Identification and characterization of lymphoid precursors in the murine intestinal epithelium
Abstract
Although in vivo evidence supports a role for the murine intestinal epithelium in the extrathymic generation of certain intraepithelial T lymphocytes (IEL), no intraepithelial cells with in vitro lymphoid progenitor potential have yet been demonstrated. Using reaggregate fetal thymic organ culture techniques, we show that a subset of CD3– cells isolated from the intestinal epithelium of young mice is capable of generating T cells (α β and γ δ) and NK1.1+ cells in vitro. A novel IEL subset bearing a low level of CD45 was identified and found to comprise cells expressing highly immature lymphoid markers including CD34, c-kit, CD122, CD127 and high levels of CD16 and CD44. This subset represents 20–30% of intraepithelial CD45+ cells from 4-week-old wild-type and nude mouse strains and contains cells with in vitro T cell differentiation capacity. The identification of such an early pluripotent precursor phenotype within the intestinal epithelium implies that the potential for T cell generation exists at this site, and suggests that extrathymic T cell generation may occur within the epithelium itself.
Abbreviations:
-
- IEL:
-
Intraepithelial lymphocyte
-
- RAG:
-
Re-combination activating gene
-
- SCID:
-
Severe combine immunodeficiency
-
- FTOC:
-
Fetal thymic organ culture
1 Introduction
Considerable experimental evidence supports a role for the murine intestinal epithelium in the extrathymic generation of a local population of intraepithelial lymphocytes (IEL). This is based largely on the analysis of IEL in two murine models that lack conventional peripheral T cell populations–athymic 1–10, and stem cell reconstituted thymectomized mice 11–15. There is general agreement that whichever model is used, γ δ T cell receptor (TCR)-bearing IEL are present within the intestine despite the absence of a thymus, albeit in smaller numbers in athymic animals when compared to wild type 1, 2, 5. However, the degree to which α β TCR-bearing IEL are independent of the thymus, appears to vary significantly between models. While certain authors have been unable to detect these cells in the congenitally athymic ("nude") mouse 8, 13 most of the wide panoply of IEL phenotypes have been demonstrated in such animals, although relative proportions may vary between individuals 7. Similarly, in mice thymectomized within 3 days of birth, the presence of α β TCR-bearing IEL is variably reported 2, 3, 10. In contrast, mice that are thymectomized, lethally irradiated and subsequently reconstituted with stem cells from bone marrow or fetal liver appear capable of regenerating a normal complement of IEL bothin terms of number and phenotype 11–14. The potentially artifactual effects of radiation have been overcome by the use of a stem-cell factor receptor mutant strain (W/Wv) that permits stem-cell reconstitution of un-irradiated athymic animals 15. In this model, the majority of the donor-derived IEL were found to express the γ δTCR, although a population of IEL expressing the α βTCR was demonstrated. The latter expressed CD8 as the α α homodimer form in contrast to the more usual CD8α β heterodimer foundon thymically-derived T cells.
The expression of CD8α α by α βTCR-bearing IEL has been proposed as a marker of extrathymic ontogeny. This is the only phenotype of α βTCR-bearing IEL that is found in irradiated, thymectomized recombination activating gene (RAG) knockout mice reconstituted with bone marrow from nude mice 14. Furthermore, this CD8α α TCRα β phenotype demonstrates various atypical properties including non-thymic selection 16, 17, the ability to use an Fc-receptor γ chain in place of a CD3ζ moleculefor TCR-mediated signaling 18, the expression of Natural Killer (NK) cell markers and NK-like functions 19. These features would support the experimental evidence that IEL constitute a mixed population of thymically derived CD8α β and CD4+ TCRα β cells, and locally generated CD8α α TCRγ δ and TCRα β cells. However, the results of chimeric thymic regrafting of athymic mice suggest that in fact all subpopulations of IEL can be derived from the thymus 3–5, 9. Lefrancois and Olson 9 propose a model whereby partially differentiated cells from the thymus traffic to the intestine to complete development as IEL. Subsequent work in TCR transgenic mice has demonstrated that even autoreactive γ δTCR-bearing IEL may be thymus-derived and escape intrathymic selection to find sanctuary within the intestinal epithelium 20.
The available evidence therefore supports both intra- and extrathymic differentiation pathways of IEL, and the extent to which the latter occurs within the intestine in vivo is controversial. The role of the thymus in IEL ontogeny as the source of all IEL, of IEL precursors or of lymphopoieitic neurohumoral factors 10 is debated.
Studies of IEL differentiation are markedly hampered in vivo by the substantial mature secondary lymphoid presence in the gut, and by the lack of a suitable in vitro environment akin to fetal thymic organ culture techniques that have contributed much to our knowledge of thymocyte differentiation.
Putative lymphoid precursors have recently been identified in the intestinal lamina propria in mice within discreet collections termed "cryptopatches" 21. Adoptive transfer experiments into immunodeficient SCID mice have suggested that these c-kit+ cryptopatch cells may have an IEL progenitor capability. However, cryptopatch cells showed no T cell differentiation potential in whole lobe fetal thymic organ culture (FTOC) 22.
In this study we have used reaggregate fetal thymic organ culture techniques to demonstrate the presence of a lymphoid precursor population within the murine intestinal epithelium that is capable of T and NK cell differentiation in vitro and we show that the phenotype of this precursor resembles the earliest intrathymic T/NK cell progenitors.
2 Results
2.1 Selected CD3–CD45+ "IEL" survive in fetal thymic organ culture
Any potential intestinal lymphoid precursor would necessarily express a CD3–CD45+ phenotype. Using dual immunofluorescence microscopy with staining for CD3 and CD45, such cells are readily identified and quantified within the intestinal epithelium of BALB/c mice at different ages. These cells are proportionately most numerous within the epithelium at 4 weeks after birth (Fig. 1). At this age, which is around the time of weaning, there is a rapid expansion of IEL numbers which is underestimated by measurement of IEL density (expressed as a ratio of IEL:enterocytes), as the intestine itself is also growing. It is therefore possible that the overall number of CD3–45+ IEL remains constant after this age despite the subsequent apparent decrease in the proportion of these cells. Nevertheless, in view of the relative enrichment of CD3–45+ IEL at 4 weeks, animals of this age were used in all subsequent experiments in order to optimize the yield of isolated CD3–CD45+ cells.
Crude IEL isolates from 4-week-old BALB/c mouse intestine were depleted of mature B and T cells and purified from enterocytes using immunomagnetic bead techniques (Sect. 4.5). CD8+ cells were also removed in order to exclude a postulated CD3–8+ intermediate population 23. Resulting yields from 19 experiments averaged 2.1×105(±2.7×104; 95%CI) cells, with a mean purity of 97% for CD3– cells (Fig. 2). Selected CD3–8–Ig–CD45+ IEL were mixed with newborn thymic stromal cells that had been depleted of thymocytes by culture ex vivo in the presence of 2-deoxy-guanosine, and subsequently subjected to immunomagnetic depletion of CD45+ cells. Using a reaggregate technique, FTOC containing the selected IEL were established in vitro.
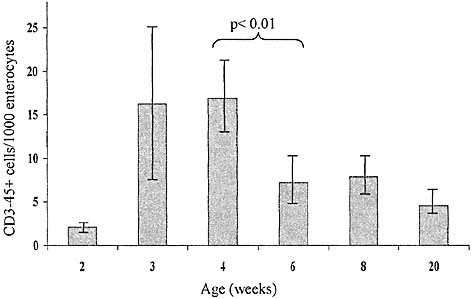
Density of CD3–CD45+ IEL in intestinal epithelium of BALB/c mice of different ages. Three-color immunofluorescence microscopy identified CD3–CD45+ cells lying on the luminal side of the basement membrane and these cells were counted relative to the number of enterocyte nuclei. At least three animals of each age were studied and sections were taken from at least two segments of proximal small bowel (distal to the ligament of Treitz) in each. Results expressed as a mean with 95% confidence intervals. p-value represents significance using Student's t-test. The density of CD3–45+ cells in the epithelium is greatest at 3–4 weeks of age, but overall numbers may remain similar despite a drop in the relative density compared to enterocytes in view of the increase in size of the intestine after this age.
Following periods of up to 4 weeks in culture, live lymphoid cells could be harvested from FTOC seeded with CD3–8–CD45+ IEL. In 19 equivalent experiments, the average increase in cell number over those added to the culture amounted to 32%±27% (95%CI). Aliquots of thymic stroma removed before the addition of the intestinal lymphoid population were also set up in reaggregate organ culture and on no occasion could lymphoid cells be isolated from these parallel control cultures (Fig. 3). This confirms that thymocyte depletion from the explanted thymic tissue was complete, and that no endogenous thymocyte precursors were carried over into the reaggregate cultures. Any resulting lymphoid cells from these cultures must therefore have originated from the intestinal lymphoid population added.
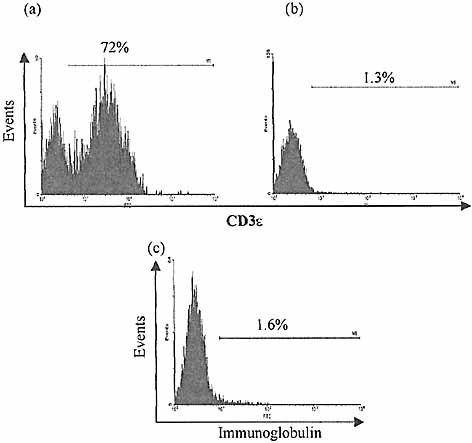
Purity of selected CD3–CD45+ IEL populations. IEL were isolated from 4-week-old BALB/c mouse intestine and purified from enterocyte contamination using Percoll density centrifugation. B and T cells were depleted by successive rounds of depletion using anti-CD3 and anti-mouse immunoglobulin-coated magnetic beads (Dynal). IEL were further purified from residual enterocytes using positive selection with anti-CD45.2 FITC-antibodies and anti-FITC-coated mini-magnetic beads (MACS). Flow cytometric analysis of initial (a) and post-purification populations (b, c) reveals significant depletion of CD3+ and sIg+ cells.
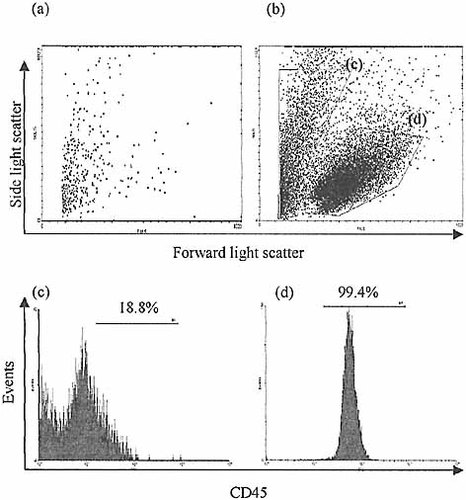
Results of selected IEL co-culture with deoxyguanosine treated, CD45-depleted thymic stroma. Fetal thymic stroma from BALB/c mice was cultured in the presence of deoxyguanosine in medium for 5 days and disaggregated with trypsin before undergoing depletion of CD45+ cells by successive rounds of depletion with anti-CD45-coated magnetic beads (Dynal). The residual thymic stromal cells were divided into two aliquots of approximately 1×106 cells. Reaggregate cultures were constructed either with the addition of approximately 2×105 selected CD3–8–sIg–CD45+ ‘IEL’ or without additional lymphoid input (control). Total cell populations harvested after 21 days from control (a) and lymphoid input (b) reaggregate cultures are shown by light scatter characteristics on flow cytometry. Expression of CD45 by gated populations (c and d) clearly demonstrates the live lymphoid population (d). Few cells are apparent from the control culture, and almost none in the gate corresponding to (d). This confirms the adequacy of thymocyte depletion of the thymic stroma and that resultant lymphoid progeny are derived from intestinal precursors added.
2.2 Lymphoid precursor potential of CD3–CD45+ cells isolated from the intestinal epithelium
After at least 2 weeks in fetal thymic organ culture, a population of CD4+8+ cells was reliably detected constituting 6.4%±2.1% (95%CI) of the resultant lymphoid cells in all of ten consecutive experiments (Fig. 4). Single-positive CD4+ (4.2±2.8%) and CD8+ (11.2±5.2%) cells were also detected in all experiments (Fig. 4). This suggests that the crude CD3–CD45+ population of IEL added to the thymic stroma contained cells capable of T-lymphocyte differentiation.
Despite control cultures proving that the thymic stroma contained no residual cells with lymphoid developmental potential, confirmation that the CD4+8+ population was not due to the carry-over of thymocytes was performed using selected CD3–8– IEL from AKR (Thy-1.1) strain mice cultured in BALB/c (Thy-1.2) FTOC and analyzed for Thy-1.2, CD4 and CD8 expression. All resulting CD4+8+ cells lacked Thy-1.2 expression, in contrast to CD3–8– BALB/c IEL cultured under identical conditions (Fig. 4).
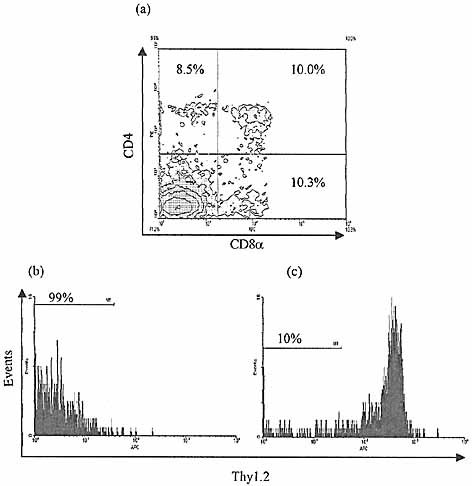
Development of CD4+, CD8+ and CD4+8+ cell populations from IEL precursors in FTOC. Progeny of CD3–8–sIg–CD45+ BALB/c ‘IEL’ co-cultured with BALB/c fetal thymic stroma for 23 days, analyzed for CD4 and CD8 expression by flow cytometry (a). Cells pre-gated by light scatter as for Fig. 3d. Significant populations of CD4+ and CD8+ single-positive cells and CD4+8+ double-positive cells are demonstrated, suggesting lymphoid development has occurred from precursors present in the input population (representative of ten consecutive experiments). Use of AKR (Thy-1.1) mice for selected IEL input into BALB/c (Thy-1.2) fetal thymic stroma demonstrates that gated CD4+8+ cell progeny after 21 days uniformly lack Thy-1.2 expression (b), confirming that these cells are derived from the intestinal input source rather than potential carry over of thymocyte precursors with the thymic stroma. In contrast, the majority of CD4+8+ gated progeny of selected IEL from BALB/c mice express Thy-1.2 (c).
The rate of development of CD4+8+ cells in FTOC from CD3–8– IEL was markedly retarded compared to thymocyte precursors – termination and analysis of progeny after less than 2 weeks in culture failed to reveal a CD4+8+ population. In addition, splitting the mixture of selected IEL and thymic stroma into two cultures and terminating after 1 and 2 weeks, respectively, revealed the presence of CD4+8+ cells in the two week culture only (Fig. 5). Analysis of native freshly isolated IEL showed very few cells to co-express CD4 and CD8 in 4–week-old mice, in common with previous reports that show this population to increase with age 24. Such CD4+8+ IEL lack the expression of CD8β, however, CD4+8+ progeny of CD3– IEL in FTOC express CD8α β exclusively and are therefore unlikely to represent contamination with mature CD4+8+ IEL (Fig. 6).
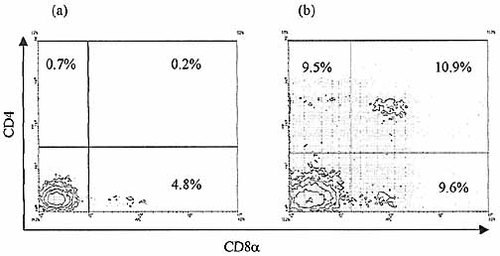
Time course of CD4+8+ cell development from selected IEL in FTOC. Purified CD45+ cell-depleted thymic stroma was split into three aliquots and the selected IEL suspension was divided between two of these aliquots prior to reaggregation, leaving the third as control. No lymphoid cells developed in the control culture after 14 days (not shown). No CD4+8+ cells are apparent after terminating the culture at 7 days (a) in contrast to the harvesting at 14 days (b). A small population of CD8+ cells is apparent in the 1-week culture which may represent contamination with CD3–8+ cells, as the selected IEL were not depleted of CD8+ cells in order to maximize numbers of CD3–sIg–CD45+ cells.
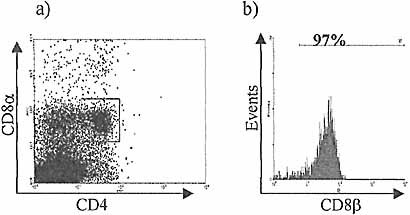
CD8β expression by CD4+8+ culture progeny of selected CD3–-8–sIg–CD45+ IEL in BALB/c FTOC after 21 days. Selected IEL co-cultured with fetal thymic stroma as shown previously with CD4 and 8 expression by harvested cells after 21 days in culture (a). The gated population of CD4+8+ cells shown in (a) uniformly expresses CD8β, demonstrating that these cells are not equivalent to the mature CD4+8α+β– IEL found in older mouse intestinal epithelium (b).
In common with thymocyte differentiation, the CD4+8+ progeny of CD3–8– IEL cultured in FTOC were found to express TCRα β (Fig. 7). In addition, a substantial proportion of cells harvested from the culture revealed a CD3+4–8– phenotype comprising TCRα β+ and TCRγ δ+ cells (Fig. 8).
Analysis of NK cell differentiation was performed using C57/B10 mice as the source of intestinal lymphoid precursors. Up to 40% of the resulting progeny were found to express NK1.1 (Fig. 8).
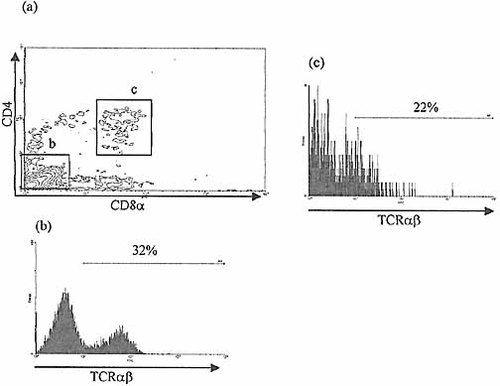
TCRα β expression by CD4+8+ and CD4–8–-populations of culture progeny. Three-color flow cytometric analysis of progeny of selected IEL in BALB/c FTOC after 21 days stained for CD4, CD8 and TCRα β expression. Intermediate levels of TCRα β expression are demonstrated by CD4+8+ cells, compared to a higher level by CD4–8– progeny.
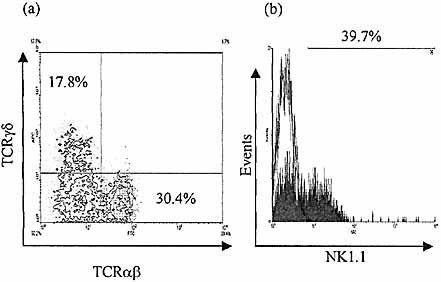
Expression of TCR types and NK1.1 by culture progeny of selected IEL in FTOC. Both TCRα β and TCRγ δ receptors are expressed by culture progeny of CD3–8–sIg–45+ IEL in FTOC after 21 days in culture (a). A significant proportion of cells are shown to express NK1.1 after culture under similar conditions (b) – C57 B10 mice were used for the selected IEL input population (BALB/c thymic stroma as before).
2.3 Characterization of the intestinal lymphoid precursor phenotype
Having demonstrated the presence of a precursor capable of T lineage α β, γ δ and NK cell differentiation in a thymic stromal environment in vitro, we further examined the nature of the CD3–8– IEL input population to determine its homogeneity. Strikingly, a subpopulation of cells expressing a low level of CD45 could be detected (Fig. 9), and comprised around 44% of the selected CD3–8– IEL. These CD45low cells were prominently detectable in crude IEL isolates from 4-week-old animals, comprising 12.0±1.6% (95%CI) of all IEL in both C57/B10 and BALB/c strains (based on 19 consecutive experiments). The proportion of CD45low cells diminished with age to around 1.5% at 8 weeks of age and remained at this level in aged mice. Given the expansion of IEL numbers between 4 and 8 weeks of age, this is most likely to represent a constant population size. An even higher proportion of CD45low IEL was detected in nude mice (up to 30% at 4 weeks), and in contrast to euthymic mice this proportion remains constant with age.
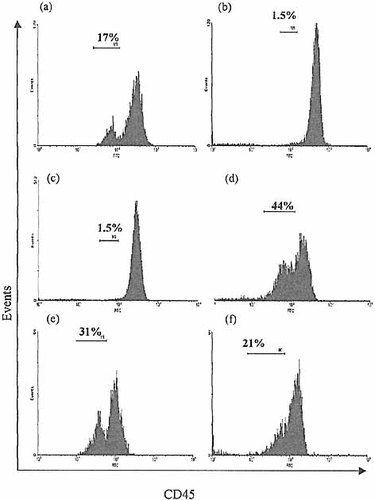
Demonstration of two levels of CD45 expression by freshly isolated IEL. A CD45low IEL population is demonstrated as shown in IEL from (a) 4-week-old BALB/c, (b) 8-week-old BALB/c, (c) 8-month-old BALB/c, (d) selected CD3–8–sIg–CD45+ IEL from 4-week-old BALB/c, (e) 8-week- old BALB/c nude and (f) 8-month-old BALB/c nude mice. Note that the proportion of CD45low IEL is greater in the 4-week-old BALB/c mice than at 8 weeks of age and remains constant thereafter. In view of the expansion of the CD3+ IEL population after 4 weeks of age, this does not necessarily indicate a reduction in total numbers of CD45low IEL, which may remain relatively constant throughout the life of the animal. This is further indicated by the high proportion of CD45low IEL found in both young and aged nude mice. CD45low IEL are notably enriched in the selected CD3–8–sIg–CD45+ population of cells. IEL were pooled from at least three animals; results characteristic of at least three experiments.
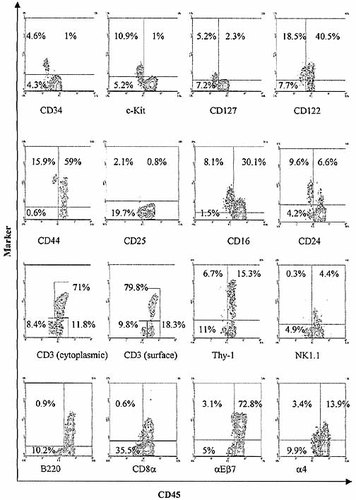
Phenotypic characterization of the CD45low IEL population. Freshly isolated IEL analyzed by two color flow cytometry to demonstrate characteristics of CD45low IEL. The relevant ordinate marker for each is shown below separate graphs. All graphs show surface staining of freshly isolated 4-week-old BALB/c IEL except for: cytoplasmic staining of CD3, NK1.1 expression (C57/B10 mice), and CD8α (selected CD3–sIg–CD45+ BALB/c IEL).
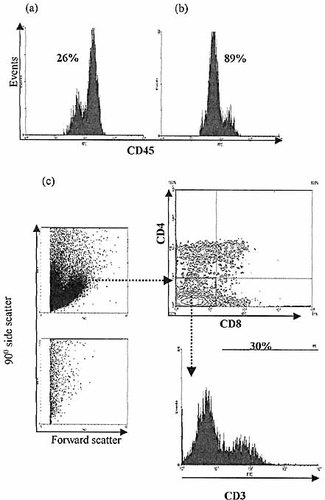
CD45low IEL selected by CD34 expression and cultured in FTOC for 21 days demonstrate lymphoid development. IEL were selected for CD34 expression from 8-week-old BALB/c nude mice using magnetic bead techniques. Enrichment for CD45low IEL is demonstrated (a) before, (b) after selection. CD4, 8 and 3 expression is demonstrated as for crude CD3–8–sIg–CD45+ IEL isolates after 21 days in FTOC (c). Control cultures without lymphoid input again show no significant lymphoid cell population.
Phenotypic analysis of CD45low IEL (Fig. 10) showed the majority to lack expression of lineage markers including CD3 (both surface and cytoplasmic), surface immunoglobulin, B220, CD4, CD8, NK1.1, and Thy-1. Furthermore, most CD45low cells expressed high levels of CD44, CD16, CD24 (Heat stable antigen), c-kit, CD127 (the IL-7 receptor α chain) and CD122 (the IL-2 receptor β chain), but lacked CD25 (the IL-2 receptor α chain). Significantly, a high level of CD34 expression could be detected on about half of the CD45low cells. The lack of CD103 (αEβ7) expression by these cells led us to question whether they could be sub-epithelial contaminants, perhaps from cryptopatches. However, two characteristic populations of cryptopatches, CD4+3– – and class II MHC+sIg, – cells could not be detected amongst IEL isolates (data not shown) implying an intraepithelial rather than cryptopatch origin for the CD45low cells. Furthermore, few CD45low IEL were found to express the α4 integrin chain.
To determine whether the CD45low population of CD3– IEL contained the progenitor phenotype present within the CD3–8–-IEL isolates, CD34+ IEL were enriched from 8-week-old nude mice using immunomagnetic bead techniques and co-cultured with fetal thymic stroma in FTOC. Analysis of the culture progeny after 21 days showed identical results to those described above, with CD4+, CD8+, CD4+8+ and CD3+4–8–-populations apparent (Fig. 11). Whether any T lineage potential was present amongst cells of CD45high phenotype could not be tested due to inadequate depletion of CD45low IEL using immunomagnetic selection for CD34. However, it would appear that progenitors with T lineage potential are contained within the CD45low IEL population.
3 Discussion
The presence of a lymphoid precursor cell type within the murine intestinal epithelium has long been suspected, and this population is demonstrated for the first time in this study by using fetal thymic organ culture techniques. T cell differentiation from CD3– IEL is shown by the development within this system of CD4+, CD8+ and CD4+8+ cell populations that were absent from the selected input cells. The CD4+8+ culture progeny resemble intermediates in thymocyte differentiation rather than double positive IEL by virtue of expression of CD8β. However, these cells clearly derive from the intestine rather than thymic stroma due to incompete lymphoid depletion: control cultures using the same thymic epithelial cells without CD3– IEL input failed to support such a population, much longer culture times were required compared to the differentiation of thymic precursors, and the intestinal provenance of these cells is confirmed using Thy-1 discordant strains. In addition, thymic precursors cultured under these conditions predominantly follow a CD4+8+ route to single positive T cells, whereas only a minority of cells deriving from intestinal precursors expressed this phenotype with a large proportion of CD3+4–8– cells in the culture progeny. Whether this suggests an early environmental influence on subsequent developmental events is unclear, however, it suggests again that the behavior of the precursors differs from that of thymic progenitors.
The development of TCRα β, TCRγ δ and NK cells is consistent with an immature multipotent lymphoid precursor, however, non-T cell developmental fates were not tested in this system.
The CD3–CD45+CD8– IEL population selected in this study for co-culture with thymic stroma comprises several different phenotypic sub-groups, and if only one of thesecell types had lymphoid precursor potential it would explain the relatively small numbers of CD4+8+ cells harvested from the cultures.
The novel finding of a low level of CD45 expression on certain CD3– IEL enabled a previously undescribed phenotype of IEL to be determined, and enrichment for this subset using immunoagnetic selection of CD34+ cells demonstrated lymphoid differentiation potential within this population. Whether the CD45high population also showed such potential could not be tested as deple-tion of CD34+ cells was incomplete using this technique. Nevertheless, the striking phenotypic similarity of the CD45low IEL subset to early intrathymic lymphoid precursors 25–28 suggests that the intestinal lymphoid precursors reside within this population. The expression by these cells of the stem cell factor receptor, c-kit,the α chain of the IL-7 receptor and the β, but not the α chain of the IL-2 receptor, is in concordance with data supporting the role of IL-7 and 15 and stem cell factor in the ontogenyof IEL 29–31.
The demonstration of CD34 on these intraepithelial lymphoid precursors suggests marked immaturity, as this molecule implies a stem cell phenotype and is expressed by the earliest intrathymic immigrants, being rapidly down-regulated early in thymocyte differentiation 28. Intriguingly, the CD45low cells lack both α4 and CD103 integrins. The former appears to play a major role in thymic migration of thymocyte precursors, and its lack profoundly effects intrathymic T cell differentiation 32, but strikingly has little effect onIEL numbers. It may be that such differences allow for the differential homing of precursors to the intestine and thymus. The reaggregate thymic culture technique used overcomes any such potential homing barriers and could explain why it was possible to demonstrate developmental potential of these intestinal lymphoid precursors in a thymic microenvironment. The absence of expression of CD103 by the CD45low cells may imply that these cells are recent intra-epithelial immigrants, or reside in sub-epithelial structures. In this regard it is notable that a similar phenotype of cells has been described within "cryptopatches" 21, 22. However, other characteristic cell types are found within these structures such as CD4+3– and MHC class II+, surface Ig–-cells, and the lack of such phenotypes within the IEL isolates used (data not shown) supports an epithelial rather than cryptopatch origin for the CD45low intestinal lymphoid precursors.
The use of FTOC in this study has permitted the demonstration of lymphoid precursors that are capable of T cell differentiation within a permissive surrogate environment in vitro, andthe phenotypic identification of the putative progenitor. The mere presence of such cells does not necessarily confirm that they do indeed differentiate into T cells within the intestinal epithelium, and it is highly unlikely that they follow thymocyte-like pathways of differentiation, even though they are capable of this in a thymic stromal microenvironment. However, a substantial body of experimental data strongly supports the likelihood of such a capability within the murine intestinal epithelium 1–15, and that these cells express a lymphoid precursor phenotype and differentiate into T cells in vitro makes it likely that these are the precursors of extrathymically generated IEL. Similarly, those IEL co-expressing CD4 and CD8 are thought not to be developmental intermediates akin to thymocytes of similar phenotype in view of the high level of CD3 expression 33, their lack of CD8β, and functional characteristics 34, 35 that suggest these to be mature cells. Instead, evidence currently supports a CD3–8α+ IEL population as developmental intermediate, possibly arising as a result of the inductive effect of the intestinal epithelium on CD8α expression 36, 37. Intriguingly, the pathway of IEL differentiation mayresemble more closely an alternative pathway of thymocyte differentiation in which CD4 and CD8 are not expressed and mature CD3+4–8–-cells result 38.
That such substantial proportions of very immature putative lymphoid progenitors (up to 20% of IEL numbers at 4 weeks of age) can be demonstrated within the intestinal epithelium of young micesuggests that theories regarding the site of origin and differentiation of IEL need to be re-addressed. The presence of CD34 on these intestinal lymphoid precursors implies that they are unlikely to have been re-exported from the thymus, indeed these cells are notably present within nude mouse intestinal epithelium. Furthermore, the epithelial location of these precursors is in keeping with the inability to demonstrate T cell differentiation from cryptopatches or cryptopatch cells in fetal thymic organ culture (personal observation and 22) and suggests that the role of these structures in the extrathymic generation of IEL needs to be re-addressed.
4 Materials and methods
4.1 Mice
BALB/c (euthymic and nude), AKR and C57/B10 mice were taken from long-term colonies maintained by the University animal facility. Weaning was formally commenced at 21 days after birth.
4.2 Antibodies and immunoconjugates
The following monoclonal antibodies were used in this study and were obtained from PharMingen: 2C11, anti-CD3ϵ (and FITC-conjugated); GK1.5, anti-CD4 (and PE-conjugated); 53–6.7, anti-CD8α (FITC- and APC-conjugated); 53–5.8, anti-CD8β (PE-conjugated); 2.4G2, anti-CD16/32 (PE-conjugated); J11d, anti-CD24 (biotinylated); 7D4, anti-CD25 –(PE-conjugated); RAM34, anti-CD34 –(biotinylated); IM7, anti-CD44; 104, anti-CD45.2 –(FITC-conjugated); RA3–6B2, anti-B220 –(PE-conjugated); 14.8, anti-CD45RA –(biotinylated); 5H10–27, anti-CD49d; 53–2.1, anti-Thy-1.2 (and FITC-conjugated); M290, anti-CD103; 2B8, anti-CD117 –(biotinylated); TM-b1, anti-CD122; B12–1, anti-CD127; PK136, anti-NK1.1 –(biotinylated); H57, anti-TCRβ; GL3, anti-TCRδ; KT3, anti-CD3 was supplied by Serotec. Anti-Collagen (IV) was supplied by ICN. Biotinylated anti-mouse immunoglobulin and streptavidin-phycoerythrin were supplied by Amersham. Other fluorescent conjugates included streptavidin-FITC (Sigma), Extra-avidin-rhodamine (PharMingen), anti-rabbit Ig-AMCA (Dako) and Streptavidin-APC (PharMingen).
4.3 Immunofluorescence microscopy
After removal, a length of proximal small intestine was stripped of mesentery and cut into 1-cm sections and flushed with cold PBS using a glass pipette. The intestine was everted and mounted in OCT compound (Raymond Lamb, London) before snap freezing in liquid Nitrogen and storage at –20°C. Sections of 5 μm thickness were cut and allowed to dry before fixing in methanol at –20°C for30–45 min. Slides were washed in PBS (Sigma) containing 0.5% Tween 20 (Sigma) for 30 min then blocked with a commercial blocking agent (NEN Lifesciences). Following a 60-min primary incubation withrat anti-CD3 antibodies diluted in PBS, slides were washed and a mixture of rabbit-anti Collagen-IV and biotinylated sheep anti-rat immunoglobulin antibodies were applied for 45 min. After subsequent washes, a mixture of mouse anti-CD45.2 FITC, anti-rabbit-AMCA and extra-avidin-rhodamine were added for 30 min. Following final washing steps, slides were mounted in anti-fade glycerol and viewedusing a Zeiss Axioplan microscope with ×40 and ×100 objectives.
4.4 IEL quantitation
Two or more sections from each of at least three animals were examined at 2, 3, 4, 6, 8, and 20 weeks and the number of CD45+, CD3– cells lying on the epithelial side of the basement membrane (stained with AMCA-conjugated anti-Collagen IV) were counted. Background staining was digitally enhanced to permit visualization of enterocyte nuclei which were enumerated over theareas in which IEL were counted. CD3–45+ IEL were therefore enumerated as a ratio to enterocytes.
4.5 IEL isolation
Established techniques of IEL isolation were adapted 39. Briefly, intestines from 3–4-week-old mice were removed free of mesentery and flushed with medium before opening longitudinally and cutting into 1-cm pieces. The pieces were washed in calcium/magnesium-free PBS (Sigma) and then incubated with constant stirring at 37°C for 30 min in a 2 mM solution of EDTA (Sigma)containing dithiothreitol (Sigma). The supernatant was filtered through glass wool (Fisher), and IEL were purified by Percoll (Sigma) density centrifugation at 650×g with IEL harvested from a 42/70% interface and washed in PBS.
4.6 IEL subset isolation
Magnetic beads (Dynal) were coated by overnight incubation at 4°C with rat mAb to CD3 or CD8. A cocktail of anti-CD3, anti-CD8 and anti-mouse immunoglobulin-coated magnetic beads was added to the IEL preparation, and at least three rounds of magnetic depletion were performed to remove CD3+, CD8+ and B cells. CD45+ cells were subsequently separated from contaminating enterocytes by incubation successively with anti-CD45.2FITC and anti-FITC-coated microbeads (Miltenyi Biotec), followed by selection on a magnetic column. The eluted CD45+ cells were subjected to a further round of magnetic depletion of CD3+ and CD8+ cells.
CD34+ IEL were isolated by incubation of crude IEL isolates with biotinylated anti-CD34 antibody and subsequently with streptavidin-linked microbeads (Miltenyi Biotec), and se-lected on a magnetic column.
4.7 Fetal thymic organ culture
Established techniques were employed 40. BALB/c fetal thymic lobes (15-day) were dissected and cultured in the presence of 1.35 mmol 2-deoxyguanosine for 5 days. Lobes were subsequently disaggregated with 0.25% trypsin (Sigma) in 0.02% EDTA (Sigma). The resulting cell suspension was subjected to two rounds of depletion using anti-CD45-coated magnetic beads (Dynal). Approximately 1×106 thymic stromal cells were added to around 2×105 selected IEL, and a similar aliquot served as a control culture to confirm depletion of thymocytes. Following centrifugation, each pellet was carefully deposited (using a drawn glass pipette) onto the surface of a filter and cultured at the medium-air interface on Dulbecco's modified Eagle medium for periods of up to 4 weeks.
4.8 Immunofluorescence flow cytometric analysis and statistics
IEL or cells harvested from the composite reaggregate cultures were stained with fluorochrome-conjugated mAb on ice for 30 min and washed in PBS to tag surface markers. For detection of cytoplasmic CD3ϵ, cells were first labeled using mouse anti-CD45.2 FITC-conjugate, then washed and the pellet resuspended in 40 μl PBS and 250 μl Ortho Permeafix (Johnson and Johnson) for 1 hin the dark at room temperature. After washing, cells were suspended in a solution of anti-CD3-PE in PBS, for 30 min then washed. Immunostained cells were stored in 200 μl PBS-containing 1.5% paraformaldehyde and detected using a Coulter Elite dual laser flow cytometer (Coulter Electronics Inc., Hialeah, Florida). Analysis of data was performed using Epics Elite Workstation (v3) or Windows MDI (v2.7) run on an Elonex PC-450 computer. Errors are provided as 95% confidence intervals.
Acknowledgements
The authors are grateful to Miss Deirdre McLoughlin and Mr. Alan Murdoch for flow cytometry expertise. This work was supported by an NHS Executive Sheldon Fellowship and a Medical Research Council Clinical Training Fellowship.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




