Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts
Abstract
The glycosylphosphatidylinositol-anchored receptor CD14 plays a major role in the inflammatory response of monocytes to lipopolysaccharide. Here, we describe that ceramide, a constituent of atherogenic lipoproteins, binds to CD14 and induces clustering of CD14 to co-receptors in rafts. In resting cells, CD14 was associated with CD55, the Fcγ-receptors CD32 and CD64 and the pentaspan CD47. Ceramide further recruited the complement receptor 3 (CD11b/CD18) and CD36 into proximity of CD14. Lipopolysaccharide, in addition, induced co-clustering with Toll-like receptor 4, Fcγ-RIIIa (CD16a) and the tetraspanin CD81 while CD47 was dissociated. The different receptor complexes may be linked to ligand-specific cellular responses initiated by CD14.
Abbreviations:
-
- FRET:
-
Fluorescence resonance energy transfer
-
- HSP70:
-
Heat shock protein 70
-
- LTA:
-
Lipoteichoic acid
-
- TLR:
-
Toll-like receptor
-
- CAD:
-
Coronary artery disease
1 Introduction
CD14, a glycosylphosphatidylinositol–anchored receptor (GPI-R) on monocytes, plays a major role in the inflammatory response to bacterial pathogens (reviewed in Stelter et al. 1). Monocytic CD14 expression is reduced in systemic inflammation 2 and an atherogenic lipoprotein profile is associated with an expansion of a more differentiated monocyte subpopulation with a lower expression of CD14 and expression of Fcγ-RIIIa (CD16a) 3, 4. Moreover, a functional genetic polymorphism of CD14 is associated with myocardial infarction 5, suggesting a role of CD14 in inflammation and atherogenesis.
Although lipopolysaccharide (LPS) is the major ligand, recent data indicate 1 that CD14 can also interact with other ligands including lipoteichoic acid 6, soluble peptidoglycan, muramyldipeptide, polymannuronic acid and lipoarabinomannan 1. In addition anionic phospholipids compete with LPS for CD14 binding 7. Similarities in the cellular interaction of ceramide and LPS have pointed to the possibility that ceramide may also act as a CD14 ligand 8. Recently, the stress inducible heat shock protein 70 (HSP70), a binding protein for sulfogalactosylceramide 9, was shown to stimulate cytokine production of monocytes by a CD14-dependent pathway 10.
GPI-R specifically associate with cholesterol and sphingolipid-rich membrane microdomains also termed rafts 11. CD14 mediates selective binding of LPS to this membrane compartment 12. CD14 as a GPI-R lacks a transmembrane domain and the membrane proteins Toll-like receptors (TLR) 4 13 and TLR2 14 have been suggested to be involved in the signal transduction to LPS. Physical proximity of TLR4 and CD14 in LPS-activated cells 15 suggests TLR4 as a major signaling molecule.
Here we characterized the potential of different CD14-ligands to induce the formation of different multimeric membrane receptor complexes in human monocytes and analyzed the effect of cholesterol loading and depletion. Fluorescence resonance energy transfer (FRET) was used to determine the sterical co-association between the proteins. In addition, receptor co-localization was confirmed by multiparameter high-resolution microscopy 16 and sucrose density gradient floatation after detergent extraction 17.
2 Results
2.1 Ligand-induced clustering of CD11b and CD14 within rafts
In a previous study, co-association of CD11b and CD14 on LPS-activated neutrophils was demonstrated using FRET technology 18. Therefore, human monocytes were stimulated in vitro with LPS, fMLP and PMA and FRET between CD11b and CD14 was measured. Co-assembly was induced by LPS, but not by fMLP or PMA (Fig. 1a). This effect reached a maximum near 20 ng/ml of LPS (Fig. 1b). On resting monocytes, in contrast, neither FRET analysis nor confocal microscopy revealed proximity between CD14 and CD11b (Fig. 1c). Microscopy confirmed the co-localization of CD14 and CD11b following activation by LPS (Fig. 1d).
Ceramide-stimulation partially mimics LPS-responses in different cellular systems 8. In our experiments ceramide induced FRET between CD11b and CD14 similar to LPS (Fig. 1b) and saturation was reached near 2 nM. Confocal microscopy again confirmed the co-association (Fig. 1e). The potent LPS-antagonist compound-406 19 which did not induce clustering of CD14 and CD11b (Fig. 1f) significantly reduced the co-association of CD14 and CD11b induced by either LPS or ceramide indicating a similar mechanism of interaction. Further evidence for an interaction of ceramide and CD14 was provided by the specific binding of [14C]-N-Palmitoyl-D-Sphingosine (C16-ceramide) to CD14-transfected CHO-fibroblasts (Fig. 1g).
Cytochalasin D, a potent inhibitor of LPS internalization 20, inhibited the uptake of a fluorescent LPS derivative (FITC-LPS) by 59±8% as assessed by flow cytometry. The LPS- or ceramide dependent co-localization of CD14 and CD11b, in contrast, was not attenuated (Fig. 1f), suggesting that receptor clustering was probably not dependent on ligand internalization.
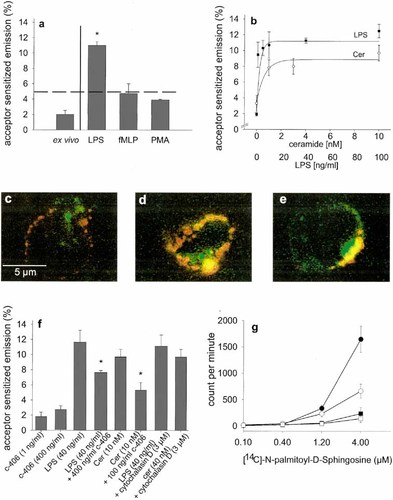
Ligand-dependent conformational activation of the CD14/CD11b complex. (a) Monocytes were stimulated with LPS, fMLP and PMA or control incubated with PBS. Energy transfer values are expressed in terms of emission of Cy5 excited by 488 nm (equation 1) and significantly different from unstimulated controls at p<0.05 (*). Data are the mean ± SD of ten experiments. (b) Dose-dependent effects of LPS and ceramide on CD11b and CD14 co-assembly. Data are the mean ± SD of ten experiments. Membrane distribution of CD11b and CD14 on (c) resting monocytes and the effect of (d) LPS and (e) ceramide visualized by confocal microscopy. CD11-PE is shown in green, CD14-Cy5 in red, co-localization in yellow. (f) Incubation with LPS, ceramide (Cer), compound-406 (c-406) and cytochalasin D. Energy transfer values were significantly different from the respective incubation without inhibitor at p<0.05 (+). Data are the mean ± SD of five experiments. (g) CHO-cells transfected with human CD14 (-unlabeled ceramide (•), + unlabeled ceramide (○)) or with pPOL-DHFR vector (-unlabeled ceramide (▪), + unlabeled ceramide (□)) were incubated with [14C]N-Palmitoyl-D-Sphingosine at the indicated concentrations and cell bound 14C was determined. Each data point represents the mean ± SD of triplicate measurements. The experiment has been repeated twice with similar results.
To further identify agonists inducing co-assembly of CD14, monocytes were incubated with lipoteichoic acid, sulfogalactosylceramide, and different glycero-phospholipid liposomes. Only lipoteichoic acid, phosphatidylethanolamine and phosphatidylinositol induced a co-assembly of CD11b and CD14 (Fig. 2a).
Recently, HSP70 was found to stimulate cytokine production through a CD14 dependent pathway 10. HSP70 in its native form in our experiments induced co-assembly of CD11b and CD14 (Fig. 2b), while the delipidated form (dHSP70) was not effective. Since HSP70 binds sulfogalactosylceramide 9, monocytes were incubated with dHSP70 recombined with this ceramide derivative. Complexes of sulfogalactosylceramide and dHSP70 had the same effect on clustering of CD11b and CD14 as native HSP70 (Fig. 2b), while sulfogalactosylceramide alone was not effective (Fig. 2a).
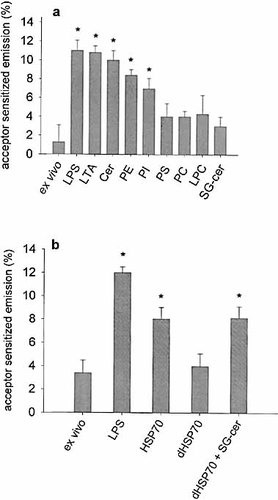
Alternative agonists inducing co-assembly of CD11b and CD14. Effects of (a) LPS, lipoteichoic acid (LTA), ceramide (Cer), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylcholine (PC), lyso-phosphatidylcholine (LPC) and sulfogalactosylceramide (SG-cer) and (b) heat shock protein 70 (HSP70), delipidated HSP70 (dHSP70) and SG-cer + dHSP70 on co-assembly of CD11b and CD14. Energy transfer values were significantly different from unstimulated controls at p<0.05 (*). Data are the mean ± SD of ten experiments.
CD14 has been shown to be associated with lipid-enriched low density domains resembling rafts in monocytic THP-1 cells 12. This was further analyzed by modification of plasma membrane cholesterol composition with cyclodextrins 21. Cholesterol loading led to decreased expression of CD14 and CD11b (Fig. 3a) and completely inhibited the LPS induced co-assembly of CD14 and CD11b (Fig. 3b). A relatively milder cholesterol depletion, in contrast, did not affect the expression densities of CD14 and CD11b. The co-association of both receptors upon stimulation with LPS or ceramide, however, was significantly reduced. Finally, raft specific staining (Fig. 3c–e) revealed that cholesterol loading strongly increased the size and number of rafts while cholesterol depletion did not affect raft structure.
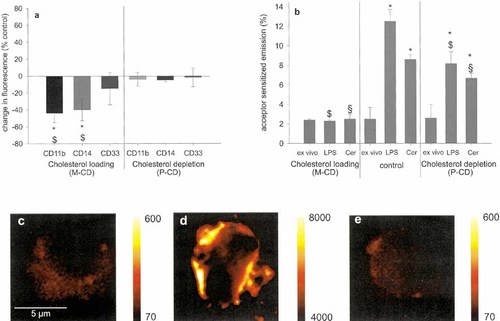
Effect of modifications of raft structure on the CD14/CD11b complex. (a) Expression density of receptors was analyzed upon cholesterol loading (methyl-cyclodextrin/ M-CD) or depletion (propyl-cyclodextrin, P-CD) relative to the untreated control. *, significantly different from controls; $, significantly different from CD33 (p<0.05, each); mean ± SD of five experiments. (b) Effect of cholesterol modification using cyclodextrins on LPS and ceramide induced co-assembly of CD11b and CD14. *, significantly different from unstimulated controls; $, significantly different from LPS; §, significantly different from ceramide; mean ± SD of five experiments. (c) Visualization of rafts on human monocytes using the raft specific lipid probe DMPE-Cy5. (d) Effect of cholesterol loading and (e) cholesterol depletion on raft structure.
2.2 Characterization of surface receptors in the proximity of CD14
Because large signaling receptor complexes with numerous protein components have been reported for example for T cells 22, we now investigated the sterical association of further receptors with CD14 on LPS- or ceramide-activated monocytes. In resting cells, CD14 was found to be clustered with the GPI-R decay accelerating factor (CD55), a complement regulatory protein, the Fcγ-receptors CD32 and CD64, and with the integrin associated protein CD47 (Table 1). LPS stimulation, in comparison, induced clustering of CD14 with CD11b and CD18, TLR-4, the Fcγ-receptor CD16a and the scavenger receptor CD36. CD47 was no longer associated with CD14 following LPS stimulation but the tetraspanin CD81 appeared in the complex. LTA induced the clustering of the same receptor complex components as LPS.
The ceramide-induced cluster differs from that induced by LPS and includes: CD14, CD11b/CD18, CD55, CD47, CD32 and CD64, and CD36 (Table 1). Complexes of sulfogalactosylceramide and HSP70 induced a receptor cluster which was similar to that induced by ceramide.
|
a |
ETp (%) |
control |
LPS |
LTA |
Cer |
HSP70 |
|---|---|---|---|---|---|---|
|
|
CD11b / CD14 (x 8) |
2.1 ± 0.5 |
11.0 ± 0.5* |
10.8 ± 0.7* |
10.0 ± 0.8* |
8.0 ± 1.0* |
|
|
CD18 / CD14 (x 8) |
2.4 ± 2.1 |
9.3 ± 1.1* |
6.0 ± 2.1* |
5.4 ± 0.8* |
6.0 ± 2.1* |
|
|
CD36 / CD14 (x 8) |
3.8 ± 0.8 |
9.9 ± 0.9* |
6.7 ± 0.9* |
10.1 ± 1.0* |
8.2 ± 1.8* |
|
|
TLR-4 / CD14 (x 8) |
2.2 ± 0.6 |
9.1 ± 0.6* |
7.8 ± 2.3* |
3.2 ± 2.5 |
5.4 ± 0.6 |
|
|
CD81 / CD14 (x 8) |
1.8 ± 0.5 |
7.3 ± 0.5* |
6.0 ± 1.4* |
2.8 ± 0.7 |
3.8 ± 2.5 |
|
|
CD55 / CD14 (Uchm1) |
6.6 ± 2.5 |
8.5 ± 2.0 |
7.2 ± 2.0 |
9.7 ± 1.5 |
6.0 ± 0.5 |
|
|
CD32 / CD14 (Uchm1) |
7.8 ± 1.5 |
6.5 ± 3.5 |
6.0 ± 2.0 |
11.6 ± 3.4 |
5.3 ± 2.1 |
|
|
CD64 / CD14 (Uchm1) |
9.4 ± 2.5 |
10.0 ± 2.2 |
7.5 ± 1.8 |
10.4 ± 1.3 |
10.1 ± 1.5 |
|
b |
||||||
|
|
CD47 / CD14 (Uchm1) |
10.5 ± 1.0 |
4.0 ± 0.9* |
5.8 ± 0.9* |
11.9 ± 3.1 |
10.2 ± 1.5 |
|
|
CD16a / CD14 (Uchm1) |
0.5 ± 2.4 |
24.1 ± 4.2* |
35.7 ± 3.2* |
3.2 ± 5.6 |
0.5 ± 1.0 |
- a) Monocytes were incubated with LPS (40 ng / ml), LTA (1 μg / ml), ceramide (40 nM) and HSP70 (7 nM) and labeled with mAb. CD14 clones were selected based on the highest transfer upon stimulation with LPS. Data are mean ± SD of five independent experiments. Energy transfer values were significantly different from controls at p < 0.05 (*).
Thus, LPS, LTA, ceramide, and sulfogalactosylceramide/HSP70 induce the partitioning of membrane receptors into two different clusters, despite ligation of the same receptor, CD14. As control for unspecific Fc-receptor binding, energy transfer was measured between CD16a, CD32, CD64 and irrelevant (non-)monocytic monoclonal antibodies (CD3, Vß8, CD25 and CD91). Furthermore, stimulation of cells with fMLP or PMA as a control did not lead to a significant energy transfer between any pair of the antibodies (data not shown).
The different patterns of receptor co-clustering which are detected by FRET in LPS- or ceramide-stimulated monocytes may represent both minor changes in the intermolecular distances of receptors within a given membrane microdomain as well as lateral sorting of receptors between membrane compartments. Therefore, rafts were isolated from monocytes as Triton X-100-insoluble complexes that float to a low density (0.65 and 0.5 M sucrose) during density gradient centrifugation 17. Both CD11b and CD14 floated in these low-density fractions independent of the activation state of monocytes (Fig. 4). CD16a, however, was detected in these compartments only upon stimulation with LPS.
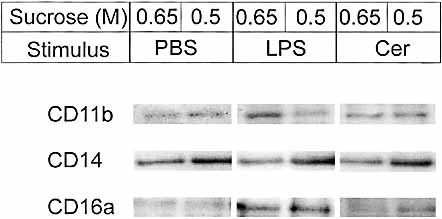
Western blot analysis of receptors in the vicinity of CD14. Effects of LPS (40 ng/ml) and ceramide (40 nM) on association of CD11b, CD14 and CD16 with lipid rafts. Density gradient fractions (0.65 and 0.5 M sucrose) were analyzed by Western blot of cell lysates.
2.3 Co-assembly of monocyte receptors with CD14 in inflammatory diseases
CD14 activation plays a role in the pathogenesis of infectious diseases and other inflammatory diseases such as coronary artery disease (CAD). Therefore, FRET analysis was performed on monocytes from patients suffering from sepsis, stroke and CAD (Table 2a). Monocytes from all patients but not the control group showed a co-association of CD11b and CD14 without prior in vitro stimulation.
While LPS represents a candidate for monocyte activation in sepsis, it is unlikely to interact with monocytes in the two groups of patients with inflammatory disorders of the vessel wall which are linked to disturbances in lipid metabolism. Since atherogenic lipoproteins have been reported to contain elevated levels of ceramide 23, plasma ceramide levels of patients and controls were determined by tandem mass spectrometry. Significantly different plasma concentrations of the ceramide species C22, C23, C24:0, and/or C24:1 were found in CAD, stroke and sepsis patients as compared to controls (Table 2b). Sepsis showed the most pronounced alteration in ceramide species with elevated C24:1 and C22, and decreased C24:0 and C23 levels.
|
a |
|
Control |
Sepsis |
CAD |
Stroke |
|---|---|---|---|---|---|
|
|
|
n = 20 |
n = 7 |
n = 20 |
n = 7 |
|
|
Stimuli |
|
|
|
|
|
|
Unstimulated (ETp (%)) |
1.4 ± 0.5 |
7.7 ± 1.2* |
7.9 ± 0.6* |
13.3 ± 3.2* |
|
|
LPS (ETp (%)) |
12.1 ± 0.5* |
9.3 ± 0.8 |
9.9 ± 0.6 |
15.2 ± 3.0 |
|
b |
|||||
|
|
Plasma ceramice (μM) |
|
|
|
|
|
|
C24 : 0 |
2.9 ± 0.6 |
2.1 ± 0.8* |
3.0 ± 0.8 |
3.0 ± 0.5 |
|
|
C24 : 1 |
1.6 ± 0.5 |
3.0 ± 0.9* |
2.3 ± 0.6* |
2.3 ± 0.3* |
|
|
C23 |
0.8 ± 0.2 |
0.5 ± 0.1* |
0.8 ± 0.3 |
1.0 ± 0.3* |
|
|
C22 |
0.8 ± 0.2 |
1.1 ± 0.2* |
0.9 ± 0.2 |
1.0 ± 0.2 |
|
|
C16 |
0.7 ± 0.6 |
1.1 ± 0.3 |
0.8 ± 0.2 |
0.7 ± 0.1 |
- a) (a) Co-assembly of CD11b and CD14 was analyzed with or without stimulation with LPS (40 ng / ml). The mean energy transfer efficiencies for all monocytes are expressed in terms of emission of Cy5 excited by 488 nm (ETp acceptor sensitized emission, equation 1). Energy transfer values of LPS-stimulated control samples were significantly different from unstimulated control samplesat p < 0.05 (*). Energy transfer values of unstimulated patient samples were significantly different from unstimulated control samples at p < 0.05 (#). (b) Plasma ceramide levels were quantified by tandem mass spectrometry. Differences were significantly different from controls at p < 0.05 (*).
Finally, the receptor clusters on monocytes in patients with sepsis and CAD were analyzed in more detail. Based on a cell-by-cell determination of FRET efficiency, co-assembly of CD11b and CD14 was observed on the majority of monocytes of patients with sepsis and CAD (Fig. 5a). Next we measured the co-association of CD81 with CD14 which had differed in vitro between LPS and ceramide stimulation. A co-assembly of CD81 and CD14 was only observed in the sepsis patients but not in CAD patients (Fig. 5b).
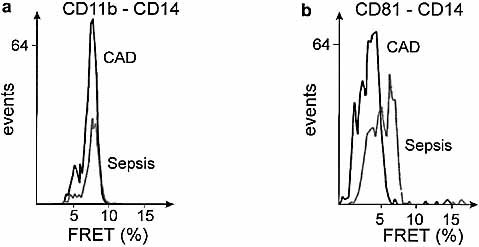
Patterns of receptor-co-association in patients with sepsis and CAD. Ex vivo washed monocytes from patients with sepsis and CAD were labeled with monoclonal antibodies. (a) Co-assembly of CD11b and CD14. (b) Co-assembly of CD81 and CD14. Energy transfer values are given individually for more than 300 cells (sepsis) and more than 600 cells (CAD), respectively, as a one-parameter histogram obtained by conversion according to equation 1.
3 Discussion
Here we characterized the interaction of CD14 with multiple ligands based on its sterical interaction with co-receptors within rafts. Upon stimulation of monocytes with LPS or membrane impermeable long chain ceramide we detected increased proximity of CD11b and CD14 similar to that described for neutrophils 18. A specificity of this response for the interaction of either ligand with CD14 was shown with compound-406 19 which selectively blocks CD14-dependent LPS-induced signaling and by specific binding of 14C-labeled ceramide to CD14 transfected cells. A number of further monocyte receptors which play an important role in the innate immune response were differentially clustered to CD14 in either LPS- or ceramide-activated cells (Fig. 6). The modulation of rafts based on cholesterol loading indicates that an intact raft structure is the basis of the formation of these different receptor clusters upon LPS or ceramide stimulation. Finally, based on the isolation of rafts, a selective partitioning of receptors into these membrane domains was identified as an underlying mechanism. The precise mechanisms that induce the formation of these receptor clusters are unknown. The demonstration of a ligand-specific response initiated by CD14, however, has important implications for the role of rafts in cellular signaling as well as the mechanisms involved in initiating an immune response.
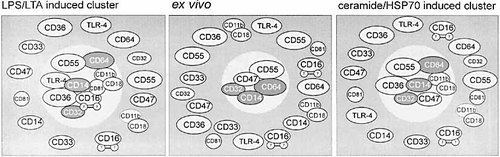
CD14 receptor complexes on human monocytes. Illustration of the receptor complexes on human monocytes induced by LPS, LTA, ceramide or sulfogalactosylceramide/HSP70.
After stimulation with LPS not only the β2-integrin CD11b/CD18 was associated with CD14, but also the integrin associated protein CD81, the Fcγ-R CD16a, the scavenger receptor CD36, and the LPS-signal transducer TLR4 (Table 1, Fig. 6). Interestingly, the cluster induced by ceramide differed by the absence of CD16a, TLR4 and CD81 but presence of CD47 as a different integrin associated protein. Expression of TLR4 has been shown to be associated with CD14-dependent LPS signaling 13 and clustering of CD14 and TLR4 upon LPS stimulation further supports the functional significance of TLR4 for LPS-dependent signal transduction 15. LPS induced signal transduction, however, is far more diverse than signaling mediated by TLR4 alone. Therefore, it is tempting to speculate that additional components of the LPS-induced cluster participate in LPS signaling. In this context, CD16a is known to induce signaling pathways as well as cellular effects similar to those observed after LPS treatment (for review see 24). The tetraspan CD81, via a selective interaction with α4β1-integrins as well as the formation of multimolecular complexes with other members of the tetraspanin family 25 may play an important role in functionally coupling further membrane proteins. Interestingly, complexes of tetraspanins with integrins were recently found to associate with lipid rafts confirming our observations 26. The pentaspan transmembrane protein CD47 that was shown to associate with CD14 in resting but not LPS-activated cells may also exert an important function in CD14 dependent activation. As an integrin associated protein with specificity for β3-integrins, the function of CD47 was shown to depend on raft association 27.
A variety of non-LPS ligands of CD14 have been identified 1. In our experiments, in addition to LPS and ceramide, sulfogalactosylceramide complexed to HSP70, phosphatidylinositol, phosphatidylethanolamine and lipoteichoic acid induced assembly of CD14 and CD11b. The clusters induced by ceramide or sulfogalactosylceramide/HSP70 were similar and differed from those observed after LPS stimulation (Fig. 6). In contrast, the gram-positive bacterial compound LTA induced a receptor complex similar to LPS. This suggests that multiple ligands signal through CD14 into a limited number of alternative pathways of cellular activation.
Interestingly, analysis on a single-cell basis also revealed that different patterns of CD81-coassociation similar to those induced by LPS or ceramide can be observed in patients with sepsis and myocardial infarction suggesting that different mechanisms of CD14 ligation are also relevant in vivo. This analytical approach seems promising for a more detailed analysis of patterns of receptor activation in inflammatory disease. The increased ceramide plasma levels in all groups of patients with inflammatory processes analyzed in this study might point to the stimulus initiating the receptor clustering. The finding that healthy controls alsoexhibit high plasma ceramide concentrations raises the possibility, that ceramide exists in an active or inactive form similar to LPS. This might be due to its lipoprotein association. A potential role of extracellular ceramide as a modulator of immune function in atherogenesis is supported by the finding of increased ceramide levels in atherosclerotic lesions 23.
Independent of the potential role of ceramide as a soluble modulator of immune function in inflammation, the interaction between ceramide and CD14 might be involved in another physiological context. It is interesting to note that CD11b as well as CD36 are involved in the phagocytosis of apoptotic cells. This process depends on the recognition of an as yet undefined ligand exposed by apoptotic cells which interacts with a domain of CD14 that is identical to the LPS binding site 28. Since ceramide is known to accumulate in membranes of apoptotic cells, it is conceivable that ceramide acts as a ligand for CD14. Interestingly, the interaction of CD14 with apoptotic cells in contrast to LPS does not result in a production of proinflammatory cytokines supporting the concept of differential signaling through this receptor 28. At this point, it remains to be established by which mechanisms LPS and ceramide induce a differential recruitment of receptors into a receptor cluster in rafts despite their binding to the same receptor. Interestingly, the multimerization of receptors into clusters known as capping has been shown to involve a cytoskeletal-based mechanism 29, wherever the reorganization within membrane microdomains of the receptors in our experiments depends on similar mechanisms is unclear. The differential co-association and most likely functional cooperation of the GPI-R CD14, however, with its co-receptors seems to represent an important mechanism for regulating a diversity of cellular signals through a limited number of receptors.
4 Methods
4.1 Blood samples
Heparinized blood samples were obtained from healthy volunteers. After informed consent and with the approval of the local Ethical Committee (No. 00/33), blood samples were also obtained from patients with sepsis, coronary artery disease and stroke. The diagnosis of sepsis was based on the consensus criteria published by the American College of Chest Physicians and the Society of Critical Care Medicine. The diagnosis of infection was based on the criteria of the Center of Disease Control, Atlanta, GA. Coronary artery disease (CAD) was defined by coronary angiography and blood samples were obtained the day following acute myocardial infarction or during angina. Blood samples of patients with acute stroke were obtained within 12 h after infarction. All patients presented middlecerebral artery territory infarction proven by cranial computer tomography. Blood samples of all patients were analyzed for LPS in a chromogenic limulus amebocyte lysate (LAL) assay (QCL-100, BioWhittaker, Walkersville, MD). Half of the sepsis patients showed a positive result whereas all other samples were negative for LPS.
4.2 Preparation of phospholipid liposomes
Phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylinositol (PI) and phosphatidylserine (PS) were obtained from Sigma (Deisenhofen, Germany) as chloroform solutions. Lipids were dried under vacuum and resuspended in Dulbecco's modified phosphate buffer saline without Ca2+ and Mg2+ (PBS). Lipid content was determined by thin layer chromatography.
4.3 Delipidation of HSP70
HSP70 was obtained from Stressgen (Victoria, Canada). HSP70 (200 μg) was delipidated in ethanol/diethylether (3:1) at –20°C overnight. After centrifugation (15 min at 1,000 g) the pellet was delipidated in diethylether at –20°C for 4 h. After centrifugation (15 min, 1,000) delipidated HSP70 (dHSP70) was transferred into 50 nM Tris-HCl pH 7.5, 100 mM NaCl, 1 mM dithiothreitol and 0.1 mM phenylmethylsulfonyl fluoride.
4.4 Stimulation of blood samples
Whole blood aliquots (100 μl) were incubated for 15 min at 37°C with the following stimulatory compounds: LPS from Salmonella minnesota (1–100 ng/ml), ceramide containing primarily stearic and nervonic acids (Cer, 1 nM – 0.5 μM); lysophosphatidylcholine (10 μM); phorbol 12-myristate 13-acetate (PMA, 1 μM); N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP, 1 μM); PE, PC, PI and PS-liposomes (10 μM) prepared as described above. All reagents were obtained from Sigma. Cells were also incubated with LTA (1 μg/ml), HSP70 (7 nM), dHSP70 (7 nM) and sulfogalactosylceramide (Sigma, 40 nM) together with dHSP70 (7 nM). LTA (bacillus subtilis) was prepared as described before 30. After incubation the cells were washed with cold PBS containing 0.1% NaN3. The synthetic LPS antagonist compound-406 (1–400 ng/ml) was kindly provided by Dr. Shoichi Kusumoto. The cells were preincubated with the LPS antagonist for 15 min at 37°C and then stimulated with LPS or ceramide without washing. All compounds despite LPS used for cellular stimulation were negative for LPS in the LAL assay.
4.5 Inhibition of LPS internalization
Whole blood was incubated with cytochalasin D (Sigma, 3 μM) for 30 min at 37°C, then further stimulated with LPS (40 ng/ml) and ceramide (40 nM) as described and the energy transfer between CD14 and CD11b was measured. Cells were also further incubated with FITC-LPS (Sigma, 400 ng/ml) and internalization was measured by flow cytometry using trypan blue as quencher of extracellular fluorescence.
4.6 Binding of [14C]N-palmitoyl-D-sphingosine to CHO-cells
CHO cells transfected with human CD14 or with pPOL-DHFR vector were cultured and prepared for labeling as previously described 31. CHO-cells (4×105) were preincubated on ice in the presence of a 50-fold excess of unlabeled N-Palmitoyl-D-Sphingosine (Sigma) for 15 min. Then [14C]N-palmitoyl-D-sphingosine (American Radiolabeled Chemical Inc., St. Louis, MO) was added and cells were incubated for another 15 min. Cells were washed and the pellet was dissolved in 100 μl of 1% SDS, 10 mM EDTA and added to 3 ml of scintillation fluid. Cell-bound radioactivity was determined by liquid scintillation counting.
4.7 Immunostaining of cells
Stimulated or control washed whole blood samples (200 μl) were incubated for 15 min on ice with saturating concentrations of the fluorochrome conjugated or biotinylated mAb as shown in Table 3. Similar results were obtained for both antibodies against CD81. Lysis of erythrocytes and washing were performed as described 3. After the last washingstep, samples were divided and one part of each sample was incubated with a saturating amount of SA-Cy5 (0.6 μg/ml) for 15 min on ice and washed before flow cytometric measurement. For analysisof receptor co-association by confocal multiparameter microscopy, lysed cells were seated on borosilicated coverslips (Nunc, Wiesbaden, Germany).
|
Reagent |
Clone |
Label |
Source |
|---|---|---|---|
|
CD16a |
3G8 |
R-PE |
Coulter / Immunotech, Krefeld, Germany |
|
CD18 |
7E4 |
R-PE |
|
|
CD14 |
RM052 |
R-PE |
|
|
CD33 |
My9-RD1 |
R-PE |
|
|
CD81 |
Js64 |
R-PE |
|
|
CD25 |
B1.49.9 |
Biotin |
|
|
CD11b |
D12 |
R-PE |
BD, Bioscience, Heidelberg, Germany |
|
CD36 |
CB38 |
R-PE |
|
|
CD47 |
B6H12 |
R-PE |
|
|
CD81 |
JS-81 |
R-PE |
|
|
CD55 |
IA10 |
R-PE |
|
|
Vβ8 |
JR2 |
Biotin |
|
|
CD14 |
Uchm1, x8 |
Biotin |
Dianova, Hamburg, Germany |
|
CD3 |
Cris-7 |
Biotin |
|
|
CD91 |
8G1 |
Biotin |
Progen, Heidelberg, Germany |
|
CD32 |
C1KM5 |
R-PE |
Caltag, Burlingame, CA |
|
CD64 |
10.1 |
R-PE |
|
|
anti-TLR4 |
HTA125 |
Pure |
Provided by Dr. Kensuke Miyake |
|
Streptavidin |
|
Cy5 |
Dianova |
|
anti-IgG2a |
|
PE |
Southern Biotechnology Associates, Birmingham, AL |
4.8 Modifications in the raft structure
Mononuclear cells were isolated from blood by Histopaque 1077 (Sigma) density gradient centrifugation. Methylated β-cyclodextrin (Sigma) was used for cholesterol loading of the cell membrane 21. Aliquots of mononuclear cells containing 5×105 cells/μl were re-suspended in 3 ml of PBS and incubated for 30 min at 37°C with complexes of cholesterol (100 μg/ml) and methyl-β-cyclodextrin (4.6 mg/ml) prepared as described 21. Cells were incubated with 2-hydroxy-propyl-β-cyclodextrin (Sigma, 0.15 mg/ml) for 30 min at 37°C to deplete cholesterol. After incubation with cyclodextrins the cells were washed, immunostained as described above, and the expression of the antigens was measured by flow cytometry. For energy transfer experiments 200-μl aliquots of whole blood were treated with cholesterol/methyl-cyclodextrin (C/C) (100 μg/ml / 4.6 mg/ml) or 2-hydroxy-propyl-β-cyclodextrin (0.15 mg/ml) at 37°C for 30 min, then stimulated with LPS (40 ng/ml) or ceramide (40 nM) as described and the energy transfer between CD14 and CD11b was measured. For microscopic determination of raft size and structure, monocytes from healthy volunteers were isolatedby leukapheresis followed by elutriation. Cells were cultured on borosilicated coverslips (Nunc, Wiesbaden, Germany) in macrophage SFM medium (Gibco BRL, Karlsruhe, Germany) overnight. Cells were loaded with cholesterol or cholesterol was depleted for 12 h as described above.
4.9 Measurement of FRET by flow cytometry
A dual-laser FACSCalibur flow cytometer and Cellquest software (Becton Dickinson, San José, CA) were used for the measurements. Measurements were performed without compensation. 50,000 leukocytes were acquired in list mode using forward scatter (FSC) as the triggering signal.
4.10 Determination of FRET efficiency (acceptor sensitized emission or donor quenching)
The principle of FRET first described by Förster in the late 1940s allows the measurement of distances between surface molecules 32. The energy transfer parameter (ETp), which is proportional to FRET efficiency (ET), was calculated according to (1) where A is acceptor, D is donor, FL2 is mean fluorescence in channel 2 (488 → 530 nm) (donor, R-PE), FL3 is mean fluorescence in channel 3 (488 → 585 nm), FL4 is mean fluorescence in channel 4 (633 → 670 nm, acceptor, Cy5) (each value obtained after autofluorescence subtraction):
ETp = FL3(D,A) – FL2(D,A)/a – FL4(DA)/b FL3(D,A) (1)
a = FL2(A)/FL3(A)
b = FL4(D)/FL3(D)
FRET efficiency (ET) was calculated from quenching according to (2):
ET = FL2(D) – FL2(D,A) FL2(D) (2)
According to the publication by Szöllösi et al. 33 an ET = 5% was defined as the threshold level for significant transfer efficiency in preliminary experiments. This level was experimentally confirmed as the detection limit for the proportional ETp value (data not shown).
4.11 Measurement of plasma ceramide levels
Plasma ceramide levels were quantified by Tandem mass spectometry as described previously 34. The measurement was performed with an API 365 triple quadrupol system (Perkin Elmer, Norwalk, CT) equipped with a turbo ion spray interface.
4.12 Microscopic characterization of raft structure
For analysis of raft structure samples were labeled with 50 μg/ml raft-specific 1,2-dimyristoyl-sn-glycero-3-phospho-ethanolamine)-Cy5 (DMPE-Cy5) and analyzed in epi-illustration (Axiovert 135TV, Zeiss, Jena, Germany) as described earlier 16.
A TCS 4D inverted confocal laser scanning microscope (Leica Lasertechnik, Heidelberg, Germany), with an argon-crypton mixed ion laser was used for multiparameter cell analysis. Monocytes were selected according to positive CD14-expression. Overlay images were generated after separate analysis of R-PE (CD11b) and Cy5 (CD14) dependent fluorescence.
4.13 Cell harvesting
For raft-specific microscopy and western blot analysis peripheral blood monocytes from healthy volunteers were separated by leukapheresis as described above and cultured in ultra-low attachment plates (Corning, Wiesbaden, Germany) in macrophage SFM medium over night and stimulated with LPS (40 ng/ml) or ceramide (40 nM) in the presence of 5% human serum for 15 min at 37°C. Cells were washed twice in 50 ml ice-cold TNE-buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 5 mM EDTA and 1 mM PMSF) and pelleted at 500 g for 5 min.
4.14 Detergent lysis and sucrose flotation gradients
Triton X100 lysis and sucrose density gradient floatation was performed as described 17 using a SW55Ti ultracentrifuge (Beckman/Coulter/Immunotech).
4.15 Immunoblotting
Immunoblotting was performed using the ECL plus detection system (Amersham Pharmacia Biotech, Braunschweig, Germany). The following antibodies were used: anti-CD14 (goat polyclonal, clone N-15, Santa Cruz, Heidelberg, Germany), anti-CD11b (goat polyclonal, clone C-19, Santa Cruz), anti-CD16a (mouse monoclonal, clone 3G8, Dianova).
4.16 Statistical analysis
Data are given as mean ± standard deviation (SD). For statistical comparison Wilcoxon signed-ranks test was used. (*) designates p<0.05.
Acknowledgements
This work was supported in part by a joined grant from Deutscher Akademischer Austauschdienst and Magyar Ösztöndij Bizottság and the Deutsche Forschungsgemeinschaft collaborative research group on macrophages (AN 111/6–6 project 5). A.J.U. and H.H. were supported by Deutsche Forschungsgemeinschaft (SFB 367, project C5). We thank R. Görlich for experttechnical assistance, Kensuke Miyake (Saga Medical School, Nabeshima, Japan) for providing the anti-TLR4 monoclonal antibody and Shoichi Kusumoto (Osaka University, Japan) for providing the LPS antagonist, compound-406.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




