Abundance of unconventional CD8+ natural killer T cells in the large intestine
Abstract
Natural killer T (NKT) cells are mainly present in the liver and thymus, and the majority of these T cells express either a CD4+ or a double-negative (DN) CD4–8– phenotype. In the present study, we examined whether such NKT cells were present in the intestine. NKT cells were rare in all sites of the small intestine, including an intraepithelial site. However, aconsiderable number of NKT cells were found at an intraepithelial site in the large intestine. This result was confirmed by both immunofluorescence and immunohistochemistry. In contrast to conventional NKT cells, NKT cells in the large intestine were CD8+ or DN CD4–8–. In the case of conventional NKT cells, their existence is known to depend on non-classical MHC class I-like antigens (i. e. CD1d) but not on classical MHC class I antigens. However, the NKT cells in the large intestine were independent of the presence of both CD1d and classical MHC class I antigens. These results were obtained using knockout mice lacking the corresponding genes and molecules. NKT cells in the large intestine were mainly α βTCR+ (> 75 %) but did not use an invariant chain of Vα14Jα281, which is preferentially used by conventional NKT cells. These NKT cells did not bias the TCR-Vβ usage toward Vβ8. These findings suggest that the large intestine is a site in which unconventional NKT cells carrying the CD8+ phenotype (or DN CD4–8–) are abundant and that these cells are independent of MHC andMHC-like antigens.
Abbreviations:
-
- DN:
-
Double-negative
-
- β2m:
-
β2-micro-globulin
-
- IEL:
-
Intraepithelial lymphocytes
1 Introduction
Attention has been recently focused on natural killer T (NKT) cells in the liver, thymus, and other immune organs in mice 1 – 8. In contrast to conventionalT cells, NKT cells are known to recognize glycolipid or peptide antigens in the context of non-classical MHC class I-like antigens (i. e. CD1d) or MHC class I antigens 9, 10. In spite of the interaction with MHC class I-related antigens, their phenotype is either CD4 or double-negative (DN) CD4–8– 11 – 14. NKT cells are generated by an alternative intrathymic pathway, whereas conventional T cells are generated through the mainstream of T cell differentiation in the thymus 15, 16. Concerning the unique properties ascribed to NKT cells, these cells are at an intermediate position between NK cells and conventional T cells in phylogeny.
It is still controversial whether NKT cells seen at sites other than the thymus (e. g. the liver, spleen, and bone marrow) are of thymic origin or of extrathymic origin 6, 17. To anser this question, we need to further characterize the properties of NKT cells that exist at sites other than the thymus. Furthermore, the function of NKT cells in the extrathymic organs is an important subject which requires elucidation. We therefore examined NKT cells in the large intestine. Although extrathymic T cells are known to be present at an intraepithelial site in the small intestine 18 – 20, NKT cells are extremely rare at this site. On the other hand, NKT cells were found to be abundant at an intraepithelial site in the large intestine (up to 10 % of the whole lymphocytes). More interestingly, these NKT cells in the large intestine were found to have many properties distinct from those of conventional NKT cells, in terms of phenotype, MHC restriction, TCR repertoire, and cytokine production.
2 Results
2.1 Identification of NKT cells at an intraepithelial site of the large intestine
Lymphocytes were isolated from an intraepithelial site in both the small intestine and large intestine of various mice. Two-color staining for α βTCR and NK1.1 was then conducted to identify α βTCR+ NKT cells (Fig. 1). Liver lymphocytes were also examined as a positive control. It was confirmed that NKT cells were rare (< 2.0 %) in the small intestine of normal B6 mice. However, a significant proportion of NKT cells (i. e. α βTCR+NK1.1+) (up to 11 %) were present in the large intestine of B6 mice.
Conventional NKT cells seen in the liver and thymus are known to be positively selected in the presence of CD1d and to express an invariant chain of Vα14Jα281 6, 9. Therefore, such NKT cells are reduced in the liver of Jα281 (− / −) mice, β2m (− / −) mice, and CD1d (− / −) mice (see NKT cells of knockout mice in Fig. 1). However, there was no reduction in the proportion of NKT cells in the large intestine of these mice (7 – 8 %). In the case of B6 mice and other knockout mice, approximately 75 % of the NKT cells in the large intestine were α βTCR+ (data not shown).
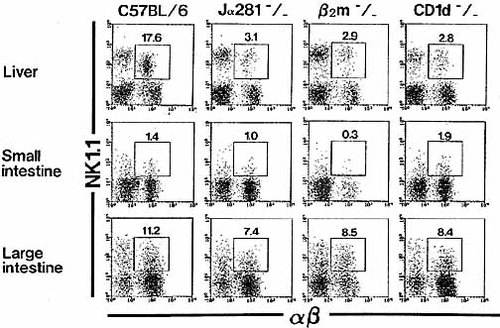
Identification of NKT cells in the liver and intestine of various mice. Lymphocytes were isolated from the liver, small intestine, and large intestine of various strains mice. All mice were used at the age of 8 weeks. To identify α βTCR+ NKT cells, two-color staining for α βTCR and NK1.1 was conducted. Numbers represent the percentages of fluorescence-positive cells. The data shown are representative of three experiments.
|
Organs |
C57BL / 6 |
Jα281 (− / −) |
β2m (− / −) |
CD1d (− / −) |
|---|---|---|---|---|
|
Liver |
16.0 ± 2.3b) |
3.7 ± 0.5 |
2.2 ± 0.8 |
3.5 ± 0.6 |
|
Thymus |
0.5 ± 0.2 |
0.2 ± 0.1 |
0.1 ± 0.1 |
0.1 ± 0.1 |
|
Spleen |
1.9 ± 0.8 |
0.3 ± 0.2 |
0.3 ± 0.2 |
0.3 ± 0.1 |
|
Large intestine |
9.6 ± 1.7 |
7.0 ± 0.4 |
8.5 ± 0.7 |
9.5 ± 1.5 |
- a) All mice were used at the age of 8 weeks (n = 6 in each strain).
- b) Values represent percentages.
All these findings were confirmed by repeated experiments (n = 6 in each group) in various mice (Table 1). In these experiments, the proportion of α βTCR+ NKT cells in the liver, thymus, and spleen was also determined. NKT cells were most abundant in the liver and the proportion of NKT cells in this organ was reduced in Jα281 (− / −), β2m (− / −), and CD1d (− / −) mice. However, this was not the case in the large intestine.
We then determined the absolute number of CD8+ NKT cells and whole lymphocytes at the intraepithelial site of the small and large intestines (Fig. 2). Although the number of whole lymphocytes in the large intestine was smaller than that in the small intestine, the absolute number of CD8+ NKT cells in the large intestine was greater than that in the small intestine.
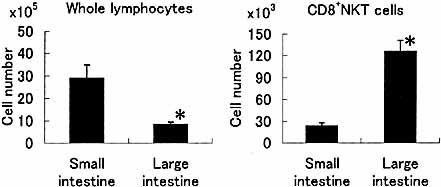
Acomparison of the absolute number of lymphocytes and CD8+ NKT cells between the small intestine and the large intestine. Lymphocytes were isolated from the intraepithelial sites in the small intestine and large intestine of 8-week-old mice (n = 4). The mean + SD are represented.
2.2 Identification of NK1.1+ cells in the intraepithelial site of the large intestine by immunohistochemistry
The localization of NKT cells in the large intestine was directly determined by immunohistochemical staining (Fig. 3). The spleen and large intestine (bowel) were examined in parallel in B6 (NK1.1+ strain) and BALB / c (B / c) (NK1.1– strain) mice. NK1.1+ cells were present at the intraepithelial site in the large intestine (arrowheads in Fig. 3). The majority of these NK1.1+ cells were CD3+CD8+ (see below). Although only few cells were present in the small intestine (arrowheads), they were localized in the lamina propria. We used spleen as a control for positioning. There were many NK1.1+ cells in this organ but almost all (mainly CD3– NK cells in this organ) were localized in the red pulp. Conventional T cells which existed in the white pulp did not express NK1.1 antigens. The results from the NK1.1– mouse strain showed no false-positive stainings.
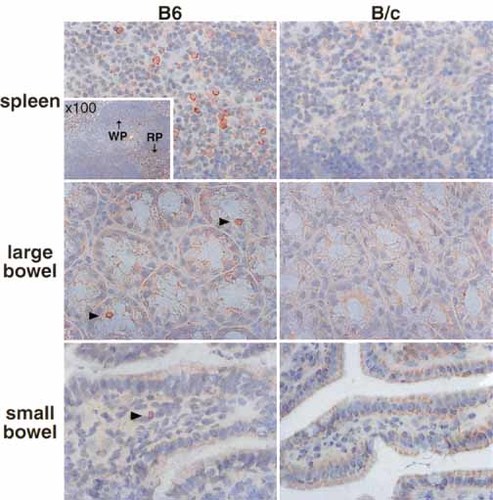
Localization of NK1.1+ cells in the spleen, small intestine, and large intestine (× 200). B6 (NK1.1+ strain) and BALB / c (B / c) (NK1.1– strain) mice were used in this experiment. Immunohistochemical staining was conducted using biotin-conjugated anti-NK1.1 mAb. The small compartment in the spleen figure of B6 mice (× 100) shows localization of NK1.1+ cells in the red pulp (RP), but not in the white pulp (WP).
2.3 NKT cells in the large intestine are CD8+ or DN CD4–8–
The expression of CD4 and CD8 antigens on α βTCR+ NKT cells was then examined in various organs of B6, CD1d (− / −), and β2m (− / −) mice (n = 4 in each strain) (Table 2), using three-color staining for α βTCR, NK1.1, and CD4 (or CD8). By gated analysis, the percentages of CD4+, CD8+, and CD4–8– cells among α βTCR+ NK1.1+ were enumerated. In B6 mice, the majority of NKT cells in the liver, thymus, and spleen were CD4+, whereas the next most abundant was DN CD4–8–. CD8+ NKT cells were confirmed to be almost completely absent in the thymus (0.3 %) and to constitute relatively small populations in the liver (7 %) and spleen (20 %). In sharp contrast, almost all NKT cells in the large intestine were found to be CD8+ (58 %) or DN CD4–8– (38 %) in B6 mice. CD4+ NKT cells were extremely rare in this organ (3 %) in CD1d (− / −) mice, the percentages of CD8+ NKT cells were elevated in the liver (60 %) and spleen (31 %), but was not affected in the large intestine (45 %). The proportion of CD8+ NKT cells was slightly less in all these organs of β2m (− / −) mice in comparison with those in CD1d (− / −) mice.
|
|
C57BL / 6 |
CD1d (− / −) |
β2m (− / −) |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Organs |
CD4 |
DN |
CD8 |
CD4 |
DN |
CD8 |
CD4 |
DN |
CD8 |
|
Liver |
68.1 ± 3.5a) |
24.1 ± 3.1 |
7.8 ± 2.3 |
8.8 ± 6.4 |
23.3 ± 4.1 |
60.0 ± 9.7 |
13.5 ± 6.4 |
57.5 ± 7.2 |
29.0 ± 4.2 |
|
Thymusb) |
51.1 ± 5.3 |
48.2 ± 4.4 |
0.3 ± 0.1 |
30.5 ± 5.2 |
68.1 ± 4.9 |
2.4 ± 1.3 |
17.0 ± 3.7 |
80.5 ± 4.2 |
2.5 ± 1.7 |
|
Spleenc) |
52.3 ± 5.2 |
27.6 ± 10.3 |
20.2 ± 6.1 |
19.3 ± 6.5 |
49.6 ± 18.1 |
31.1 ± 15.2 |
17.0 ± 7.6 |
65.1 ± 4.5 |
18.0 ± 6.6 |
|
Large intestine |
3.4 ± 1.4 |
38.0 ± 4.3 |
58.6 ± 5.3 |
3.6 ± 2.7 |
51.3 ± 1.9 |
45.1 ± 4.6 |
3.7 ± 1.9 |
60.4 ± 4.9 |
35.9 ± 6.6 |
- a) Values represent percentages; n = 4 in each experiment.
- b) HSA– fraction was used.
- c) B cell-depleted fraction was used.
|
|
C57BL / 6 |
CD1d (− / −) |
β2m (− / −) |
|||
|---|---|---|---|---|---|---|
|
Organs |
CD8α α |
CD8α β |
CD8α α |
CD8α β |
CD8α α |
CD8α β |
|
Liver |
71.9 ± 4.6a) |
28.1 ± 4.6 |
79.5 ± 6.0 |
20.5 ± 6.0 |
92.6 ± 1.5 |
7.4 ± 1.5 |
|
Thymusb) |
n.d. |
n.d. |
n.d. |
|||
|
Spleen |
54.8 ± 5.2 |
45.2 ± 5.2 |
41.3 ± 7.0 |
58.7 ± 7.0 |
80.9 ± 13.2 |
19.1 ± 13.2 |
|
Large intestine |
66.0 ± 6.6 |
34.0 ± 6.6 |
73.7 ± 7.9 |
26.6 ± 7.9 |
92.7 ± 2.8 |
7.3 ± 2.8 |
- a) Values represent percentages; n = 6 in each experiment, n.d. = not detectable.
- b) HSA– fraction was used.
- c) B cell-depleted fraction was used.
We also examined the distribution of CD8α α+ and CD8α β+ cells among CD8+ NKT cells in the large intestine, liver and spleen (Table 3). The proportion of CD8α α+ cells was greater than that of CD8α β+ cells in the large intestine and liver of both B6 mice and CD1d (− / −) mice. In the spleen, the proportions of CD8α α+ and CD8α β+ cells were comparable in B6 mice, while CD8α β+ cells were somewhat abundant in CD1d (− / −) mice. In β2m (− / −) mice, most CD8+ NKT were CD8α α+ cells in all organs tested.
2.4 NKT cells in the large intestine do not use an invariant chain of Vα14Jα281
CD4+ (or DN CD4–8–) NKT cells are known to use an invariant chain of Vα14Jα281 for TCRα 6. Therefore, we investigated whether NKT cells in the large intestine used such an invariant chain (Fig. 4). Whole lymphocytes were purified from the liver, thymus, spleen, and large intestine, CD4+ NKT cells and CD8+ NKT cells were purified from the liver, and CD8+ NKT cells were purified from the large intestine. The reverse transcription (RT)-PCR method was applied to detect mRNA of the Vα14Jα281 gene. In contrast to the other organs, no Vα14Jα281 mRNA was detected in lymphocytes in the large intestine. Among the purified fractions, only CD4+ NKT cells in the liver showed signs of Vα14Jα281 mRNA. No Vα14Jα281 mRNA was found in CD8+ NKT cells in the liver or large intestine.
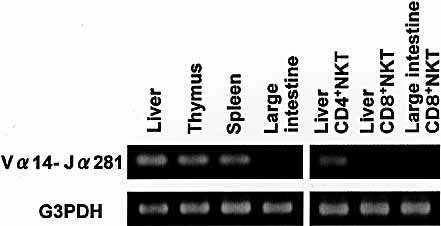
No expression of Vα14Jα281 gene mRNA in CD8+ NKT cells of the large intestine. Lymphocytes were isolated from various immune organs of 8-week-old B6 mice. CD4+ NKT cells and CD8+ NKT cells were purified from the liver and CD8+ NKT cells were purified from the large intestine. Expression of Vα14Jα281 gene mRNA was determined by the RT-PCR method.
2.5 Usage of Vβ seen in NKT cells in the large intestine
NKT cells in the liver, thymus, and other organs preferentially use Vβ8 (mainly Vβ8.2), including Vβ2 and Vβ7 15. We investigated whether there was a similar skewed usage of Vβ in NKT cells in the large intestine (Fig. 5) using three-color staining for α βTCR, NK1.1, and Vβ8 (or Vβ5). By gated analysis, the proportion of each type of Vβ+ cells among NKT cells was enumerated in the liver and large intestine. Vβ8+ cells were much more dominant among NKT cells in the liver (61.0 %). However, this was not the case in the large intestine (17.4 %). The TCR-Vβ usage of the large intestine was not biased toward Vβ8, but was diverse, i. e. Vβ5 (20.7 %) was used as much as Vβ8. There was no skewed usage of Vβ2 and Vβ7 in NKT cells of the large intestine (data not shown).
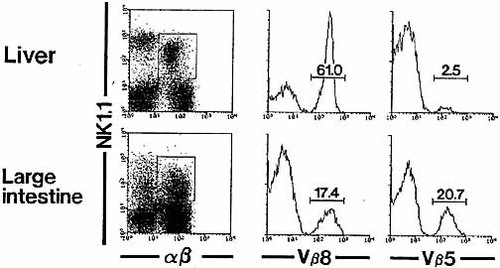
Three-color staining for α βTCR, NK1.1 and TCR-Vβ8 or -Vβ5 was conducted in the liver and large intestine. The proportion of Vβ8+ and Vβ5+ cells among NKT cells was enumerated.
2.6 Cytokine production of NKT cells in the large intestine
Conventional NKT cells produced large amounts of both IL-4 and IFN-γ upon in vitro administration of anti-CD3 mAb. We isolated total lymphocytes from the liver and large intestine and stimulated them in vitro with anti-CD3 or anti-NK1.1 mAb (Fig. 6). In our preliminary experiments, the NK1.1+ cell-depleted faction in the liver and large intestine did not produced either IL-4 or IFN-γ upon the stimulation with anti-CD3 mAb (data not shown). However, the total liver lymphocyte fraction produced both IL-4 and IFN-γ upon the stimulation with anti-CD3 mAb. On the other hand, the total large intestinal lymphocytes produced only IFN-γ upon the stimulation with anti-CD3 mAb. A similar tendency was seen on stimulation with anti-NK1.1 mAb, although in vitro stimulation with anti-NK1.1 mAb was not effective for IL-4 production. Since the large intestine contained a small proportion of NK cells, it is possible that IFN-γ was produced by these NK cells.
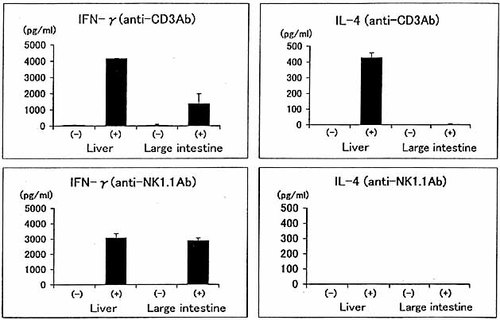
A comparison of cytokine production by NKT cells between the liver and large intestine. Total lymphocytes were purified from the liver and large intestine and were stimulated with either anti-CD3 mAb or anti-NK1.1 mAb in vitro. The culture supernatants were harvested 48 h after stimulation and the activity of IL-4 and IFN-γ was measured by the ELISA.
3 Discussion
In the present study, we demonstrated that unconventional NKT cells are abundant at an intraepithelial site in the large intestine. In contrast, the small intestine which comprises the major portion of the digestive tract, including the intraepithelial site, lacked such NKT cells. Furthermore, almost all NKT cells in the large intestine were either CD8+ or DN CD4–8– cells, and these NKT cells were not completely dependent on either non-classical MHC class I-like antigens (i. e. CD1d) or classical MHC class I antigens. Conventional NKT cells are mainly present in the thymus, liver, and spleen, preferentially express CD4 antigens, use an invariant chain of Vα14Jα281 for TCRα, and recognize some glycolipid antigens (e. g. α-galactosylceramide) as well as peptide antigens in the context of non-classical MHC class I-like antigens 1 – 9. NKT cells in the large intestine were not found to use the invariant chain of Vα14Jα281. In the present study, it was found that CD8+ and DN CD4–8– NKT cells in the large intestine had properties completely different from those of conventional NKT cells.
Several groups have studied unconventional CD8+ NKT cells in the peripheral immune organs, including the spleen liver, bone marrow, etc. 21 – 23. However, the proportion of CD8+ NKT cells in these peripheral immune organs was much less than that of CD8+ NKT cells in the large intestine. We therefore emphasize that the largeintestine, but not the small intestine, is the site where CD8+ NKT cells are most abundant. Generalized features of CD8+ NKT cells in the large intestine seem to be quite similar to those of the peripheral CD8+ NKT cells so far described. Such features include the non-skewed usage of Vα14Jα281 and non-dependency on CD1d.
Since CD8+ NKT cells are almost completely absent in the thymus but are abundant in the large intestine, we speculated that CD8+ NKT cells in the large intestine are of extrathymic origin. However, our subsequent study revealed that CD8+ NKT cells are absent in the large intestine of athymic nude mice (our unpublished observation). This was true for both young (8-week-old) and old (1-year-old) nude mice. As discussed by Zeng et al. 23 for CD8+ NKT cells in the bone marrow, several interpretations of their origin are possible. Conclusions, however, will have to await further functional characterization of the nude gene itself, which has been recently demonstrated to encode the whn transcription factor 24, 25.
Recent studies have shown that some conventional T cells acquire NK1.1 antigens during culture in vitro 26 and that influenza-specific T cells acquire NK1.1 antigenunder in vivo and in vitro activated conditions 27, 28. Considering the above possibilities, however, in the case of CD8+ NKT cells inthe large intestine, this does not seem to be the case. A significant proportion (66 %) of these NKT cells in the large intestine of wild-type B6 mice and the majority (70 – 90 %) of NKT cells in the large intestine of CD1d (− / −) and β2m (− / −) mice expressed a homodimer of CD8α α. Taken together with their unique localization, these results suggest that NKT cells in the large intestine may be unconventional T cells.
In a previous study, we reported both similar and differenting properties of T cells between an intraepithelial site in the small intestine and such a site in the large intestine 29; similar findings have also been reported by other groups 30 – 32. The small and large intestines contain both α β T cells and γ δ T cells. γ δ T cells at both sites were found to have a similar usage pattern of Vγ and Vδ genes, i. e. Vγ2, 4, 7 and Vδ4, 6, 7. However, T cells in the small intestine always contain double-positive (DP) CD4+8+ α β T cells, while those in the large intestine do not, whereas T cells in the large intestine contain DN CD4–8– α β T cells, and those in the small intestine do not. We also showed that the lymphocyte fraction from the large intestine contains NKT cells.
We confirmed that the proportion of NKT cells in the liver decreased in Jα281 (− / −), β2m (− / −), and CD1d (− / −) mice, due to the fact that conventional CD4+ NKT cells and some DN NKT cells are dependent on CD1d antigen and use an invariant chain of Vα14Jα281 [1, 6, 9. Even in these Jα281 (− / −), β2m (− / −), and CD1d (− / −) mice, a small number of NKT cells remained in the liver. These liver NKT cells were mainly CD8+ or DN CD4–8–, andincreased in proportion as a compensatory response. In sharp contrast, NKT cells in the large intestine were CD8+ or DN CD4–8–, and the proportion of NKT cells at this site did not decrease in Jα281 (− / −), β2m (− / −), nor in CD1d (− / −) mice. NKT cells did not show any signs of mRNA of the Vα14Jα281 gene. Moreover, NKT cells in thelarge intestine did not respond to α-galactosylceramide (our unpublished observation).
In the present study, we characterized the distribution pattern of DN CD4–8–, CD8+, CD8α α+ and CD8α β+ in NKT cells in the large intestine and other peripheral immune organs. The lack of dependence of NKT cells on both CD1d and classical MHC class I antigens seems most prominent in the large intestine. Among CD8+ NKT cells in the large intestine (and those in other peripheral organs), the CD8α β+ subset was found to be more highly dependent on the presence of polymorphic MHC class I antigen thanthe CD8α α+ subset.
Vβ usage for TCRα β was also unique in CD8+ NKT cells in the large intestine, with the usage of Vβ5 by NKT cells being similar to that of Vβ8. We speculate that some tissue-specific antigens are recognized by NKT cells in the large intestine, independently from polymorphic MHC class I antigens and non-classical MHC class I-like antigens.
NKT cells in the large intestine produced IFN-γ, but not IL-4, upon stimulation with anti-CD3 and anti-NK1.1 mAb. This result was unique when compared with the cytokine profiles of conventional NKT cells 33 – 36. At present, we do not known whether they have the ability to produce IL-4 under different stimulation conditions. Although the actual function of NKT cells in the large intestine remains unknown, the restricted localization of CD8+ (and DN CD4–8–) NKT cells in the large intestine is very interesting in conjunction with their unusual MHC restriction.
4 Materials and methods
4.1 Mice
C57BL / 6 (B6), Jα281 (− / −) 10 β2m (− / −) 37, 38, and CD1d (− / −) mice 39, 40 were used at the age of 8 – 15 weeks. All mice were fed under specific pathogen-free conditions in the animal facility of Niigata University (Niigata, Japan).
4.2 Cell preparations
Mice anesthetized with ether were killed by exsanguination through incised axillary arteries and veins, and the liver, thymus, spleen, small intestine, and large intestine were removed 41. The liver was pressed through a 200-gauge stainless steel mesh and then suspended in Eagle's MEM supplemented with 5 mM Hepes (Nissui Pharmaceutical Co., Tokyo, Japan) and 2 % heat-inactivated newborn calf serum. After washing, the cells were fractionated by centrifugation in 15 ml of 35 % Percoll solution (Amersham Pharmacia Biotech AB, Uppsala, Sweden) for 15 min at 2,000 rpm. The pellet was resuspended in erythrocyte-lysing solution (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA, and 170 mM Tris pH 7.3).
The thymus and spleen were also pressed through a 200-gauge stainless steel mesh. After centrifugation, the pellet was treated with the erythrocyte-lysing solution. To enrich NKT cells from thymocytes, heat-stable antigen (HSA)+ thymocytes were depleted by incubation with anti-HSA (J11D) antibody (PharMingen Co., San Diego, CA) followed by rabbit complement. For enrichment of splenic T cells, B cells were depleted by anti-B220 (RA3-6B2) mAb and subsequent incubation with sheep anti-rat IgG antibody conjugated with immunomagnetic beads (Dynal A.S., Oslo, Norway).
The small intestine and large intestine were removed and flushed with PBS to eliminate luminal contents 41. The mesentery or Peyer's patch lymphoid follicles were then resected. To obtain intraepithelial lymphocytes (IEL), the intestine was cut longitudinally into 1 – 2-cm pieces. These fragments were incubated for 15 min in 15 ml Ca2 +- and Mg2 +-free PBS containing 5 mM EDTA in a 37 °C shaking-water bath. The supernatant containing the cell suspensions were collected and centrifuged on a discontinuous 40 % / 80 % Percoll gradient solution at 2,800 rpm for 25 min. Cells from the 40 % / 80 % interface were collected.
4.3 Immunofluorescence
The surface phenotype of cells was analyzed using mAb in conjunction with two-color or three-color immunofluorescence 2. The mAb used in this study included FITC-, phycoerythrin (PE)-, or biotin-conjugated anti-CD3 (145-2C11), anti-TCR-α β (H57-597), anti-CD4 (RH4-5), anti-CD8 (53-6.7) and anti-NK1.1 (PK136) mAb (PharMingen). Biotin-conjugated anti-Vβ5 (MR9-4) and Vβ8 (F23.1) mAb were also used (PharMingen). Biotin-conjugated reagents were developed with PE-conjugated streptavidin (Becton Dickinson, Mountain View, CA) or TRI-COLOR-conjugated streptavidin (CALTAG Lab., Burlingame, CA). To prevent nonspecific binding of mAb, CD32 / 16 (2.4G2) was added before staining with labeled mAb. Dead cells were excluded by propidium iodide (PI) gating. The fluorescence-positive cells were analyzed with FACScan using Lysis II software (Becton Dickinson).
4.4 Immunohistochemical procedure
The spleen and ∼ 10-mm lengths of longitudinally opened small and large intestines were embedded in O.C.T. compound (Tissue-Tek, Miles Inc., Elkhart, IN) at − 80 °C 3. The tissue segments were sectioned with a cryostat at 6 μm and applied to poly-L-lysine-coated glass slides (Matsunami Glass IND., Japan). The tissue sections that had been air-dried and fixed in acetone for 10 min at room temperature were preincubated with Block-ace (Dainippon Pharmaceutical Co., Osaka, Japan) for 10 min at 37 °C to block nonspecific binding of the primary mAb. The sections were then incubated with appropriately diluted biotin-conjugated anti-NK1.1 mAb for 30 min at 37 °C or overnight at 7 °C, and rinsed three times with PBS and then incubated with avidin-biotin peroxidase complexes (Vectastatin ABC kit; Vector Laboratories). Histochemical color development was achieved by a Vectastaitin 3,3-diaminobenzidine (DAB) substrate kit (Vector Laboratories) according to the manufacturer's instructions. The sections were counterstained with hematoxylin for microscopy. Endogenous peroxidase activity was blocked with 0.3 % H2O2 and 0.1 % NaN3 in distilled water for 10 min at room temperature.
4.5 RT-PCR for Vα14Jα281 gene mRNA
Total RNA was extracted from lymphocytes (2 × 106 cells) by the acid guanidium thiocyanate-phenol-chloroform method 41. To detect Vα14Jα281 gene mRNA, RNA was reverse-transcribed using the oligo(dT)18 primer, and the resulting cDNA was further amplified by the PCR method as previously described 42. For Vα14Jα281, a 5′ primer, 5′-AAAGATGCTAAGCACAGCACGCTGCACA-3′, and a 3′ primer, 5′-AGGTATGACAATCAGCTGAGTCCC-3′, yielding a 132-bp fragment, were used.
In some experiments, RNA was extracted from purified cell fractions (2 × 105 cells). Such cell fractions included CD4+ NKT cells and CD8+ NKT cells in the liver and CD8+ NKT cells in the large intestine which were purified by a cell sorter (FACSVantage, Becton Dickinson) after two-color staining for NK1.1 and CD4 (or CD8).
4.6 Enzyme-linked immunosorbent assay
Lymphocytes isolated from the intraepithelial site in the large intestine were cultured in the presence of anti-CD3 or anti-NK1.1 mAb (3 × 105 cells / 0.2 ml / well) at 37 °C in RPMI 1640 medium supplemented with 1 % fresh mouse serum. The antibodies (10 μl of 10 μg / ml) were cross-linked on plastic culture plates at 4 °C overnight. Culture supernatants were obtained 48 h after the stimulation. IFN-γ and IL-4 levels in the culture supernatants were evaluated using specific ELISA kits for mouse IFN-γ and IL-4, respectively (Amersham, Little Chalfont, England).
Acknowledgements
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture, Japan. The authors wish to thank Dr. van Kaer, Vanderbilt University (Nashville, TN) for kindly furnishing us with CD1d-deficient mice and Mrs. Masako Watanabe for preparation of the manuscript.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




