
7.0 |
CoEO/glycidol 500 (R,S) |
0.22 |
7.0 |
Diglycerin |
0.66 |
4.6 |
Polyglycerin 310 |
0.49 |
4.8 |
500 |
0.32 |
5.6 |
750 |
0.33 |
5.8 |
Polyglycidol 13 300 ( R ) |
NG |
7.0 |
13 300 ( S ) |
NG |
7.0 |
PTMG 200 |
NG |
7.0 |
PVA n=500 |
NG |
7.0 |
As PEG and PPG-monoalkyl derivatives may be assimilated in a similar manner to free PEG and PPG, yet dialkyl PEG/monoalkyl PPG acetate cannot be utilized by PEG/PPG-utilizers, at least one free alcohol group is necessary for metabolism (Kawai, 1993). These results – intracellular PPG-DH, a need for the terminal free alcohol group for growth, and oxidized metabolic products – suggested that PPG is incorporated into cells at least through the outer membranes, and is oxidatively metabolized. The principal mechanism is possibly similar to that for PEG – that the terminal oxidation precedes the ether cleavage.
Several PPG dehydrogenase (PPG-DH) activities were found in PPG-utilizing S. maltophilia (Tachibana et al., 2002). During growth on PPG 2000, three PPG-DH peaks appeared in 36 h, 7 days, and 9 days: the majority (88%) of the first peak at the early logarithmic phase was localized in the cytoplasm. In the second peak (the highest) at the stationary phase of growth, activity was found in the membrane (54%), the periplasm (34%), and the cytoplasm (12%). The third peak may not contribute significantly to the assimilation of PPG, because PPG was already consumed in 9 days. As well as differing in their localization and induction times, these PPG-DHs also showed differences in their specificity towards electron acceptors. Further characterization of these enzymes is eagerly awaited.
6 Biodegradation of PTMG
PTMG is waxy and water-insoluble at the ambient temperatures which are used exclusively for the synthesis of polyurethanes. As water-soluble oligomers are unsuitable for synthesizing polyurethanes, they are removed with water from a mixture of polymers and so will pass into the wastewater of a synthetic chemical plant. PTMG-utilizing microorganisms might then be used for the biological treatment of this wastewater. Although the oxidation of 1,4-butanediol by strains of Gluconobacter and Acetobacter, or bacterial utilization of 1,4-butanediol as a sole carbon source have been reported, no report other than that of Kawai and Moriya (1991) has been found on the degradation and metabolism of PTMG. PTMG-utilizing bacteria were obtained by enrichment culture techniques, namely Alcaligenes denitrificans subsp. denitrificans and Xanthomonas maltophilia. PTMG 265 or 200, which includes monomer to octamer, completely disappeared in 7 days from the culture filtrate of both strains.
Prior to growth and biodegradation studies with these two strains, the number and quantity of components contained in PTMG samples were analyzed in Toyo Soda TSK by high-performance liquid chromatography (HPLC) and liquid chromatography/mass spectrometry (LC/MS), and eight peaks were detected with either PTMG 200 or 265. Oligomers up to a degree of polymerization (DP) ∼8 (molecular weight 594 Da) appeared to be water-soluble. The intact cells (Figure 9) were also seen to metabolize PTMG 265, with oligomers of a higher DP disappearing more rapidly than those of lower DP. Metabolites such as carboxylated PTMG could not be differentiated, as organic acids are eluted at almost the same position as inorganic phosphate. Furthermore, sonic extracts of A. denitrificans dehydrogenated PTMG coupling with artificial electron acceptors, suggesting that PTMG is degraded via the oxidation process linked to an electron transport system of the bacterium. With regard to the bacterial oxidation of PEG and PPG, a precedence of oxidation of terminal alcoholic groups over the cleavage of an ether bond was suggested, as described earlier. When the terminal structure of PTMG [R-O-(CH2)4OH] is perhaps oxidized to R-O-(CH2)3COOH and then cleaved by β-oxidation, R-O-CH2COOH, a structure similar to carboxylated PEG is produced, and this might explain why the organism can grow on PEG 400. As neither strain was able to grow on PPG or PBG, it is possible that neither organism is able to metabolize secondary alcohols.
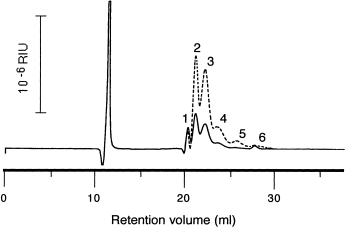
Degradation of PTMG 265 by intact cells of A. denitrificans ssp. denitrificans. PTMG 265 was analyzed by HPLC before incubation (broken line) and after incubation (solid line). The numbers 1 to 6 correspond to monomer to hexamer. (Modified from Kawai and Moriya, 1991.)
7 Biodegradation of Miscellaneous Polyethers
To date, a wide range of polyethers have been synthesized (see Figure 1). PBG is a polyether composed of the C4 monomer (as is PTMG), but its structural backbone is similar that of to PPG, where the C2 monomer with methyl or ethyl group is polymerized. As PPG-utilizing S. maltophilia is able to grow on PBG 400 and 4000 (see Table 4), the organism (actually metabolic enzymes) must be unable to discriminate between methyl and ethyl groups. No report on PBG-utilizing microorganisms has been made, but the biodegradability of PBG is also clear from the results of biodegradability testing with activated sludge (Kawai, 1993). A PPG-utilizing strain grew minimally on diglycerine, but not on polyglycidol (isotactic, S or R: Mn 13,300). Matsumura et al. (1987) synthesized polycarboxylate oligomers containing ether linkages and tested their biodegradability, among which poly(sodium glycidate) and poly(disodium epoxysuccinate) were biodegraded by 25% and 40%, respectively in five-day BOD tests. PEG-utilizing Sphingomonads were able to grow on PEG carboxylate as well as PEG (Kawai, 1993); this was not surprising given that PEG is first oxidized to carboxylated compounds and then depolymerized by one glycol unit. In general, polyethers are biodegradable, though the degradation is dependent on the Mn (lower-molecular weights are degraded more rapidly than higher), the compound's water solubility (depending on Mn), and its physical state (liquids are more easily degraded than waxes and solids) (Kawai, 1995).
8 Physiology
As described earlier, the metabolism of PEG has been elucidated, both aerobically and anaerobically. The most well-known pathway of aerobic metabolism of PEG is oxidation of the terminal alcohol groups to carboxylic aids, followed by cleavage of an ether bond to produce PEG which is depolymerized by one glycol unit. This single glycol unit was suggested to be GOA (Kawai, 1985, 1987, Kawai and Yamanaka, 1986), which may then enter central metabolic routes by known pathways, e.g., the oxidative dicarboxylic acid cycle, TCA cycle, and the glycerate pathway (see Figure 3). Glycolic acid is another product (proposed by Schöberl, 1985) which can be readily converted to GOA by glycolate oxidase. It was suggested by Thélu et al. (1980) that an unstable hemiacetal was formed, and this was then cleaved nonenzymatically into glyoxylate, though no further investigations into this have since been carried out.
Pearce and Heydeman (1980) proposed a nonoxidative removal of EG units as acetaldehyde, which can be introduced into the TCA cycle after being converted to acetic acid and then to acetyl-CoA. Acetaldehyde was later suggested to be a product of the anaerobic metabolism of PEG (Schink et al., 1991, 1992), and formed by a shift of the terminal OH group to the C2-position. In this way an unstable hemiacetal group would be formed and then rearrange to the product shown in Figure 4. These authors were unable to purify or characterize the enzyme involved in this reaction, but the assumption was supported by growth experiments and anaerobic degradation of phenoxyethanol (Schink, 1994). Dwyer and Tiedge (1986) also suggested that acetaldehyde was a direct metabolite of PEG by Bacteroides and Desulfovibrio strains. A different method of degradation via an unknown depolymerization mechanism and conversion of oligomers into acetaldehyde was suggested, but never tested experimentally. Acetaldehyde is linked to methane generation under anaerobic conditions.
Far less information is available on the metabolic pathway of PPG than on that of PEG, with only one group having successfully isolated PPG-utilizing bacteria (Kawai et al., 1987). Fincher and Payne (1962) noted that a PEG-utilizing isolate could assimilate 1,2-propylene glycol and its dimer, mainly because these compounds contain primary alcohol groups (as does PEG), and some primary alcohol-oxidizing enzymes act also on secondary alcohols. Tachibana et al. (2002) reported the existence of several PPG dehydrogenases in PPG-utilizing Stenotrophomonas maltophilia, which differ in their cellular localization, induction time, electron acceptor specificities and electrophoretic behaviors. A crude extract of this organism was shown to dehydrogenate PEG and PVA as well as PPG, suggesting the existence of primary and secondary alcohol dehydrogenases or the broad substrate specificity of PPG-DH. As the strain could grow on PPG-monoalkyl derivatives, but not on monoalkyl PPG acetate, at least one free alcohol group is necessary for metabolism (Kawai, 1993). As described in Section 5, the principal mechanism is possibly similar to that of PEG; oxidation of the terminal OH group must precede the cleavage of an ether bond. If this suggestion is correct, the metabolic products of C3 might be pyruvaldehyde or pyruvate (Figure 10), both of which may then enter the TCA cycle via acetyl CoA.
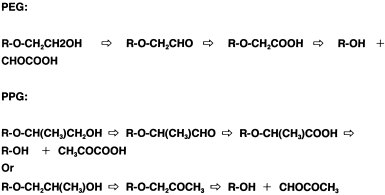
Proposed mechanism for an ether-bond cleavage in the metabolism of PEG and PPG.
Cell-free extracts of a PTMG-utilizing Alcaligenes denitrificans were shown to possess PTMG dehydrogenase activity (Kawai and Moriya, 1991) which must linked to an electron transport system of the bacterium. When PTMG was oxidized to a carboxylic acid and subjected to β-oxidation to yield acetic acid, the terminal structure of a metabolite, R-O-CH2COOH, was analogous to that of oxidized PEG. As the organism grew on PEG 400, it might have possessed the same metabolic enzymes as for PEG metabolism, and glyoxylate might be a metabolite linked with the central metabolic pathway. In the case where β-oxidation was not involved, C4 compounds might be cleaved.
In conclusion, polyethers are aerobically depolymerized in common with mechanisms analogous to those used for PEG. Initially, oxidation of the terminal OH groups produces either carboxylated or carbonylated compounds, after which the cleavage of an ether bond yields C2–4 compounds that are integrated into the carbon cycle via central metabolic pathways. The anaerobic metabolic process proposed for PEG seems reasonable, though as yet no report has been made on the anaerobic digestion of other polyethers.
PEG is oxidized by several alcohol dehydrogenases that originate from several organisms, whether grown on PEG or not. These findings strongly suggest that PEG can be recognized as a primary alcohol rather than as a specific xenobiotic. Based on its kinetic parameters for several substrates, PEG-DH cloned and expressed in E. coli was suggested to be a benzyl alcohol/long chain alcohol dehydrogenase (Table 5) which belongs to the GMC flavoprotein family, with one molecule of FAD bound to the monomer protein of the homodimeric protein enzyme (Sugimoto et al., 2001). Thus, PEG can be recognized by ubiquitous alcohol dehydrogenases, and not by specifically designed PEG-DH, in which case their active sites fit the size of the polymers. An ether bond-cleaving enzyme (DGA-DH) from S. terrae showed a high similarity of the N-terminal region with that of α-hydroxy acid dehydrogenase (unpublished data) as well as its substrate specificity on PEG-carboxylic acid, GOA and glycolic acid. These results might solve the problem of whether PEG-utilizing ability already existed, or whether it was acquired as a result of mutations, though assimilation of the polymers requires other factors that alcohol and aldehyde dehydrogenases and ether bond-splitting enzyme are already equipped in cells and the polymers are incorporated into cells via membrane barriers. As polyethers are genuine xenobiotics and have no close relatives in nature, it remains unclear whether the same results will be found for other polyethers.
Substrate |
Vmax [U mg−1 protein] |
Km [mM] |
|
|---|---|---|---|
Benzyl alcohol |
17.8 |
0.6 |
|
1-Heptanol |
23.5 |
0.4 |
|
1-Hexanol |
17.0 |
0.5 |
|
1-Propanol |
3.3 |
6.6 |
|
TEG |
14.7 |
14.0 |
|
TEG-monoethyl ether |
6.6 |
4.2 |
|
PEG 300 |
10.8 |
0.8 |
|
PEG 400 |
10.2 |
0.7 |
|
PEG 1000 |
12.1 |
1.6 |
|
PEG 6000 |
10.5 |
2.4 |
- a Reproduced from Sugimoto et al. (2001).
Based on current knowledge of the physiology of polyethers, the metabolism of xenobiotics is seen to depend totally on the conversion rates of xenobiotics to common metabolites that can directly enter central metabolism. In addition, the number of steps/enzymes required to transform xenobiotics into common metabolites, and whether a utilizer is equipped with all of these, determine the assimilation either by a pure culture or by a consortium, each organism of which has different metabolic steps. As was found in the assimilation of PEG (Kawai and Yamanaka 1986), factors other than metabolic steps may be causes for symbioses.
9 Production
Poly(alkylene glycol)s are generally synthesized by ring-opening polymerization from alkylene oxides. The most representative 1,2-epoxide polymer synthesized from ethylene oxide is classified into two groups: PEGs and poly(ethylene oxide) (PEO). The former has relatively low molecular weights ranging from 200 to 20,000 Da, these being synthesized on base-catalytic polymerization from ethylene oxide, using water or EG as a starter. They are used in ceramics, cosmetics, lubricants, pharmaceuticals, polyurethane, inks, and rubbers. Depending on their molecular weights, PEGs change physical state, from liquid to wax and powder. The latter forms have high molecular weights, ranging from 1×105 to 5×106; they also have high viscosity, and are used as detergents, adhesives, lubricants, and formulation coatings for sustained release of coagulants.
The synthesis of PEO is based on a different mechanism from that used for PEGs. Irrespective of the high molecular weight, PEOs are equally water-soluble as PEGs. The latter are available commercially under various brand names such as Carbowax (Union Carbide), Polyglycol E (Dow), Jeffox (Texaco Chemical), PolyG (Olin), Pluracol E (BASF Wyandotte), poly(ethylene glycol)s (Hodag), and ATEG (ICI Americas). PEOs are available under brand names such as Polyox (Union Carbide), Alkox (Meisli Chemical Work), and Peo (Seitetsu Chemical).
PPG is synthesized using a mechanism similar to that used for PEG, and with a molecular weight of <5000 Da. The material is generally water-insoluble, and highly viscous.
Copolymers of ethylene oxide and polypropylene oxide are used as polyols for synthesizing polyurethanes, as block copolymers for surfactants, the most famous being Pluronic (BASF Wyandotte), and as random copolymers for hydraulic fluids and other functional fluids. More than 450×103 tonnes of propylene oxide and 45×103 tonnes of ethylene oxide are utilized annually as polyols in USA in the synthesis of polyurethanes (Kroschwitz, 1990).
The annual worldwide production of PEG is in the range of one million metric tons (Houston, 1981). At the time of writing, the production level has been increased due to an expansion in the need for nonionic surfactants and other products including PEGs, though no exact data are available on the total amounts of PEG and PPG produced and used. This is partly due to the fact that nonionic surfactants and other derivatives are synthesized from ethylene oxide or propylene oxide, and not from PEG or PPG. The production level of PPG is greater than for PEG, but most PPG is transformed into polyurethanes. The amount of PEG entering water streams is considered to be far greater than that of PPG, based on these applications. Polyethers other than PEG and PPG are not listed in the yearbooks of the chemical industry statistics.
10 Outlook and Perspectives
Aerobic as well as anaerobic bacteria – either in pure culture or as a consortium – have been found which are able to degrade PEGs. Aerobic bacteria have also been reported which can grow on PPG/PBG and PTMG. Most of these aerobes are Gram-negative, but a Gram-positive PEG-utilizing actinomycete which is able to depolymerize PEG or oxidize PEG was recently added as a new member of this group. No eukaryote has been reported to use PEG as sole carbon source, and this is perhaps related to the enormous metabolic diversity of bacteria, thereby providing the opportunity to adapt an already existing machinery to the conversion of xenobiotics with only a few mutations. In the case of PEG degradation, either “new” enzymes or enzymes related to alcohol/α-hydroxy acid dehydrogenases and diol dehydratases may be involved, the latter case being the most likely.
The partial metabolism of PEG by bacteria has also been reported, either by one of the member organisms of a consortium, or adventitiously (as co-metabolism) by an organism with an enzyme that is suited to attack PEG. Whether aerobically or anaerobically, PEG is degraded intracellularly by enzymes which are either periplasmic (PEG-DH, aldehyde dehydrogenase and DGA-DH in Sphingomonads) or cytoplasmic (PEG acetaldehyde lyase in anaerobes). As yet, it appears that the restrictions of any one bacterium with regard to the size of PEG that can be used is due not to the limitations of the degrading enzyme active site, but to the transportation properties of the cell's outer membrane (Kawai and Yamanaka, 1989), the nature of which has yet to be established.
Unique enzymes and mechanisms may be expected in the degradation of PPG, and PPG-DH of very broad specificity towards primary and secondary alcohols, stereoselectivity towards R- and S-isomers, or cooperativity towards several different alcohol dehydrogenases await further purification. Moreover, even if enzymes relevant to the metabolism are, in time, well characterized, the identification of a barrier for substrate uptake, as in the case of PEG, must also await clarification.
The existence of a biological Fenton reaction has led to the suggestion of a new degradation pathway for polyethers. As many polyethers find their way into the aquatic environment, where accumulation of neither H2O2 nor Fe2+is expected, this may be of minimal significance in the fate of polyethers. However, a biological system might well be applicable to the pretreatment of either wastewater or sewage in cases where a conventional activated sludge system is, for any reason, inappropriate.
The characterization of metabolic enzymes might result in the finding of either new or unique enzymes that are available for environmental biotechnology or industrial purposes, as has already been suggested for PEG-DH (Geerlof et al., 1994).
PEGs are commonly used solvents for the administration of compounds either to intact animals or to perfused organs, and they appear in general to be metabolically inert and nontoxic (Gilman et al., 1980). PEGs are sulfated in vitro by rat and guinea-pig liver (Roy et al., 1987), and repeated topical application of a PEG-based antimicrobial cream to open wounds in rabbits and burn patients was shown to cause a syndrome that was related to the metabolism of PEGs to compounds including mono- and diacids (Herold et al., 1982). PEG 400 may be toxic towards mitochondrial function, and inhibit oxidative phosphorylation in rat lymphocytes and hepatocytes (Gordienko and Kudokotseva, 1980). The biodegradation of PEG yields oligomeric and monomeric EG, the toxicity of which is far greater than PEGs: hence, biodegradability might produce an additional risk due to metabolite production. In this respect, information on the biological fate of polyethers and their metabolites, both in the environment and in vivo, is essential.
Although the presence of acetaldehyde lyase is strongly supported by the finding that acetaldehyde is formed quantitatively from PEG, the instability of the enzyme and lack of its purification have led to uncertainty regarding the anaerobic metabolism of PEG and the relevant enzyme(s). The sequencing of PEG-DH (Sugimoto et al., 2001) and co-oxidation of PEG by both a well-characterized QH-ADHs type I and a mammalian enzyme verified that alcohol oxidation is the primary process of aerobic PEG metabolism. Aldehyde produced by ADHs is oxidized by alcohol/aldehyde dehydrogenases, after which the ether cleavage acts on the carboxylated PEG, yielding PEG depolymerized by one glycol unit. The ether bond-cleaving enzyme seemed to be α-hydroxyl acid dehydrogenase, based on its substrate specificities toward caroboxylated PEGs and α-hydroxy acids, and most likely was glycolate dehydrogenase. As yet, there are no data available on the mechanism of the ether cleavage however. If it is assumed that the enzyme catalyzes the ether cleavage, it may be indispensable for the biodegradation of PEG by Sphingomonads. A newly isolated Gram-positive Pseudonocardia sp. might be a useful tool to establish the aerobic metabolic route and enzymes involved, especially of ether cleavage. In this respect, it would be valuable to know whether the aerobic metabolism process conducted among different genera, and in both Gram-positive or negative organisms, is either variable or has a common root.
The biodegradation mechanism of PEG is, in the main part, similar to that for PPG, PBG, and PTMG, and growth substrate specificities of the PPG/PTMG-utilizing bacteria on PBG/PEG, oxidized metabolic products of PPG and dehydrogenase activities of their cell-free extracts with these substrates have supported this assumption. Confirmation of these suggestions requires the continuation of these studies, though the very limited numbers of isolates assimilating these polyethers restrict these investigations to a large extent.
11 Patents
Irrespective of its long history used as a commodity chemical, only a limited number of patents exist on the biodegradation of PEG homologues. Nagashima (1976) claimed microbial degradation of chemical compounds including PEG (Mn unknown) and triEG by Alcaligenes cellosolvus and A. polyphillus. Suzuki and Kusunoki (1979) claimed treatment of wastewater containing polyalkylene glycol (Mn 200–2000) with activated sludge under conditions such that the wastewater contains ≦ 200 g polyalkylene glycol per kg suspended solids per day. Kawai et al. (1999) claimed microbial treatment of polyesters composed of PEG and phthalate/isophathalate/terephthalate by consortia constituted by PEG-utilizing Sphingomonads and phthalic ester-utilizing Comamonas acidovorans. No other patents were found through the Internet searches available. The patents are summarized in Table 6.
No. of patent |
JP 76/41496 |
JP 79/69253 |
JP 99/2971757 |
|---|---|---|---|
Patent holder |
Tekkosha Co., Ltd. |
Toray Industries, Inc. |
Dai-ichi Kogyo Seiyaku Co., Ltd. |
Inventors |
Nagashima Y. |
Suzuki T. and Kusunoki E. |
Kawai F., Rikyu K. and Nakano T. |
Title of patent |
Microbial decomposition of chemical compounds |
Treatment of wastewater containing polyalkylene glycol |
Microbial degradation of aliphatic and aromatic polyesters |
Date of publication |
April 7, 1976 |
June 4, 1979 |
November 8, 1999 |
12 Acknowledgments
The author is grateful to Dr. A. Tani and Mrs. M. Genba for their valuable help in writing this review. The author also expresses her thanks to Dr. T. Morino, Sanyo Chem. Ind., Japan for information on the industrial production of polyethers and their application.
-
- BOD
-
- biological oxygen demand
-
- CoA
-
- coenzyme A
-
- COD
-
- chemical oxygen demand
-
- Da
-
- daltons
-
- DCIP
-
- 2,6-dichlorophenolindophenol
-
- DP
-
- degree of polymerization
-
- EG
-
- ethylene glycol
-
- GA
-
- glycolic acid
-
- GC
-
- gas chromatography
-
- GC/MS
-
- gas chromatography/mass spectrometry
-
- GMC
-
- glucose-methanol-choline
-
- GOA
-
- glyoxylic acid
-
- HPLC
-
- high-performance liquid chromatography
-
- LC/MS
-
- liquid chromatography-mass spectrometry
-
- Mn
-
- number-average molecular weight
-
- Mw
-
- weight-average molecular weight
-
- PBG
-
- polybutylene glycol
-
- PEG
-
- polyethylene glycol
-
- PMS
-
- phenazine methosulfate
-
- PPG
-
- polypropylene glycol
-
- PQQ
-
- pyrroloquinolinequinone
-
- PTMG
-
- polytetramethylene glycol
-
- TCA
-
- tricarboxylic acid



