Two-step activation of T cells, clonal expansion and subsequent Th1 cytokine production, is essential for the development of clinical autoimmune encephalomyelitis
Abstract
Lewis rats immunized with guinea pig myelin basic protein (GPBP) emulsified with incomplete Freund's adjuvant (IFA) do not develop experimental autoimmune encephalomyelitis (EAE). However, we found that GPBP/IFA with pertussis toxin (PT) administration induced full-blown EAE. By comparing the immunological status of rats immunized with GPBP/IFA plus PT [PT (+) rats] with that of rats immunized with GPBP/IFA alone [PT (–) rats], we tried to elucidate the pathomechanisms of EAE. Analysis of the TCR clonality by CDR3 spectratyping revealed that Vβ8.2 and Vβ10 expansion of T cells occurred in both PT (–) and PT (+) rats, indicating that activation of T cells at this level is not sufficient for the development of clinical EAE. Quantitaion of cytokine mRNA and protein revealed that PT (–) rats showed a Th2-dominant, while PT (+) rats showed a Th1-dominant, cytokine profile. Furthermore, administration of IL-12, but not of IFN-γ and TNF-α, induced clinical EAE in GPBP/IFA-immunized animals. Taken together, two-step activation, activation of T cells bearing a particular type of TCR by antigen immunization and subsequent overproduction of Th1 cytokines, mainlyIL-12 production, induced by appropriate adjuvants is essential for the development of clinical EAE.
Abbreviations:
-
- GPBP:
-
Guinea pig myelin basic protein
-
- PT:
-
Pertussis toxin
-
- PT (+) rats:
-
Rats immunized with GPBP/IFA plus PT
-
- PT (–) rats:
-
Rats immunized with GPBP/IFA alone
-
- CDR3:
-
Complementarity-determining region 3
-
- SC:
-
Spinal cord
1 Introduction
Organ-specific autoimmune disease is inducible in susceptible animals by immunization with organ-specific antigen emulsified with CFA. The route of antigen administration and kind of adjuvant used for challenge are key factors for the disease induction. Unlike antigen injections in CFA (containing mycobacterial proteins in mineral oil), injections of antigen in IFA (mineral oil only) were thought to be prone to induce tolerance. For instance, immunization with guinea pig myelin basic protein (GPBP) in IFA does not elicit clinical signs of experimental autoimmune encephalomyelitis (EAE) in Lewis rats and rather induces resistance to EAE upon subsequent challenge with GPBP/CFA 1–3. Similarly, intravenous injection and oral administration of the antigen induce protection against subsequent challenge with GPBP/CFA 4, 5. With regard to IFA, it has been suggested that Th2 immune responses induced by immunization with not only encephalitogenic, but also non-encephalito-genic, antigen in IFA protect animals from EAE 6. Another group observed a lower overall antigen-induced cytokine production in encephalitogenic antigen/IFA-immunized mice 7.
In the process of examining the effects of bacterial toxins on the development of EAE, we found that simultaneous administration of pertussis toxin (PT) to Lewis rats immunized with GPBP/IFA, but not with GPBP/IFA alone, elicits severe EAE. Here, we have analyzed the immunological events occurring in rats with clinical EAE elicited by immunization with GPBP/IFA plus PT compared with those in asymptomatic rats immunized with GPBP/IFA alone. TCR repertoire analysis by CDR3 spectratyping revealed that the activation pattern of spectratypes of symptomatic rats receiving GPBP/IFA plus PT was essentially the same as that of asymptomatic rats immunized with GPBP/IFA alone, indicating that activation of T cells bearing a particular type of TCR is not related to the presence or absence of PT and further suggesting that additional signals are necessary for the development of clinical EAE. Consistent with this finding, cytokine analysis demonstrated that PT administration to GPBP/IFA-immunized rats induced overproduction of Th1 cytokines in the lymphoid organ. Furthermore, administration of IL-12, but not of IFN-γ and TNF-α , induced clinical EAE in GPBP/IFA-immunized animals. These findings strongly suggest that two-step activation of T cells, i.e. clonal expansion of disease-inducing T cells and subsequent production of Th1 cytokines, is essential for the development of autoimmune diseases.
2 Results
2.1 PT administration induced severe EAE in GPBP/IFA-immunized rats
There is consensus that immunization of Lewis rats with encephalitogenic antigen, GPBP, emulsified with IFA fails to induce clinical EAE but rather confers resistance to EAE upon subsequent challenge with GPBP/CFA 8. While examining the effects of bacterial toxins such as PT and staphylococcal enterotoxins, we found that intraperitoneal injection of PT induced severe EAE in Lewis rats immunized with GPBP/IFA. By comparing the immunological status of rats with clinical EAE after immunization with GPBP/IFA plus PT with that of asymptomatic rats after GPBP/IFA immunization alone, we tried to elucidate factors involved in the development of the organ-specific autoimmune disease.
We first examined the clinical course of EAE in rats immunized with GPBP/IFA plus PT [PT (+) rats] or with GPBP/IFA alone [PT (–) rats]. As shown in Fig. 1, all the PT (+) rats developed clinical EAE (closed squares). With this protocol, the clinical signs appeared slightly earlier and were more severe than those seen in rats with EAE induced with GPBP/CFA (Table 1). The mean maximal disease severity was 3.9±0.2 with a 28.6% mortality rate. In sharp contrast, none of the PT (–) rats developed clinical EAE (closed circles in Fig. 1).
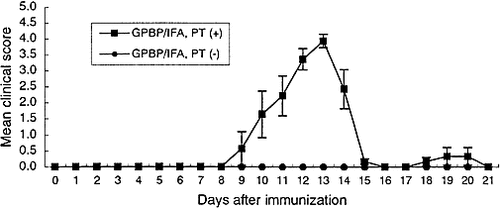
Clinical course of EAE induced by GPBP/IFA plus pertussis toxin [PT (+)] and GPBP/IFA alone [PT (–)]. Two groups of Lewis rats were injected in the hind footpads on both sides with an emulsion of 100 μ g GPBP in IFA with (squares) or without (circles) PT. Mice were examined daily for signs of EAE and scored as follows: 0, no disease; 1, floppy tail; 2, mild paraparesis; 3, severe paraparesis; 4, tetraparesis or moribund condition. In the GPBP/IFA PT (+) group, the mean maximal severity was 3.9±0.2. Two rats died on days 12 and 13, respectively. The data are presented as the mean of EAE scores ± SEM in each group (n=7).
|
|
Clinical status |
Pathology |
|||
|---|---|---|---|---|---|
|
Immunogen |
PT |
Incidence |
Mean clinical score at histological examination |
Inflammation/SC segmentb) |
Mean histological score |
|
GPBP/IFA |
(+) |
5/5 |
4.0 |
16/16 (100 %) |
3.2 ± 0.8 |
|
GPBP/IFA |
(–) |
0/11 |
0 |
16/37 (43.2 %) |
0.5 ± 0.7 (1.2 ± 0.4)c) |
|
GPBP/CFA |
(–) |
6/6 |
2.9 |
18/18 (100 %) |
2.8 ± 0.7 |
- a) Lewis rats were immunized with GPBP in IFA with or without intraperitoneal injection of PT. On day 14 post-immunization when PT (+) rats showed full-blown EAE, the indicated number of rats were killed for histological examination.
- b) Hematoxylin and eosin-stained sections were prepared from three to four segments of the lumbar SC. The number of segments containing inflammation/total number of segments examined is shown.
- c) The mean histological grade of sections containing inflammatory lesions.
Using hematoxylin and eosin-stained sections of the lumbar spinal cord (SC), pathology of the central nervous system (CNS) lesion was evaluated on day 14 post-immunization when PT (+) rats showed maximal clinical signs (Table 2, Fig. 2A, B). PT (+) rats showed severe inflammation characterized by extensive perivascular cuffing and severe parenchymal cell infiltration (Fig. 2A). However, there was no polymorphic neutrophil infiltration. The PT (+) rats showed a severer inflammation with a mean histological score of all the lumbar SC segments of 3.2±0.8, compared to the rats immunized with GPBP/CFA whose mean histological score was 2.8±0.7 (Table 1). Interestingly, 43.2% of the segments from PT (–) rats exhibited mild inflammation (Fig. 2B), although the rats did not develop clinical signs at all (Table 1).
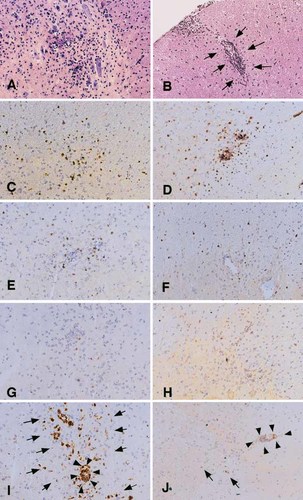
Immunopathology of the SC lesion of PT (+) rats (A, C, E, G and I) or PT (–) rats (B, D, F, H and J). Frozen sections were prepared from lumbar SC segments taken on day 14 post immunization and stained with hematoxylin and eosin (A, B) and T cell (C and D, R73; E and F, R78; G and H, G101) and macrophage (I and J, ED1) markers. Arrows and arrowheads in I and J indicate parenchymal and perivascular macrophage infiltration, respectively. A–J ×110.
To characterize the nature of inflammatory lesions in PT (+) and PT (–) rats in more detail, an immunohistochemical study was performed with mAb against TCRα β (R73), Vβ 8.2 (R78), Vβ 10 (G101) and infiltrating macrophages (ED1). There was no significant difference in T cell markers between PT (+) and PT (–) rats, although inflammation in the former was far severer than in the latter (Fig. 2A, B). TCRα β + T cells were the major components (Fig. 2C, D) and Vβ 8.2+ T cells (Fig. 2E, F) were more frequently found than Vβ 10+ T cells (Fig. 2G, H). A striking difference was noted in macrophage staining. In PT (+) rats, there was extensive macrophage infiltration of the parenchyma (arrows in Fig. 2I). In sharp contrast, very few macrophages were found in the parenchyma of PT (–) rats (arrows in Fig. 2J).
These findings indicate that Lewis rats immunized with GPBP/IFA do not develop clinical EAE, although some rats show mild inflammation in the CNS. Simultaneous administration of PT induces clinical EAE and strongly enhances the severity of inflammation in the CNS.
2.2 The TCR repertoire of SC and lymph node T cells in PT (+) rats was essentially the same as that in PT (–) rats
The difference in clinical and histological features of EAE between PT (+) and PT (–) rats may be attributable to several factors. Since clonal expansion of encephalitogenic T cells bearing particular types of TCR is essential for the development of EAE 9–11, we first examined the TCR repertoire of SC and lymph node (LN) T cells from these rats by CDR3 spectratyping. As shown in our previous study 12, the spectratype pattern of T cells from normal and adjuvant control rats shows a Gaussian distribution. It was confirmed here that SC T cells from rats immunized with saline/IFA with PT show a normal spectratype pattern without any oligoclonal expansion (Fig. 3). Rats immunized with saline/IFA without PT showed the essentially the same pattern (data not shown). These spectratypes were judged to be derived from T cells trapped in the blood vessels because there was no inflammatory lesion in the SC of control rats. An important finding was that administration of IFA and PT does not induce clonal expansion of TCR.

CDR3 spectratyping analysis of TCR β -chain of SC T cells from a rat immunized with saline/IFA plus PT administration.
We then examined the TCR repertoire of SC and LN T cells taken from PT (+) and PT (–) rats (n=3 in each group) on day 14 post-immunization and essentially the same results were obtained. A representative result is shown in Fig. 4. SC T cells from PT (+) rats showed marked Vβ 8.2 and Vβ 10 expansion (arrows in Fig. 4A) as well as additional spectratype expansion (indicated by arrowheads in Fig. 4A). In the LN, only Vβ 8.2 and Vβ 10 expansion was recognizable. Interestingly, the spectratype pattern of SC and LN T cells from a PT (–) rat with mild inflammation in the SC (Fig. 4C, D) was essentially the same as that of PT (+) rats. There was Vβ 8.2 and Vβ 10 (arrows in Fig. 4C) plus additional (arrowheads) expansion. The spectratype pattern of LN T cells from PT (–) rats (Fig. 4D) was indistinguishable from that from PT (+) rats (Fig. 4B). Peripheral blood lymphocytes from PT (+) and PT (–) rats showed a similar pattern to that of LN T cells (data not shown). These findings strongly suggest that the TCR repertoire of PT (+) and PT (–) rats is essentially the same, although there was a marked difference in clinical and histological features between the two groups.

CDR3 spectratyping analysis of the TCR β -chain of SC (A and C) and LN (B and D) T cells from rats (n=3 in each group) immunized with GPBP/IFA plus PT [PT (+), A and B] or with GPBP/IFA alone [PT (–), C and D]. Oligoclonal expansion of Vβ 8.2 and Vβ 10 spectratypes and additional spectratype expansion are indicated by arrows and arrowheads, respectively. G0, no clinical signs; G4, tetraparesis.
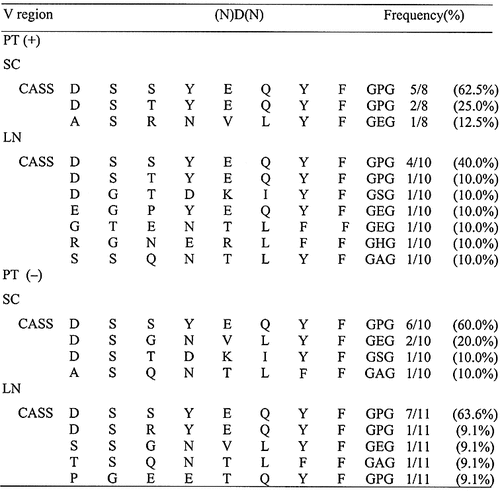
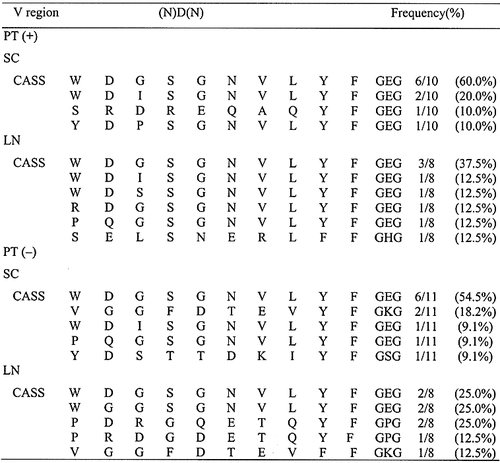
2.3 Sequencing of the CDR3 region of TCR clones derived from oligoclonally expanded spectratypes
To examine the nature of spectratypes showing oligoclonal expansion in more detail, we extracted DNA from bands of interest and determined the nucleotide and amino acid sequences of the CDR3 region of TCR clones after cloning (Table 2 and 3). As shown in Table 2, the dominant population of Vβ 8.2 clones in the SC and LN of PT (+) rats possessed the DSSYEQYF sequence in the CDR3 region. Essentially the same results were obtained using SC and LN T cells from PT (–) rats. Although Vβ 10 showed more diversity than Vβ 8.2, the predominant sequence of SC and LN T cells from PT (+) and PT (–) rats was WDGSGNVLYF (Table 3). Thus, the most frequently found sequence of Vβ 8.2 and Vβ 10 was essentially the same in both PT (+) and PT (–) rats. Moreover, the CDR3 sequences of Vβ 8.2 and Vβ 10 TCR clones other than these clones from PT (–) rats were also very similar to those found in PT (+) rats (Table 2 and 3). These findings indicate that rats immunized with GPBP/IFA and with GPBP/IFA plus PT show essentially the same state of T cell activation, and suggest that induction of severe EAE by PT administration results from immunological events other than modulation of the TCR repertoire.
- a) CDR3 size spectratyping was performed using PCR products amplified with TCR Vβ1-20-specific primers. CASS, C-terminal sequence of the V region of TCR connecting to the (N)D(N) region. cDNAwas extracted from bands representing oligoclonal expansion of Vβ 8.2 at day 14 PI with maximal clinical score of EAE. Each sample was reamplified by nested PCR, cloned and sequenced. Clones with the same amino acid sequence in this table possess the same nucleotide sequence.
- a) cDNA was extracted from bands representing oligoclonal expansion of Vβ 10 at day 14 PI with maximal clinical score of EAE. Clones with the same amino acid sequence in this table possess the same nucleotide sequence.
2.4 PT administration induces strong Th1 deviation in rats immunized with MBP/IFA
As we did not see any significant difference in the TCR repertoire between PT (+) and PT (–) rats, we next examined the cytokine profile in the SC and LN of PT (+) and PT (–) rats by competitive PCR (Fig. 5). Our previous competitive PCR analysis revealed that IFN-γ , TNF-α and IL-10 mRNA was not detectable in the CNS of normal rats. 13. In PT (+) rats, pro-inflammatory cytokines were generally up-regulated compared with PT (–) rats. IL-2, IL-12 and IFN-γ in the LN and TNF-α in the SC showed a significant difference between the two groups. In sharp contrast, anti-inflammatory cytokines, IL-4, IL-10 and TGF-β 1, were down-regulated especially in the LN of PT (+) rats. Thus, PT administration induces up-regulation of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines in the lymphoid organ. In addition, marked increase of IFN-γ and TNF-α occurred in the CNS in PT (+) rats.
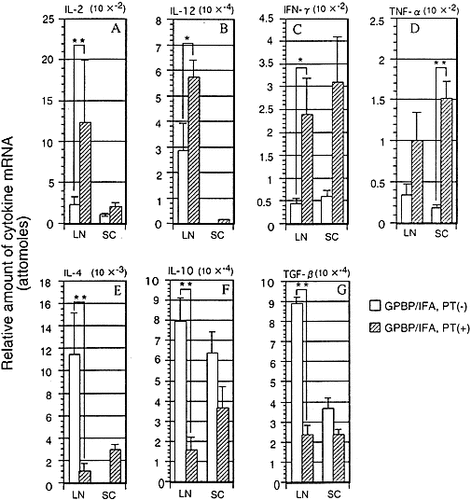
Quantitative analysis of mRNA expressions for IL-2 (A), IL-12 (B), IFN-γ (C), TNF-α (D), IL-4 (E), IL-10 (F) and TGF-β (G) in the LN and SC of PT (+) and PT (–) rats. Rats (n=4 in each group) were treated as described in the caption of Fig. 1 and killed at the peak stage of EAE (on day 11 post immunization). The results are expressed as the mean + SEM (attomoles), with (hatched bars) or without (open bars) PT. *p<0.05 and **p<0.01, significantly different by Student's t-test.
To confirm the above results at the protein level, IFN-γ and IL-4 production in PT (+) and PT (–) rats was assessed using currently available ELISA kits. An ELISA kit for rat IL-12 is not available at present. Rats were immunized with GPBP/IFA with or without PT. During the peak stage of EAE, cells taken from regional LN were cultured in the presence or absence of GPBP for 72 h and the supernatants were harvested for ELISA. The protein levels of pro-inflammatory cytokine IFN-γ were found to be five- to sevenfold higher (Fig. 6A), whereas the level of IL-4 protein was fourfold lower (Fig. 6B) in PT (+) rats than in PT (–) rats. These findings indicate that PT administration induces a Th1 predominance in GPBP/ IFA-immunized rats.
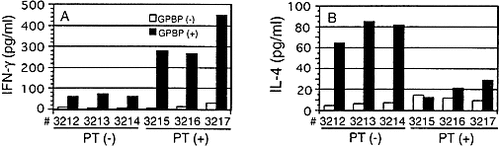
Cytokine analysis of IFN-γ (A) and IL-4 (B) by ELISA. Rats (n=3 in each group) were immunized with GPBP/IFA with or without PT and the regional LN were isolated at the peak stage of EAE (on day 10 post-immunization). LN cells (2×105 cells/well) were cultured in a 48-well plate in the presence (closed columns) or absence (open columns) of GPBP (25 μ g/ml) for 72 h. Culture supernatants were collected and tested for the cytokines by ELISA. Each sample was assayed in duplicate and essentially the same result was obtained.
2.5 IL-12 plays a central role in the development of clinical EAE induced by immunization with MBP/IFA plus PT
Simultaneous administration of PT to GPBP/IFA-immunized rats induced marked up-regulation of pro-inflammatory cytokines such as IL-12, IFN-γ , and TNF-α in lymphoid organs (Fig. 5) and induced severe EAE in Lewis rats (Fig. 1). We wished to know which cytokine plays an important role in the development of this type of EAE. For this purpose, IL-12, IFN-γ , and TNF-α were administered at the indicated doses to GPBP/IFA-immunized rats to see whether a certain cytokine can substitute the function of PT. As clearly shown in Fig. 7, simultaneous administration of IL-12, but not of IFN-γ and TNF-α , induced clinical EAE. This finding indicates that IL-12 plays a major role in the development of EAE in rats with T cells that had been activated to a certain level by GPBP/IFA immunization. However, it should be noted that IL-12 is not a sole factor for the development of clinical EAE in GPBP/IFA-immunized rats because the mean maximal clinical score of EAE in rats receiving GPBP/IFA plus IL-12 was significantly milder (p=0.01) than that in rats receiving GPBP/IFA plus PT. This finding suggests that factors other than IL-12 contribute to induce severe EAE in PT (+) rats. In this regard, there is the possibility that IFN-γ and TNF-α are involved in EAE development in PT (+) rats, although single administration of IFN-γ and TNF-α at doses employed in this study did not trigger clinical EAE in GPBP/IFA-immunized rats.
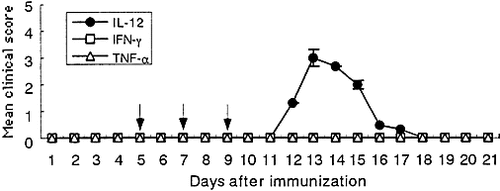
Effects of IL-12 (closed circles), IFN-γ (open squares) and TNF-α (open triangles) administration on the development of EAE. Groups of Lewis rats (n=3 in each group) were immunized with GPBP/IFA and IL-12, IFN-γ and TNF-α at the indicated doses were injected intraperitoneally on days 5, 7 and 9 post immunization (indicated by arrows). In the GPBP/IFA with IL-12 group, the mean maximal severity was 3.0. Each symbol represents the mean clinical score ± SEM.
2.6 The T cell population from PT (–) rats shows poor responses to MBP but contains encephalitogenic T cell precursors
T cells from PT (–) asymptomatic rats exhibited a Th2-type cytokine profile, although their TCR repertoire was essentially the same as that of PT (+) rats with severe EAE. One explanation for this is that immunization with GPBP/IFA without PT induces non-encephalitogenic and/or regulatory T cells with Th2 phenotype. To test this possibility, we measured the proliferative responses of T cells taken from PT (–) rats to immunization with GPBP, and then transferred in vitro stimulated T cells into naive Lewis rats. As shown in Fig. 8, LN cells taken from PT (–) rats showed marginal responses to GPBP, while LN cells from PT (+) rats responded vigorously to the antigen. However, this finding does not indicate the absence of encephalitogenic T cells in PT (–) rats. As is clearly shown in Fig. 9, transfer of in vitro GPBP-stimulated lymphoid cells from PT (–) rats induced clinical EAE in naive animals, indicating that the T cell population in PT (–) rats contains encephalitogenic T cells or their precursor cells.
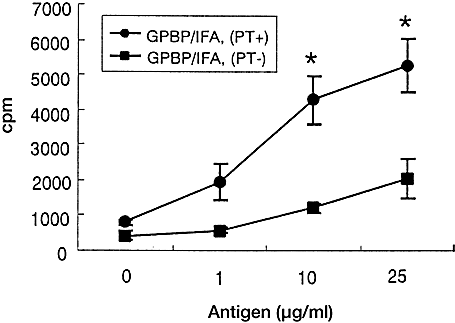
Proliferative activity of LN cells isolated from rats following stimulation with GPBP. In 96-well plates, 2×105 LN cells of either PT (+) or PT (–) rats were cultured with the indicated concentrations of GPBP. After 72 h, the plates were pulsed with [3H]thymidine for 18 h, after which they were harvested and the label uptake was determined using standard liquid scintillation techniques. SE are within 10% of the mean values. All the measurements were performed in triplicate. *Significantly different from PT (–) rats (p<0.05) by Student's t-test.
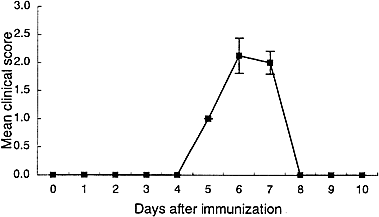
Clinical course of passive EAE induced by transfer of sensitized lymphoid cells from PT (–) rats. Lymphoid cells taken from PT (–) rats were cultured for 3 days in the presence of GPBP (5 μ g/ml) and 2×107 cells were injected intravenously into naive animals. Rats receiving GPBP-cultured lymphoid cells developed clinical EAE on day 5 post-transfer and maximal clinical signs developed on day 6.
The transfer experiment suggests that T cells are modified functionally during culture and obtain encephalitogenicity. Again in this case, there was no significant change in the TCR repertoire between T cells from PT (–) rats before and after culture (data not shown). Cytokine analysis revealed that IL-12, TNF-α and IFN-γ were markedly up-regulated after culture, whereas anti-inflammatory cytokines, IL-4 and TGF-β 1 did not show significant increase (not shown). These findings indicate that with in vitro GPBP stimulation, T cells from PT (–) rats in which Th2 cytokines are dominant are shifted to a Th1 phenotype.
3 Discussion
In the present study, we have demonstrated for the first time that immunization with GPBP/IFA plus PT, but not with GPBP/IFA alone, induces severe and sometimes fatal EAE in Lewis rats. Histological, TCR repertoire and cytokine analyses revealed the following differences between PT (+) and PT (–) rats. First, PT (–) rats did not develop clinical EAE at all but exhibited mild inflammation in the CNS not infrequently. The major difference in immunohistochemistry was that extensive macrophage infiltration was evident in the CNS of PT (+), but not of PT (–), rats. Second, no significant difference in the TCR repertoire was noticed between the two groups as demonstrated by CDR3 spectratyping (Vβ 8.2 and Vβ 10 spectratype expansion) and sequencing of the CDR3 region of oligoclonally expanded TCR clones. Third, PT administration induced marked up-regulation of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines in the lymphoid organs. Finally and most importantly, administration of IL-12, instead of PT, induced clinical EAE in GPBP/IFA-immunized rats. Similarly, T cells taken from PT (–) rats and activated by in vitro antigen stimulation secreted a large amount of pro-inflammatory cytokines and obtained encephalitogenicity. These findings suggest that changes in the cytokine profile after PT administration are closely associated with the clinical and histopathological status of EAE.
It is well known that immunization with encephalitogenic antigen emulsified with IFA does not elicit EAE in most susceptible animals but rather confers protection against subsequent challenge with antigen/CFA 1, 2. As this protection can be transferred into naive animals using lymphoid cells taken from MBP/IFA-immunized rats 2, suppressor cells were thought to conduct this event. However, it remains unclear whether suppressor cells are the major effector because transfer of lymphoid cells from MBP/IFA-immunized rats after in vitro antigen stimulation induced clinical EAE as shown in a previous 8 and this study. A recent study suggests that Th2 immune response against not only encephalitogenic, but also non-encephalitogenic antigen such as KLH elicited by immunization with antigen/IFA protects against EAE 6. Using a different EAE system, another group observed a lower overall antigen-induced cytokine production in MBP/IFA-immunized animals 7. The cytokine profile of rats immunized with GPBP/IFA alone is similar to that reported in the former study.
PT has been widely used as an adjuvant to potentiate the development of T cell-mediated diseases such as EAE 14 and autoimmune orchitis 15. Its major pharmacological action has been believed to increase vascular permeability in the CNS, thereby promoting the development of EAE 16, 17. However, it is difficult to judge this to be the major action because activated encephalitogenic T cells traverse the intact blood-brain barrier via interaction of adhesion molecules in the absence of increased vascular permeability in the CNS 18. More recently, several reports have demonstrated that in vivo administration of PT modulates the cytokine profile. Although the results reported so far are not uniform, PT seems to up-regulate both Th1- and Th2-type cytokine production 19–22. Here, we have shown that a relatively low dose of PT administered to Lewis rats that had been immunized with GPBP/IFA up-regulates IL-2, IL-12, IFN-γ and TNF-α mRNA and down-regulates IL-4, IL-10 and TGF-β 1 mRNA in the lymphoid organ (Fig. 5). Cytokine analysis at the protein level also demonstrates that PT induces a strong deviation to Th1 immune responses (Fig. 6).
It is interesting to note that the cytokine profile in the CNS is strikingly different from that in the lymphoid organ. In the CNS, IL-2 and IL-12 are found in trace amounts compared with in the lymphoid organs in both PT (+) and PT (–) rats. This cytokine status explains well the results in the previous studies showing that infiltrating T cells do not proliferate vigorously 23 and undergo apoptosis in the CNS 24, 25. On the other hand, TNF-α and IFN-γ were highly up-regulated in the CNS of PT (+) rats. Although both cytokines have disease-promoting function in the CNS 26, TNF-α appears to play more critical role in this situation. As demonstrated by Körner et al. 27 using TNF-deficient mice, TNF is essential for processes controlling initial leukocyte movement within the CNS. This result supports the present finding that extensive inflammation was not observed inPT (–) rats whose TNF-α levels were low in the CNS despite the presence of inflammatory cells. All the anti-inflammatory cytokines examined (IL-4, IL-10 and TGF-β 1) were down-regulated after PT administration. Among them, IL-10 plays a critical role in the suppression of EAE, as evidenced by the fact that (1) IL-10-deficient mice develop more severe EAE 28, 29, and (2) transgenic IL-10 prevents the induction of EAE 30. Therefore, it is possible to speculate that down-regulation of IL-10 in the lymphoid organ by PT treatment results in exacerbation of EAE.
In the present study, we have shown that PT administration greatly changed the cytokine profile (up-regulation of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines)and induced severe EAE in Lewis rats immunized with GPBP/IFA, while there was no significant difference in the TCR repertoire between PT (+) and PT (–) rats. Similarly, in vitro antigen stimulation of T cells from PT (–) rats induced marked up-regulation of Th1 cytokine secretion and rendered the T cells encephalitogenic as evidenced by the finding that transfer of cultured T cells induced EAE in naive recipients. Again, the TCR repertoire of T cells before and after culture showed no significant change. These findings suggest that a strong shift from Th2 to Th1 T cell population occurs during culture with encephalitogenic antigen.
Early studies showed that lymphocytes from Lewis rats immunized with GPBP in IFA can be activated in vitro with either GPBP or Con A and transfer EAE to naive recipients 31. The authors assumed that the second signal provided by accessory cells is required for maturation of full encephalitogenic T cells. The present study suggests that the following immunological events take place in Lewis rats (Fig. 10). Immunization with GPBP/IFA did not elicit clinical EAE but produced mild inflammatory lesions in the CNS. CDR3 spectratyping analysis revealed that Vβ 8.2 and Vβ 10 spectratype expansion occurred in the lymphoid organ. This TCR spectratype expansion basically remained unchanged in PT (+) rats (Fig. 4) and in rats immunized with GPBP/CFA 11, strongly suggesting that essentially the same TCR activation occurs after immunization of the same antigen, regardless of the type of adjuvant. However, the cytokine profile at this state, i.e. GPBP/IFA immunization, is Th2 dominant. We have shown in this study that the second signals from several molecules, i.e.in vivo administration of PT and IL-12 and in vitro antigen stimulation, can trigger the development of clinical EAE in rats with subclinically activated T cells by GPBP/IFA immunization. IL-12 plays a central role in the second step of activation, but up-regulation of other pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines is also important, as evidenced by the finding that EAE induced by GPBP/IFA plus IL-12 was significantly milder than that induced by GPBP/IFA plus PT. In rats immunized with GPBP/CFA, these two immunological events occur simultaneously or sequentially.
The events occurring in the second step in this model (Fig. 10) suggest important implications for the pathogenesis of multiple sclerosis (MS). The findings obtained in his study indicate that all the signals that are able to up-regulate the production of Th1 cytokines, mainly IL-12, stimulate subclinically activated encephalitogenic T cells to develop the clinicaldisease. It is probable that similar immunological events take place in the development and relapse of MS that occurs frequently following viral and bacterial infection 32, 33. IFN-β treatment, the trial of which is now under way, appears to be effective in blocking the second step by suppressing the overproduction of IL-12 34. However, it is essential to suppress the disease process at the first step to obtain complete cure or long-term remission. In this regard, DNA vaccination targeting pathogenic TCR to block the first step, which was shown to be effective in the animal model 35, 36, is promising as fundamental immunotherapy for MS.
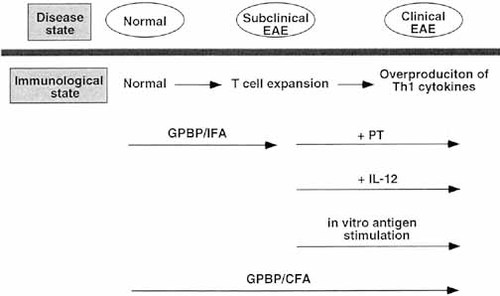
Schematic representation of immunological events occurring in rats, and rats immunized with GPBP/IFA only, GPBP/IFA + PT and GPBP/CFA.
In the present study, we have demonstrated that two-step activation, antigen-driven clonal expansion of disease-inducing T cells and overproduction of Th1 cytokines, is essential for the development of clinical EAE.
4 Materials and methods
4.1 Rats and EAE induction
Lewis rats were purchased from Seiwa (Fukuoka) and used at 8–12 weeks of age. Each rat was injected in both hind footpads with an emulsion containing 100 μ g GPBP in IFA (Difco Labs, Detroit, MI) or CFA (Mycobacterium tuberculosis H37Ra, 5 mg/ml, Difco) and observed daily for clinical signs up to day 21 post-immunization. At the time of immunization, a group of rats receivedintraperitoneal injection of 2 μ g PT (Sigma, Tokyo, Japan). In some experiments, mouse recombinant IL-12 (3 μ g, R & D Systems, Tokyo, Japan), rat recombinant TNF-α (2×104 U, Genzyme Diagnostics, Cambridge, MA) and rat recombinant IFN-γ (5×104 U, R & D Systems) were administered intraperitoneally on days 5, 7 and 9 post immunization. Doses and time schedule of cytokine administration were determined on the basis of data reported previously 37–39. The clinical stage of EAE was divided into four grades (1, floppy tail; 2, mild paraparesis; 3, severe paraparesis; 4, tetraparesis or moribund condition). The rats were killed under ether anesthesia at the indicated time point, and three to four segments of the lumbar SC were snap-frozen in OCT compound. Twenty sections, 20 μ m thick, cut in a cryostat were used for TCR and competitive PCR analysis. Sections, 10 μ m thick, stained with hematoxylin and eosin were used for histological examination. Histological findings were graded into four categories (1, leptomeningeal and adjacent subpial cell infiltration: 2, mild perivascular cuffing; 3, extensive perivascular cuffing; 4, extensive perivascular cuffing and severe parenchymal cell infiltration).
4.2 Immunohistochemical staining procedures
A single immunoperoxidase staining was performed as described previously 23. Briefly, frozen sections were air-dried and fixed in ether for 10 min. After incubation with normal horse serum, sections were allowed to react with one of the following mAb; R73 (anti-T cell receptor α β ), R78 (anti-Vβ 8.2), G101 (anti-Vβ 10) and ED1 (anti-macrophage). After washing, sections were incubated with biotinylated horse anti-mouse IgG (Vector, Burlingame, CA) followed by horseradish peroxidase (HRP)-labeled VECTSTAIN Elite ABC Kit (Vector). HRP binding sites were detected in 0.005% diaminobenzidine and 0.01% hydrogen peroxide.
4.3 cDNA synthesis and PCR amplification
Total RNA was extracted from the LN and SC tissue using RNAzol B (Biotecx Lab, Houston, TX). cDNA was then synthesized by reverse transcription using a SuperScript preamplification system (Life Technologies, Gaithersburg, MD) and amplified in a thermal cycler (Perking Elmer, Norwalk, CT) using primer pairs for TCR and cytokines. The primers for TCR and cytokines were the same as those used in previous studies 10, 13. Cycling conditions for PCR were as follows: 93°C for 1 min for denaturation, 55°C for 1 min for annealing and 72°C for 1 min for extension followed by 40 cycles of 93°C for 1 min, 55°C for 1 min and 72°C for 1 min. PCR products were then electrophoresed on 1.5 % agarose gels containing ethidium bromide, and analyzed with the fluorescence image analyzer on the FMBIO software (Hitachi Software Engineering, Yokohama, Japan).
4.4 CDR3 spectratyping
CDR3 spectratyping was performed as described previously 40 with a few modifications. cDNA was amplified with Vβ -specific and rhodamine-labeled Cβ outer primers and undiluted or diluted PCR products were added to an equal volume of formamide/dye loading buffer and heated at 94°C for 2 min. Samples (2 μ l) were applied to 6% acrylamide sequencing gels. Gels were run at 30 W for 3 h 30 min at 50°C. The fluorescence-labeled DNA profile on the gels was then recorded directly using a FMBIO fluorescence image analyzer (Hitachi).
4.5 Sequencing of PCR products
cDNA isolated from bands on the acrylamide gel was reamplified with Vβ and unlabeled Cβ internal primers. Then, PCR products were ligated into pT-Adv vector and cloned using the AdvanTAge PCR Cloning Kit (Clontech Laboratories, Palo Alto, CA) according to the manufacturer's instructions. The plasmid DNA was then sequenced using Cy5-labeled Cβ internal primer and Autoread Sequencing Kit on a ALFexpress DNA sequencer (Pharmacia Biotech, Tokyo, Japan). CDR3 length is defined as the region starting from the amino acid residue after the CASS sequence of most Vβ segments and ending before the GXG box in the Jβ region as described previously 41.
4.6 Competitive PCR analysis of cytokines
We attempted to quantify mRNA levels of pro-inflammatory (IL-2, IL-12p40, TNF-α and IFN-γ ) and anti-inflammatory (IL-4, IL-10 and TGF-β 1) cytokines by competitive PCR as described previously 13. In this analysis, the competitor DNA and target templates were amplified using the same primer pair in the same reaction. After amplification, PCR products were electrophoresed and analyzed with a fluorescence image analyzer. The amount of target mRNA was estimated from the amount of the competitor DNA added to the reaction. Densities of the target sequenceand competitor bands for each reaction were determined, and the ratios of target DNA to competitor DNA were calculated. The concentration of target DNA was determined as the point at which the ratio of target to competitor was 1.
4.7 ELISA for cytokines
For cytokine assay at the protein level, rats were immunized with GPBP/IFA with or without PT and the regional LN were isolated at the peak stage of EAE (on day 10 post immunization in this experiment). LN cells (2×105 cells/well) were cultured in a 48-well plate in the presence or absence of GPBP (25 μ g/ml) for 72 h. Culture supernatants were collected and tested for cytokines by ELISA. We used currently available IFN-γ and IL-4 ELISA kits (BioSource International, Camarillo, CA). The amount of released IFN-γ and IL-4 was determined in a sandwich ELISAusing pairs of specific capture and biotinylated detection antibodies and streptavidin-HRP according to manufacturer's instructions. Each sample was assayed in duplicate and essentially the same results were obtained.
4.8 Proliferative responses of LN cells against MBP
Proliferative responses of LN cells were assayed in microtiter wells by uptake of [3H]thymidine. After washing with PBS, LN cells (2×105 cells/well) were cultured with the indicated concentrations of GPBP for 3 days, and for the last 18 h in the presence of 0.5 μ Ci [3H]thymidine (Amersham Pharmacia Biotech, Tokyo, Japan). The cells were harvested on glass-fiber filters, and the label uptake was determined using standard liquid scintillation techniques.
4.9 Adoptive transfer of lymphoid cells
For transfer of sensitized cells, LN and spleen cells were isolated from GPBP/IFA-immunized rats 12 days after the immunization. Mixed cells were cultured for 3 days in the presence of 5 μg/ml GPBP and 2×107 cells were injected intravenously into naive animals.
Acknowledgements
We thank Y. Kawazoe, K. Kohyama and K. Nomura for technical assistance. This study was supported in part by Grants-in-Aid (10357005, 09480216 and 09670682)from the Ministry of Education, Japan.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH