T cell recognition of rat myelin basic protein as a TCR antagonist inhibits reciprocal activation of antigen-presenting cells and engenders resistance to experimental autoimmune encephalomyelitis
Abstract
The aim of this study was to assess whether T cell recognition of myelin basic protein (MBP) as a partially antagonistic self antigen regulates the reciprocal activation of professional antigen-presenting cells (APC). This study focused on the rat 3H3 T cell clone that recognized guinea pig (GP) MBP as a full agonist and self rat (R) MBP as a partial agonist. In cultures of 3H3 T cells and splenic APC, the agonist GPMBP elicited several responses by splenic APC, including production of nitric oxide, down-regulation of I-A, induction of B7.1 and B7.2, and prolongation of APC survival. RMBP stimulated a partial increase in production of nitric oxide, partially promoted survival of splenic APC, but did not alter expression of I-A, B7.1, or B7.2 on splenic APC. In the presence ofGPMBP, RMBP antagonized agonist-stimulated induction of B7 molecules, reversed the loss of I-A, and promoted the generation of I-A+, costimulus-deficient APC. Furthermore, 3H3 T cells cultured with RMBP and irradiated splenocytes reduced the severity of EAE upon adoptive transfer into naive rat recipients subsequently challenged with an encephalitogenic dose of GPMBP/CFA. Overall, this study indicates that T cell receptor antagonism blocks T cell activation, inhibits feedback activation of splenic APC, and promotes T cell-dependent regulatory activities in EAE.
Abbreviations
-
- GPMBP:
-
Guinea pig myelin basic protein
-
- irrSPL:
-
Irradiated splenocyte
-
- NO:
-
Nitric oxide
-
- RMBP:
-
Rat myelin basic protein
1 Introduction
Experimental autoimmune encephalomyelitis (EAE) in Lewis rats serves as a model for the study of multiple sclerosis (MS). The T cell repertoire in Lewis rats contains autoreactive clones specific for RMBP as well as many clones that recognize RMBP as a mixed agonist/antagonist. Typically, T cells that recognize RMBP as a partial agonist recognize the nonself peptide GPMBP as a full agonist 1, 2. GPMBP differs from RMBP in the dominant 72–86 encephalitogenic sequence by a single threonine for serine substitution at position 80. This substitution results in at least a tenfold activity difference in the induction of EAE in Lewis rats 3. Neuropeptide analogs that act as mixed agonist/antagonists have also been described in murine models of EAE 4–6. When administered to mice, these antagonistic analogs inhibit expression of EAE. TCR antagonists are known to block T cell activation, butTCR antagonists may also inhibit immune responses by preventing reciprocal activation of APC. Because T cell activation regulates activation of APC, the extent of APC activation may control the scope ofthe immune response.
This study focused on mechanisms by which TCR antagonists regulate T cell activities necessary for the reciprocal activation of splenic APC. This and a previous study focused on T cells that recognize autologous RMBP as a mixed agonist/antagonist. These studies showed that RMBP differentially regulated parameters associated with activation of both T cell 7 and APC. Specifically, RMBP inhibited agonist-induced B7 expression on T cells and APC but promoted I-A expression on both cell types and engendered T cells with the capacity to reduce the severity of EAE upon adoptive transfer into Lewis rat recipients. Hence, RMBP may inhibit EAE by promoting presentation of RMBP without sufficient costimulation.
2 Results
2.1 The self-antigen RMBP inhibits antigenic activation of 3H3 T cells
Previous studies have shown that RMBP antagonized GPMBP-induced expression of B7 molecules on 3H3 T cells 7. TCR antagonism rather than competition for the MHC glycoprotein peptide-binding groove accounted for the inhibitory action of RMBP. This conclusion was based on the observation that RMBP antagonized antigen-induced IL-2 production (Fig. 1A) and B7.1 expression (Fig. 1B) by MBP-specific 3H3 T cells but not by conalbumin-specific 9H8 T cells even though both 3H3 and 9H8 T cells were I-A restricted (Fig. 1C).
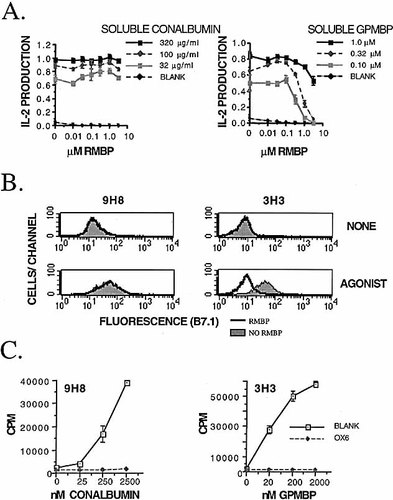
RMBP competes with GPMBP by a mechanism of TCR antagonism. (A) 3H3 or 9H8 T cells were cultured with irrSPL for 3 days with designated concentrations of RMBP (x axis) and either GPMBP (right) or conalbumin (left). (B) PKH26-labeled 3H3 or 9H8 T cells were cultured with splenic APC for 2 days in the presence (bottom) or absence (top) of 0.10 μM GPMBP or 100 μg/ml conalbumin (agonist) with (bold lines) or without (shaded histograms) 3.2 μM RMBP. Cells were stained with no mAb (mean fluorescence intensity <16) or anti-B7.1 mAb and an FITC-conjugated secondary reagent. PKH26+ T cells were analyzed for FL-1 fluorescence. (C) 3H3 or 9H8 T cells were cultured with irrSPL in the presence of designated concentrations of GPMBP (right) or conalbumin (left) with or without OX6 (anti-I-A) mAb. Cultures were pulsed with [3H]thymidine during the last day of a 2-day culture. These data are representative of two experiments.
2.2 Antagonism of 3H3 T cells results in the generation of tolerogenic APC
Previous studies have indicated that recognition of the partial agonist RMBP by 3H3 T cells may differentially regulate expression of MHC class II glycoproteins and costimulatory molecules on T cells to generate an I-A+, B7low phenotype 7 and possibly non-activated tolerogenic APC. To test whether recognition of RMBP as a TCR antagonist promotes regulatory activity in EAE, 3H3 T cells were cultured with irradiated splenocytes (irrSPL) for 2 days in serum-free medium in the presence of RMBP. Adoptive transfer of these cells into naive Lewis rats resulted in reduction in the intensity of EAE when challenged 1 week later with GPMBP in CFA (Table 1). Reduction in disease severity was dependent on the presence of RMBP because equivalent cultures lacking RMBP did not exhibit tolerogenic activity.
|
Culture conditionsa) |
Incidence (mean max. intensity) |
Mean cumulative score ± SD |
|---|---|---|
|
3H3 T cells + irrSPL |
8/9 (2.5) |
6.5 ± 3.9 |
|
3H3 T cells + irrSPL + RMBP |
18/19 (1.6) |
3.9 ± 2.9b) |
|
None |
16/16 (2.5) |
7.8 ± 2.7 |
- a) 3H3 T cells were cultured (5×105/ml) with irrSPL (5×106/ml) in RPMI media plus 2% normal rat serum with or without 4 μM RMBP. Cells were incubated for 2 days, were washed extensively, and were injected into Lewis rat recipients (i.p., 107 cells/rat). Seven days later, rats were challenged with 5 μg GPMBP in 0.1 ml CFA containing 200 μg Mycobacterium tuberculosis to induce EAE.
- b) Significantly different from 3H3 T cells cultured without antigen (1st row) and paralytic controls (3rd row) by 1-way ANOVA; p ≤ 0.05. These data are representative of four experiments.
2.3 T cell recognition of RMBP as a partial agonist negatively regulates antigenic activation of splenic APC
T cell-dependent activation of B cell and MΦ APC is critical for humoral and cell-mediated effector responses. Experiments were therefore performed to determine whether the feedback activation of splenic APC was regulated by RMBP-mediated antagonism. Splenic APC were approximately 60% I-A+ (HIS-28), 20% ED3+ (MΦ subset marker), 30–35% OX41+ I-A+/OX42+ I-A+ (MΦ markers) and 10% OX33+ I-A+ (B cell marker). During a 2-day culture with splenic APC and 3H3 T cells, a relatively low concentration of GPMBP (0.10 μM) induced a substantial loss of I-A molecules from the APC (Fig. 2). A relatively high concentration of RMBP (3.2 μM) did not affect quantitative expression of I-A on splenic APC, whereas the same concentration of RMBP effectively antagonized GPMBP-catalyzed loss of I-A from splenic APC. Similarly, RMBP antagonized GPMBP-induced expression of B7.1 and B7.2 molecules on splenic APC (Fig. 2). Induction and antagonism of B7.1 expression was primarily apparent on OX41+ and OX42+ APC (data not shown). These results indicate that TCR antagonism regulates B7 expression on splenic APC and may thereby control the extent of an immune response via provision of signal 1 (MHC/TCR ligation) without signal 2 (B7/CD28 costimulation).
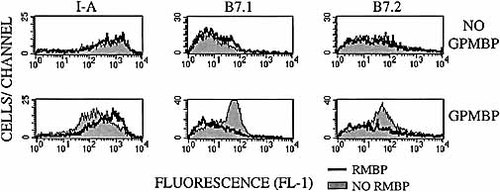
RMBP antagonizes agonist-induced up-regulation of B7.1 and B7.2 on splenic APC. PKH26-labeled 3H3 T cells were cultured with splenic APC for 2 days in the presence (bottom) or absence (top) of 0.10 μM GPMBP with (bold lines) or without (shaded histograms) 3.2 μM RMBP. Cells were stained with no mAb (mean fluorescence intensity <15), anti-I-A, anti-B7.1, or anti-B7.2 mAb and an FITC-conjugated secondary reagent. Splenic APC gated as PKH26– cells (FL-2) were analyzed for FL-1 fluorescence. These data are representative of five experiments.
2.4 TCR antagonism inhibits agonist-induced factors that promote survival of MΦ
In 3-day cultures of 3H3 T cells and irrSPL, GPMBP not only stimulated T cell activation but also resulted in increased yields of splenic APC (Fig. 3). Thus, splenic APC represented a greater percentage of the cell population in cultures of activated T cells than in non-stimulated cultures. The mechanism underlying enhanced yields of splenic APC (mostly MΦ) in antigen-stimulated cultures is not currently known but may reflect antigen-induced elaboration of M-CSF and increased survival or perhaps proliferation of MΦ. Like inhibition of B7.1 and B7.2 expression, RMBP strongly antagonized GPMBP-induced increases in the yield of splenic APC (Fig. 3, bottom left).
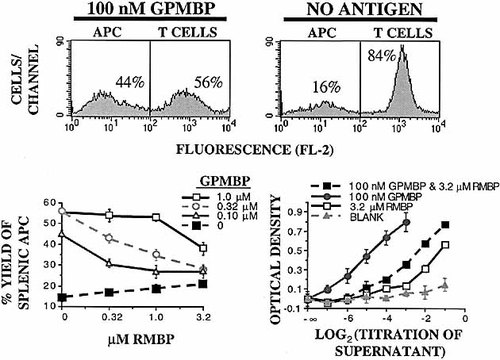
RMBP competitively antagonizes the activity of GPMBP as measured by survival of splenic APC and production of M-CSF-like cytokines. PKH26-labeled 3H3 T cells were cultured with irrSPL for 3 days in the presence of no antigen or designated concentrations of GPMBP or RMBP. Top: PKH26+ 3H3 T cells and PKH26– splenic APC were readily distinguished by FL-2 fluorescence. Bottom left: PKH26– cells as a percentage of total viable cells are plotted versus the concentration of GPMBP and RMBP. Bottom right: 3H3 T cells were cultured with splenic APC for 2 days in the presence of 0.10 μM GPMBP, 3.2 μM RMBP, both, or none. Supernatants from these cultures were titrated (x axis) and were used to assay activities that maintained viability of PEC MΦ (104/well). Aminoguanidine (1 mM) was added to each well to prevent the possible production of NO. Metabolic activity/viability of PEC MΦ was measured after a 5-day culture by addition of a MTS/PMS solution. These data are representative of three experiments.
Two approaches were used to assess mechanisms responsible for antigen-induced enrichment of splenic APC. First, 3H3 T cells were cultured for 2 days with splenic APC in the presence of no antigen, RMBP, GPMBP, or both antigens. Supernatants from these cultures were then tested for the maintenance of PEC MΦ (Fig. 3, bottom right). Culture supernatants from GPMBP-stimulated cultures maintained high levels of MΦ viability, whereas supernatants from T cells cultured with no antigen, RMBP, or both antigens exhibited reduced activity. These data indicate that the balance of agonist versus antagonist directly regulated production of soluble factors that promote MΦ survival. Second, counts of total viable cells recovered from culture were multiplied by percentages of PKH26– APC assessed by flow cytometry. These analyses revealed that GPMBP engendered an approximate doubling of the number of viable splenic APC recovered from culture (data not shown). These data indicate that TCR antagonism may directly affect accumulation of effector cells such as MΦ APC.
2.5 The self-antigen RMBP is a partial agonist that antagonizes MΦ production of NO
To test whether RMBP regulates an MΦ effector response implicated in EAE, we focused on T cell-dependent NO production (Fig. 4). Splenic MΦ were isolated by plastic adherence, were rested for 7 days in a source of M-CSF, and then were pulsed with designated concentrations of GPMBP for 45 min. 3H3 T cells were cultured for 2 days with GPMBP-pulsed splenic MΦ in the presence of designated concentrations of RMBP. MΦ pulsed with GPMBP concentrations as low as 10 nM were sufficient to stimulate NO production. This response was entirely dependent upon the presence of T cells (data not shown). These data indicated that the NO production assay was a very sensitive assay for measurement of T cell-dependent MΦ activation. RMBP behaved as a classical mixed agonist/antagonist in NO production assays. This finding provides unique evidence that TCR antagonists directly regulate T cell-dependent MΦ responses.
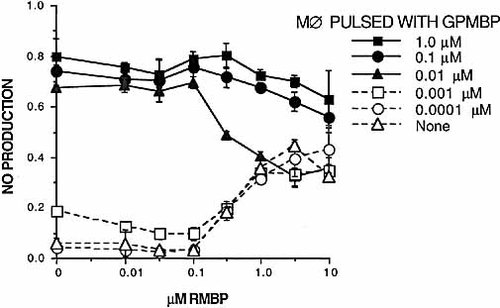
RMBP is a partial agonist/antagonist for stimulation of NO production by MΦ. Splenic MΦ were isolated by adherence and were cultured for 7 days in cRPMI and 20% vol/vol supernatant from a separate L cell culture (i.e. a source of M-CSF). MΦ were harvested and pulsed for 45 minutes (37°C) with designated concentrations of GPMBP, were washed, and were used as APC (105/well) to stimulate 3H3 T cells (2.5×104/well) in the presence or absence of RMBP (x axis). After 2 days of culture, NO production was measured by the Griess reaction. These data are representative of five experiments.
3 Discussion
This and a previous study 7 focused on the recognition of the self-antigen RMBP as a partial or mixed agonist/antagonist by 3H3 T cells. These two studies revealed a hierarchy of responses associated with the ability of RMBP to regulate expression of molecules associated with APC activity and T cell activation. In this study, RMBP was a full antagonist in regard to GPMBP-induced expression of B7.1/B7.2 on splenic APC and GPMBP-induced loss of I-A from splenic APC. RMBP was a partial agonist/antagonist in assays of MΦ survival, production of M-CSF like growth factors, and NO production. As shown in the previous study 7, RMBP was a pure antagonist in assays of GPMBP-stimulated IL-2 production and proliferation and was a mixed agonist/antagonist in assays of blastogenesis and induction of B7.1 and B7.2 on T cells. Conversely, RMBP exhibited agonistic activity in assays of I-A induction on T cells. Overall, expression of agonistic versus antagonistic activity was inversely related, and different responses required distinct strengths of agonistic signaling. The hierarchy of RMBP-mediated activities on T cells paralleled that for splenic APC, because in both cases RMBP promoted presentation of RMBP-derived I-A/peptide complexes but suppressed agonist-induced expression of costimulatory molecules.
Adoptive transfer of 3H3 T cells exposed to RMBP-derived I-A/peptide complexes effectively reduced severity of EAE (Table 1). Hence, this hierarchy of activities associated with the mixed agonistic/antagonistic activities of RMBP was also associated with regulatory activity in EAE. This inhibitory activity was dependent on recognition of RMBP by 3H3 T cells because 3H3 T cells cultured in the absence of RMBP lacked regulatory activity. The inhibitory activity of 3H3 T cells may be due to the tolerogenic presentation of RMBP by 3H3 T cells 2, 7 in vivo. Alternatively, 3H3 T cells may develop or express regulatory activities upon exposure to RMBP that are unrelated to APC activity. Whether induction of a `tolerogenic' APC phenotype is causally related to the expression of regulatory activity in vivo is an issue that warrants further study.
4 Materials and methods
4.1 Animals and reagents
Lewis rats (Harlan-Sprague Dawley, Indianapolis, IN) were maintained at East Carolina University School of Medicine. MBP was purified from rat or guinea pig spinal cords (Rockland). The anti-I-A OX6 IgG1 8, anti-SIRP OX41 IgG1 9, anti-CD11 OX42 IgG1 9, anti-rat MΦ ED3 IgG2a 10, and anti-B cell CD45 OX33 IgG1 11 mAb were produced as culture supernatants and were concentrated by ultrafiltration through Amicon spiral wound membranes (100-kDa exclusion). Anti-B7.1 IgG1 (3H5), anti-B7.2 IgG1 (24F), and anti-RT1B IgG2a (HIS28) were purchased from PharMingen. Aminoguanidine and conalbumin were from Sigma.
4.2 Derivation and culture of Lewis rat T cell lines
The CD4– subclone GP2.3H3.16 (referred to as 3H3) was derived from Lewis rats sensitized with an emulsion of GPMBP in CFA 2. The CONAL.9H8 clone was derived from Lewis rats immunized with conalbumin in CFA. Clones were maintained in complete RPMI medium (cRPMI) supplemented with IL-2 (CM). The cRPMI medium (cRPMI) consisted of 10% heat-inactivated fetal bovineserum (Summit, Boulder, CO), 2 mM glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin (Whittaker Bioproducts, Walkersville, MD), and 50 μM 2-ME (Sigma). Rat IL-2 was expressed from a recombinant rat IL-2 baculovirus.
4.3 Preparation of splenic APC
SPL were obtained from naïve Lewis rats and were purified on discontinuous Percoll gradients of 40%, 50%, and 60%. The splenic APC cell fraction was collected at the 40/50% interface and was largely comprised of cells expressing high levels of I-A, B7.1, and B7.2.
4.4 In vitro proliferation, IL-2 production, and NO production
Responder T cells (2.5×104/well) were cultured with irrSPL (5×105/well) or splenic APC (105/well) with or without antigen in cRPMI and were pulsed with 1 μCi [3H]thymidine (6.7 Ci/mmol, NEN) during the last day of a 2–3-day assay. Supernatants (100–120 μl/well) were transferred to replicate plates to measure IL-2 bioactivity, and T cells were harvested onto filters to measure [3H]thymidine incorporation by scintillation counting (Wallac). CTLL cells (104/50 μl cRPMI/well) were cultured with 100–120 μl of supernatant for 24–48 h, and 10 μl of a 2.9 mg/ml 3-(4,5-dimethylthiazol-2-yl)-5-3 (3-carboxy-methoxyphenyl). 2-(4-sulfophenyl)-2H-tetrazolium (MTS) (Promega) and 0.1 mg/ml phenazine methosulfate (PMS, Sigma) (MTS/PMS) solution were added to each well. Plates were read the next day at 492 nm on an Anthos ELISA Reader (ACCSales, Chapel Hill, NC). Antigen-stimulated IL-2 production was calculated as the mean absorbance from experimental cultures minus the mean absorbance from control unstimulated cultures. The production of NO was measured by formation of the stable decomposition product nitrite in cell-free supernatants (50 μl) after mixing with an equal volume of the Griess reagent 12.
4.5 Flow cytometric analysis
3H3 or 9H8 T cells were labeled with the PKH26 lipophilic dye (Sigma) according to manufacturer's directions. Splenic APC (5×105/ml) or irrSPL (5×106/ml) and PKH26-labeled T cells (5×105/ml) were cultured for 2–3 days in cRPMI. T cells were stained with a primary mAb (2.5 μg/ml or 1/20 titration of a concentrated supernatant) and an FITC-conjugated goat anti-mouse IgG (H+L). Washing and staining of T cells were performed at 4 °C, and normal rat serum was used to block Fc receptors. Dead cells were excluded from analysis by forward versus side scatter profiles. Data were acquired with a Becton Dickinson FACScan cytometer and were analyzed with the CellQuest software program.
4.6 Assay of M-CSF-like activity
3H3 T cells and splenic APC were cultured for 2 days with no antigen, GPMBP, RMBP, or both antigens. Supernatants from these cultures were tested for M-CSF-like activity in a subsequent culture of peritoneal exudate cell (PEC) MΦ indicator cells. To generate indicator cells for the bioassay, PEC were collected from the peritoneal cavity 2 days after i.p. injection of heat-killed C. parvum (200 μg/5 ml HBSS) into naïve Lewis rats. After 5 days of culturing MΦ with T cell supernatants, 10 μl of an MTS/PMS solution were added to each well to assess MΦ viability. Plates were read after 24 h at 492 nm on an ELISA Reader. Metabolic activity of surviving MΦ was determined as mean absorbance values. Production of MCSF-like cytokines was determined asmean absorbance for experimental cultures minus mean absorbance for control non-stimulated cultures.
4.7 Clinical assessment of EAE
The following scale was used to assign intensity of EAE; paralysis in the distal tail, 0.25; limp tail, 0.5; ataxia, 1.0; hind leg paresis, 2.0; full hind leg paralysis, 3.0. The mean maximal intensity scores were assigned to each group based on the average maximum score among afflicted rats within a group. The cumulative score for each rat consisted of the sum of daily scores for each rat. The mean cumulative score for a group was calculated by averaging the cumulative scores for all rats within a group.
Acknowledgements
This study was supported by a research grant from the National Multiple Sclerosis Society.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




