Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection
Abstract
The role of intrahepatic lymphocytes in the control of hepatitis C virus (HCV) infection and the pathology associated with it is not understood; most studies of the immunology of this infection use peripheral blood lymphocyte populations. To address this further, we examined in detail the IHL from HCV-infected patients and controls, focusing on the antigen-specific CD8+ T lymphocyte component. Individual T cells from needle liver biopsies and peripheral blood were isolated from patients with chronic HCV infection and examined directly ex vivo. We used RT-PCR spectratyping to compare the breadth of the T cell receptor usage in the liver in comparison with the peripheral blood, and applied MHC class I tetramer technology to investigate the numbers of HCV-specific CD8+ cells in the two compartments. T cell receptor usage in the liver of HCV-infected patients was broad, comparable with that in the peripheral blood of the same patients. A much higher proportion of liver CD8+ cells expressed receptors specific for HCV antigens compared with paired peripheral blood CD8+ cells. A greater proportion of the liver tetramer-positive cells expressed the activation marker CD69, compared with those in the periphery or other CD8+ cells in the liver. In the course of chronic HCV infection, HCV-specific CD8 cells, which have been recently activated, appear to accumulate specifically in the livers of infected patients but are present in much lower numbers in the peripheral circulation. Further studies are needed to determine the function of these cells and their role in protection and immunopathology.
Abbreviation:
-
- HCV:
-
Hepatitis C virus
1 Introduction
Hepatitis C virus (HCV) infection is an important cause of chronic liver disease. Up to 80 % of HCV infections become chronic and infected individuals may develop liver disease, including cirrhosis and hepatocellular carcinoma. HCV is now the most common cause of chronic viral hepatitis in the developed world and the most common cause of cirrhosis requiring liver transplantation 1.
The immune system plays an essential role in the control and elimination of acute viral infections and there is evidence that vigorous and rapid cellular immune responses with broad specificity can control and, under certain circumstances, clear persistent viruses such as HIV 2 and HBV 3. The situation during HCV infection is less clear. T cells may contribute to control of the virus 4, 5, but as has been shown for LCMV 6, 7, they may also cause hepatic disease 4, 5. T cells from both the peripheral blood and liver of infected patients can be expanded 8, 9; however, such studies may identify only a small proportion of T cells selected on the basis of retention of their proliferative and functional capacity in vitro. In addition, studies relying on in vitro expansion of T cells do not allow direct comparison of the numbers of cells with specificity for HCV in the peripheral and liver compartments. More recently, the advent of tetrameric MHC-peptide complexes, which can be used to determine the specificity of the antigen-specific receptor of T cells in combination with other phenotypic markers 10, 11, has allowed more direct quantitation of virus-specific T cells in infected patients.
In acute HCV infection, strong peripheral T cell responses can be detected 12, 13; however, such responses are frequently not sustained 14 and are more difficult to detect during the chronic phase of the disease 8, 9. It is not clear if this is due to a down-regulation of the immune response to HCV, which could help to explain the persistence of the virus, or compartmentalization of the cells to, for example, the liver, where they may be responsible for the damage associated with the infection. Characterization of the intrahepatic T cell response is, therefore, likely to be extremely important in understanding the mechanisms resulting in chronic HCV infection and the underlying liver pathology. There have been very few studies on liver-derived cells in HCV infection using MHC tetramers, and the data generated are not clear: in one study a relatively high frequency of HCV-specific T cells was found in the liver of two HCV-infected patients 15, but in another there was no evidence of such T cells in six HCV-infected liver samples 16. We have isolated individual cells from small amounts of tissue from needle liver biopsies and from peripheral blood of patients chronically-infected with HCV and provide a direct comparison of the T cells in these two compartments in terms of their specificity and TCR gene usage.
2 Results
2.1 Investigation of the diversity of the T cell repertoire within the liver
T cells from liver biopsies and paired peripheral blood cells from six HCV RNA+ patients were enriched using anti-CD3-labeled magnetic beads. RNA isolated from these cells was analyzed for variability in the size of the Vβ-Cβ region. Such variation arises from differences in D and J region usage and junctional diversity arising during recombination of the Vβ gene. The Vβ profiles for each of the patients are shown in Fig. 1. For each patient, the data for two independent PCR from liver cDNA and one PCR from peripheral blood cDNA are shown. In all but one of the patients both samples show variable Vβ profiles, comparable with that found in the peripheral blood of the same patients. In the remaining patient (75) one sample had only one peak and the other had limited variability in terms of numbers of peaks. Such variability is likely to be due to sampling errors as a result of sampling from a single liver site.

Vβ profiles of CD3 cells from liver and peripheral blood samples. Data for six paired liver and peripheral blood samples from HCV-infected patients are shown. For each liver sample the data for two independent PCR are shown, labeled as liver 1 and liver 2, respectively.
2.2 Quantitation of HCV-specific intrahepatic CD8+ T cells
Cells were dissociated from the liver biopsies of six HCV-infected, HLA-A2 patients and also prepared from paired peripheral blood samples. Data relating to these patients at the time of biopsy are given in Table 1. The cells were stained with a pool of HCV-specific HLA-A2 tetramers (Table 2) and markers for CD8 and CD69. The results are shown in Fig. 2. The quadrants were set based on the results obtained with 2 HCV-negative biopsies and one biopsy from an HCV+ patient who was not of the HLA-A2 genotype. In three of the patients (250, 261 and 344) a clear population of tetramer+ cells can be seen in the liver CD8+ populations. In a fourth patient (263) a small number of tetramer+ cells were seen in the liver; these did not form a clear cluster of cells, but were considered to be positive since they lie within the region of the graph where tetramer+ cells are found. The remaining two livers (251 and 87) appear to be negative; while some events did fall within the upper two quadrants, the majority of these were outside the area expected for tetramer+ cells. In contrast, the peripheral blood samples had much lower numbers and percentages of tetramer+ cells. Only one patient (261) showed a clear population of tetramer+ cells in the periphery.
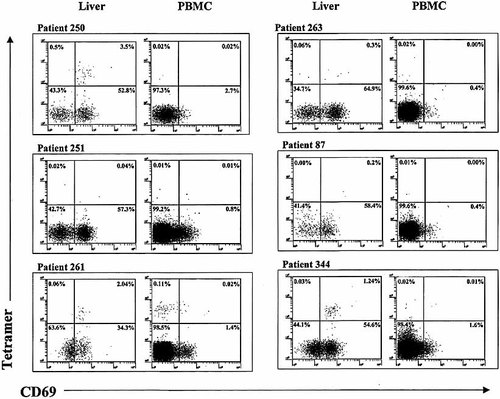
Analysis of the specificity of liver and peripheral blood CD8+ cells using HCV-specific tetramers. Data for six paired liver and peripheral blood (PBMC) samples from HCV-infected patients of the HLA-A2 genotype are shown. Cells were gated on the basis of CD8 expression and side scatter properties; staining with CD69 and HCV-specific HLA-A2 tetramers is shown. The percentages of cells in each quadrant are indicated.
The tetramer and CD69 data for liver and paired peripheral blood-derived T cells are summarized in Table 1. In general, the proportion of peripheral CD8+ cells which are tetramer+ is very low, even in those patients in whom there is a significant proportion of tetramer+ cells in the liver. There was a significant difference between the percentage of tetramer+ cells in the liver-derived and peripheral populations (p < 0.05, Wilcoxon matched pairs test). In the one patient with a clear peripheral population of tetramer+ cells the corresponding frequency in liver-derived cells was approximately 16-fold higher. Of a further eight HLA-A2 chronic HCV RNA+ patients from whom only peripheral blood-derived T cells were tested (data not shown), only one had a significant population of tetramer+ cells (0.04 %).
|
Patient |
Age |
Sex |
ALT |
Knodell |
Liver cells |
Peripheral blood cells |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
at time |
score |
CD8+ |
CD8+ |
tetramer+ |
CD8+ |
CD8+ |
tetramer+ |
|
|
|
|
of biopsy |
of biopsy |
tetramer+ |
CD69+ |
CD69+ |
tetramer+ |
CD69+ |
CD69+ |
|
250 |
39 |
M |
64 |
3 |
4.00 % |
56.3 % |
87.5 % |
0.04 % |
2.7 % |
50.0 % |
|
251 |
34 |
F |
19 |
1 |
0.06 % |
57.3 % |
66.7 % |
0.02 % |
0.8 % |
50.0 % |
|
261 |
47 |
M |
332 |
7 |
2.10 % |
36.3 % |
97.1 % |
0.13 % |
1.4 % |
15.4 % |
|
263 |
47 |
F |
108 |
3 |
0.36 % |
65.2 % |
83.3 % |
0.02 % |
0.4 % |
0.0 % |
|
87 |
35 |
F |
55 |
3 |
0.20 % |
58.6 % |
100.0 % |
0.01 % |
0.4 % |
0.0 % |
|
344 |
43 |
M |
148 |
1 |
1.27 % |
55.8 % |
97.6 % |
0.03 % |
1.6 % |
50.0 % |
- a) Percentages of CD8+, tetramer+ which were considered to be significant are indicated in bold-type.
|
Tetramer |
Peptide sequence |
Derivation of peptide |
|---|---|---|
|
NS3 1073 |
CINGVCWTV |
HCV NS3 1073 – 1081 |
|
NS3 1406 |
KLVALGINAV |
HCV NS3 1406 – 1415 |
|
NS4B 1807 |
LLFNILGGWV |
HCV NS4B 1807 – 1816 |
|
NS5 2594 |
ALYDVVTKL |
HCV NS5 2594 – 2602 |
A large proportion of the total liver CD8+ population was CD69+ with a CD8+/CD69+ to CD8+/CD69– ratio of approximately 2 : 1. Again, this is in contrast with the peripheral blood where very few CD8+ cells were CD69+. The tetramer+ liver cells had a raised proportion of CD69+ cells compared with the total liver CD8+ populatijon (p < 0.05). In the patient (261) with a clear population of tetramer+ peripheral CD8 cells, only 15 % co-expressed CD69 as compared with the same patient's liver-derived tetramer+ cells of which 88 % co-expressed CD69.
3 Discussion
Our data provide a direct comparison of the breadth and specificity of the intrahepatic and peripheral T cells in patients with chronic HCV infection. It has been suggested that an immune response with broad specificity is likely to be most effective in viral clearance. Therefore, we firstly investigated the variability of the TCR Vβ usage in CD3+ cells from the liver, in comparison with peripheral blood from the same patients, by RT-PCR. The Vβ usage in the liver-derived CD3+ cells was comparable to that found in the periphery in the same patients, in spite of the potential for the Vβ profiles of the liver-derived cells to appear clonal due to sampling from a single liver site. Our results are unlikely to be due to contamination with peripheral blood PBMC since other characteristics of the liver cells contrast markedly with those of peripheral cells from the same patients. These data are in broad agreement with previous studies 17, 18 which showed that while there may be some biases in TCR usage at a detailed level, the overall profile is not clonal. The characteristics of the liver-derived T cells are those of classical T cells, with expression of α β TCR and no obvious bias towards particular Vβ usages. We cannot rule out that non-classical T cells, such as the NKT cells ob-served in normal 19 and HCV-infected liver 20 are also present, but since such cells tend to have restricted Vβ as well as Vα usage, they are likely to be in the minority. Direct studies of the Vβ usage of tetramer+ cells in the periphery by antibody staining and flow cytometric analysis has, similarly, not revealed large clonal expansions in this antigen-specific population (Klenerman et al., unpublished data).
Analysis of the Vβ usage of T cells in the liver gives some idea of the breadth of the T cell population. However, direct analysis of the fine specificity of CD8+ cells has become possible with the development of tetrameric MHC class I molecules refolded with specific peptides in their peptide-binding grooves 10, 11. Class II tetramersare being developed 21 but such reagents displaying HCV-derived peptides are not yet available. We found clear populations of cells binding HCV-specific tetramers in three out ofsix of the HCV-infected HLA-matched livers analyzed consisting of a discrete cluster of stained cells, separated from the bulk of the CD8 cells. In each of these livers, the proportion of tetramer+ cells was greater than 1 % of the CD8+ cells, and in one liver it was as high as 4 %. The results described here are in agreement with the findings of He et al. 15 who found 1 % and 2.5 % positive cells in two HCV-infected liver samples. In contrast with our results and those of He et al. 15, Valiante et al. 16 found no tetramer+ cells in six HCV-infected livers, using the same tetramers used in this study. However, the latter study used cells derived from cirrhotic liver explants. The discrepancy in tetramer data between that study and our own raises the possibillity that the nature of the intrehepatic T cell population may change over time as liver disease progresses.
The tetramer+ population measured here is likely to be an underestimate of the total population of intrahepatic HCV-specific cells in the patient, since the cellular response to HCV is thought to be broad 8 and yet we measured the response to only four epitopes of HCV in the context of a single HLA haplotype. In addition, it is possible that HCV-specific T cells which do recognize the epitopes represented by the tetramers used in this study are missed in some patients because the viral variants in those patients have minor differences in sequence within these epitopes 22, 23 so that the fine specificity of the TCR does not perfectly match the tetramers. However, use of the same set of tetramers in the two compartments allows a direct comparison of liver and peripheral blood in the same patients. Such a comparison indicated that HCV-specific cells are significantly less frequent (⩽ 0.13 %) in the periphery. While the tetramer+ cells in the periphery were generally not activated, a large proportion (74 – 93 %) of the tetramer+ CD8 cells in the liver were stained with CD69,a short-term marker of activation, suggesting that these cells had been recently activated, perhaps in the efferent lymph nodes or in the liver itself.
The broad TCR usage of the T cells in the HCV-infected livers suggests that the cells do not arise from a clonal expansion of a small number of progenitor cells within the liver, but either represent a set of peripheral blood cells which accumulate nonspecifically in the liver, or a set of broadly reactive T cells which have expanded elsewhere and home to the liver. The former possibility is unlikely because the cells in the liver have very different characteristics from those of the periphery both in terms of their specificity and their activation state. Their expression of a short-term marker of activation suggests they have been recently activated. It has been shown that activated cells, particularly CD8+ cells, accumulate in the liver where they die by apoptosis 24; however, such a mechanism probably does not apply to cells which have been activated in lymph nodes close to the liver and selected for their liver-homing properties 25, as is likely to be the case with the HCV-specific liver-derived T cells described here. This homing of the cells to the liver may explain the disappearance of the cells from the periphery following the acute stage of infec-tion 14.
Once in the liver, during the chronic phase of disease these activated cells may play a role in keeping viral replication under control. However, in doing so they may damage the liver 6, 7 leading to the progressive inflammatory and fibrosing disease associated with HCV infection 4, 5. It is possible that in end-stage cirrhotic liver disease, the HCV-specific T cells may disappear from the liver as well as the periphery 16. Further studies are needed to address these possibilities in more detail, in particular to measure the capacity of these cells for a functional response to HCV antigens. Correlation of such an analysis with later progression to severe disease and response to treatment may be important in increasing our understanding of the role of the immune response in HCV-related liver disease.
4 Materials and methods
4.1 Source of samples
Needle liver biopsies were obtained from patients enrolled in the Trent HCV Study Group Cohort; all patients are offered a diagnostic liver biopsy, a portion of which is made available for laboratory-based studies with written informed patient consent. Negative-control biopsies were obtained from patients attending clinic for suspected non-HCV-related liver disease. The biopsies suppliedto us were of the order of 1 cm in length and 1 mm in diameter. Peripheral blood samples were also obtained from some of these patients and collected in tubes containing EDTA to prevent clotting.
4.2 Collagenase digests
Liver biopsies were immediately placed in a sterile Eppendorf tube at the bedside and were usually processed within 1 h. Hepes-buffered RPMI (500 μl) containing 5 % fetal bovine serum (FBS) and collagenase Type IV (Sigma) at 100 μg/ml was added, and the samples were incubated at 37 °C on an end-over-end mixer for 10 – 20 min. Immediately before harvesting the cells the sample was vigorously pipetted to improve release of the cells, then large pieces of tissue debris were allowed to settle out. The supernatant was removed and the cells pelleted for 2 min in a microfuge at 6000 rpm. The pelleted cells were washed in RPMI-5 %FBS, while the undigested tissue debris was further treated with collagenase. This process was repeated up to eight times, then cells harvested from each round were pooled.
4.3 Enrichment of CD3+ cells using Dynabeads
CD3 cells were enriched using Dynabeads (Dynal). The ratio of anti-CD3 coated Dynabeads to target cells and the incubation volume were calculated according to the manufacturer's guidelines. The average number of CD3+ cells in a liver cell suspension, prepared as described above, was estimated as 5 × 105. Peripheral blood CD3 cells were purified from 106 PBMC, prepared from EDTA blood on a Ficoll gradient, and also estimated to contain 5 × 105 CD3 cells. Anti-CD3 coated Dynabeads (2 × 106) were washed twice in PBS with azide (PBA) and resuspended in a final volume of 200 μl PBA. This suspension was then added to the liver cells or PBMC and the cells were resuspended. The beads and cells were incubated together for20 min on an end-over-end rotator at 4 °C, then the beads, with attached CD3 cells were washed twice in PBA, resuspended in 100 μl PBS and frozen at − 70 °C.
4.4 Vβ profile analysis by RT-PCR
RNA was extracted from the frozen CD3-enriched cells using the Stratagene total RNA extraction kit 26, according to the manufacturer's instructions. Briefly, samples were lysed in a solution containing guanidinium isothiocyanate, extracted using acid phenol-chloroform and isopropanol precipitated. cDNA synthesis was carried out using the Pharmacia First Strand kit, with random hexamers as primers, again according to the manufacturer's suggestions. RT-PCR was performed as previously described 27 using fluorescently-labeled primers to amplify across the Vβ-Cβ region using the forward primers CCTGAAGACAGCAGC (A/T)T(A/C)TA, TTCAGCTGCGTATTTCTGTG, and TGTA (C/T)CTCTGTGCCAGCAG, in combination with the reverse primer, CTCTGCTTCTGATGGCTCAAACACAGCGAC. The Cα region was also amplified as an internal control using the primers CATCCAGTTGGTGGCATTGC and GTTTCTTCGAACCTAAACTTTCAAAACCTG. The products were separated on an ABI Prism 310.
4.5 Staining with HCV-specific MHC tetramers
MHC class I peptide tetrameric complexes were made using recombinant human class I HLA-A2 heavy chain, engineered to contain a specific biotinylation site, and human β 2-microglobulin. Theproteins were expressed in bacterial inclusion bodies, solubilized in urea and refolded around the appropriate peptide derived from HCV. Tetramers were then formed using PE-conjugated streptavidin 12, 14. Table 2 shows the sequences of the peptides used 8, 28, 29.
Liver-derived cells or PBMC were pelleted in RPMI containing 5 % FBS, and the supernatant was discarded. The cells were incubated in the residual volume with 1 μg of a pool of between two and four tetramers for 30 min at 37 °C. FITC-labeled CD69 and PE-Cy5 labeled CD8 antibodies were then added simultaneously without washing away the tetramer. After a further 30 min incubation at 4 °C, the cells were washed and fixed in 500 μl of PBS containing 1 % methanal. They were analyzed on a Coulter XL 4-color flow cytometer. Cells were gated on the basis of their CD8 expression and scatter properties for analysis. Quadrants were set to allow determination of the percentage of cells positive for CD69 and/or tetramers, based on the results obtained with two HCV-negative biopsies and one biopsy from an HCV+ patient who was not of the HLA-A2 genotype.
Acknowledgements
This work was supported by grants from the Medical Research Council and Wellcome Trust. We would like to thank the staff and patients in the Liver Unit at Queen′s Medical Center for their help in obtaining the samples needed for this study.
- WILEY-VCH
- WILEY-VCH




