Perforin-independent regulation of dendritic cell homeostasis by CD8+ T cells in vivo: implications for adaptive immunotherapy
Abstract
We investigated here the effects of perforin on CTL responses during interaction of dendritic cells (DC) with cytotoxic T lymphocytes in vivo. Using MHC class I tetramers complexed with the immunodominant CTL epitope of the lymphocytic choriomeningitis virus glycoprotein (LCMV-GP33), we followed the kinetics of DC-induced CTL responses. GP33-presenting DC induced rapid primary expansion of both perforin-competent and -deficient CTL with similar kinetics. Secondary CTL responses in perforin-deficient and normal control mice after DC-booster immunization were more rapid thanthe primary responses, but never reached the high initial levels, suggesting that reactivated memory CTL eliminated the antigen-presenting DC and thereby limited the booster effect. Whereas killingof DC in vitro was strictly dependent on perforin, elimination of GP33-presenting DC by CTL in vivo was largely independent of perforin and Fas. Taken together, these results suggest that control of DC homeostasis by CTL, i. e. elimination of DC by the effector cells they had elicited, is controlled via multiple and probably redundant signals and represents an important fail-safe mechanism to avoid exaggerated CTL responses.
Abbreviations:
-
- DC:
-
Dendritic cells
-
- PKO:
-
Perforin-deficient mice
-
- LCMV-GP:
-
Glycoprotein of the lymphocytic choriomeningitis virus
1 Introduction
Optimal presentation of antigen in secondary lymphoid tissues is crucial for the initiation and maintenance of adaptive immune responses 1. Transport of antigen from peripheral non-lymphoid tissues to organized secondary lymphoid organs is most likely regulated by dendritic cells (DC). It has been shown that DC mediate translocation of infectious agents 2 – 4 and soluble antigens 5, 6 from peripheral sites to local draining lymph nodes. In secondary lymphoid organs, DC specifically home to T cell areas 7, 8; however, DC do not recirculate, suggesting that their life-cycle is locally regulated during T / DC-interactions. For example, T helper cell-derived signals may activate DC 9, 10 and prevent or delay DC apoptosis 11, 12. It appears that after initial activation of T cells by DC, DC survival is down-modulated by not yet fully characterized T cell-dependent mechanisms 13.
DC also efficiently activate CTL and thereby elicit protective antiviral 14 and antitumor immunity 15, 16. Fully differentiated CTL lyse their target cells, e. g. virus-infected cells or tumor cells, mainly via perforin-mediated, contact-dependent cytotoxicity 17, 18. In addition, it hasbeen suggested that perforin exerts important immunoregulatory functions, such as controlling activation-induced cell death of CTL 19 or mediating elimination of APC via cytotoxic T cells to prevent uncontrolled CTL activation 20, 21. However, the role of perforin in the regulation of DC persistence in vivo has not been determined to far.
Due to their migratory functions and unique immunostimulatory capacities, DC are well suited for the use in immunotherapeutical approaches 22. Thus, detailed knowledge on mechanisms regulating antigen transport via DC to secondary lymphoid organs and influencing antigen presentation by DC is important to improve further the application of DC in immunotherapy.
The aim of the current study was to investigate the role of perforin during the interaction of DC with CTL in vivo. We used MHC class I tetramers complexed with a well-characterized CTL epitope to follow primary and secondary DC-induced oligoclonal CTL responses in the presence or absence of perforin. Furthermore, we determined the relative contribution of perforin and the Fas / FasL pathway in regulating DC killing via CTL in vitro and in vivo. Our results suggest that regulation of DC homeostasis in vivo is largely independent of perforin- and Fas-mediated cytotoxicity.
2 Results
2.1 Kinetics of DC-induced oligoclonal CTL activation in vivo
In the first set of experiments, we used MHC class I tetramers complexed with the immunodominant CTL epitope (GP33) derived from the glycoprotein of the lymphocytic choriomeningitis virus (LCMV-GP) 23, 24 to follow activation of GP33-specific CTL after immunization with DC. DC (2 × 105) derived from transgenic mice ubiquitously expressing the first 60 aa of LCMV-GP including GP33 (H8-DC) or 2 × 105 control DC derived from C57BL / 6 mice (B6-DC) were injected i. v. into naive C57BL / 6 recipient mice. At different time points post immunization, blood and spleen cells were stained with H2-Db (GP33)-tetramers and the percentage of CD8 lymphocytes positive for GP33-tetramers was calculated. At the maximum of the response after H8-DC immunization, 6 – 10 % of the CD8+ lymphocytes in blood and spleen reacted with the GP33-tetramer (Fig. 1 A). CTL activation via H8-DC was specific because less than 0.1 % of the CD8+ T cells were GP33-tetramer positive after immunization with control B6-DC (Fig. 1 A). The efficient expansion of GP33-specific CTL by H8-DC in the first 8 days was followed by a rapid decrease to levels of 0.5 – 1 % GP33-specific CTL in the CD8 lymphocyte pool (Fig. 1 B).
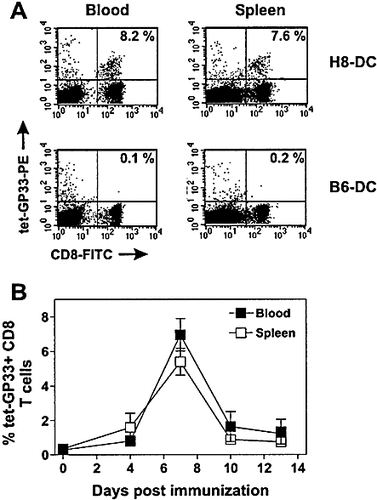
CTL activation after priming with DC as determined by staining with MHC class I tetramers. (A) C57BL / 6 mice were immunized with 2 × 105 H8-DC or control B6-DC i. v. Seven days later, the percentage of GP33-specific CD8 T cells in blood and spleen (shown in the upper right quadrant of the respective histogram) was determined by tetramer staining. (B) Kinetics of GP33-specific CTL expansion after immunization with H8-DC was followed for 2 weeks. Values represent the mean percentage (± SEM) of GP33-specific cells within the CD8+ T cell population. Three mice were analyzed per time point. One representative experiment of two is shown.
Following expansion of GP33-specific CTL after booster immunization on day 13 (Fig. 2 A) or day 30 (Fig. 2 B) post primary immunization by tetramer analysis revealed that memory CTL responded more quickly than naive CTL from untreated control mice. However, the immune responses after secondary immunization were never higher than those after the first DC application (Fig. 2). Furthermore, as with the primary immunization, the secondary response was not sustained and declined to levels of 0.5 to 1 % GP33-specific CTL in the CD8 lymphocyte population both in peripheral blood (Fig. 2) and spleen (data not shown). Taken together, these results show that after the first transient activation of CTL via DC, repeated DC immunization mainly contributed to the maintenance of CTL activity, but did not expand the CTL population above initial levels.
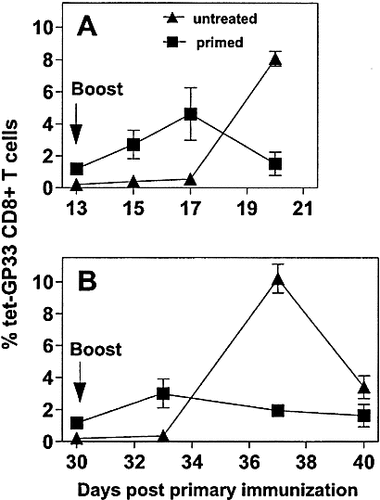
Antigen-specific CTL memory responses after booster immunization. C57BL / 6 mice were primed i. v. with 2 × 105 H8-DC or left untreated. Booster immunization of 5 × 105 H8-DC was given i. v. (A) and day 13 or (B) on day 30 and the percentage of GP33-specific CD8 T cells in blood was determined by tetramer staining at the indicated time points. Untreated C57BL / 6 mice immunized with the same dose of H8-DC served as controls. Values represent the mean percentage of GP33-specific cells (± SEM) within the CD8+ T cell population of three mice per group. One of two comparable experiments is shown.
2.2 Primary and secondary expansion of GP33-specific CTL via DC is not influenced by perforin
One possibility to explain the failure of H8-DC to expand further the GP33-specific CTL pool after secondary immunization may be due to rapid elimination of GP33-presenting APC by restimulated effector CTL. Since lysis of target cells via perforin is a major pathway of cytotoxicity, we assessed whether the presence or absence of perforin would influence the primary expansion or secondary restimulation of GP33-specific CTL via DC. C57BL / 6 (Fig. 3 A, upper panel) or perforin-deficient mice (PKO) (Fig. 3 A, lower panel) were immunized with 2 × 105 H8-DC and the frequency of GP33-specific CTL in peripheral blood and spleen was determined by tetramer analysis. CTL activation was comparable in PKO and C57BL / 6 control mice on day 7 post immunization (Fig. 3 A) and followed similar kinetics (Fig. 3 B, compare with Fig. 1). Moreover, boosting of PKO mice with H8-DC showed only a mild secondary expansion of GP33-specific CTL (Fig. 3 B). Taken together, these results indicate that perforin probably did not play a dominant role in the regulation of primary or secondary DC / CTL interactions.
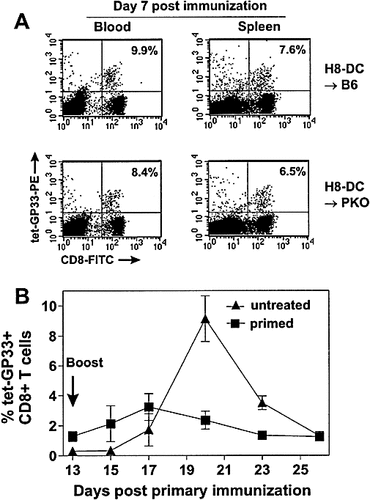
Primary and secondary CTL responses in PKO mice after priming with H8-DC. (A) C57BL / 6 or PKO mice were immunized intravenously with 2 × 105 H8-DC. On day 7, the percentage of CD33-specific CD8 T cells in blood and spleen (shown in the upper right quandrant) was determined by staining with MHC class I / GP33-tetramers. (B) Kinetics of GP33-specific CTL expansion in PKO mice after immunization with H8-DC. Untreated or primed PKO mice (2 × 105 H8-DC i. v. on day 0) were adoptively transfused with 5 × 105 H8-DC and the percentage of GP33-specific CD8 T cells in blood was determined by tetramer staining at the indicated time points. Values represent the mean percentage of GP33-specific cells (± SEM) within the CD8+ T cell population of three mice per group. One representative experiment of two is shown.
2.3 Mechanisms of in vitro and in vivo killing of DC
Next we tested whether and by which mechanisms DC may be eliminated during cognate interaction with specific effector CTL. Since beside perforin, the Fas / FasL pathway is of importance for CTL-mediated cytotoxicity 25, we assessed the susceptibility of DC to perforin and / or Fas-mediated killing by comparing B6-DC and DC derived from Fas-deficient B6.MRL-Faslpr mice (lpr-DC). Two-color FACS analysis revealed that around 90 % of the B6-DC expressed Fas (Fig. 4 A), whereas, as expected, lpr-DC were largely Fas negative (Fig. 4 B). In vitro, GP33-pulsed B6-DC (Fig. 4 C) and lpr-DC (Fig. 4 E) were susceptible to lysis by effector CTL derived from acutely LCMV-infected, perforin-competent mice. Both B6-DC (Fig. 4 D) and lpr-DC (Fig. 4 F) were not lysed by CTL from acutely infected PKO mice, indicating that CTL-mediated lysis of DC in vitro was mainly via perforin and that DC were resistant to cytotoxicity via the Fas / FasL pathway.
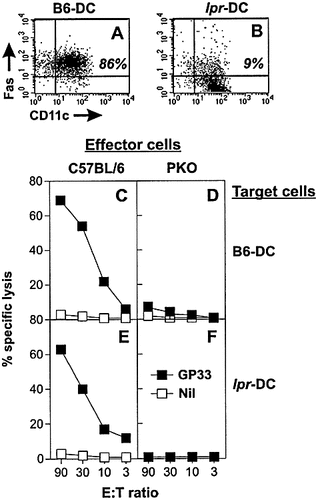
Mechanisms of DC killing in vitro. Fas expression on (A) B6-DC and (B) lpr-DC was determined by two-color FACS analysis. Percentage of CD11c+Fas+ DC are indicated in the upper right quadrant of the histograms. Susceptibility to CTL-mediated lysis in vitro. 51Cr-release assay using (C, D) B6-DC or (E, F) lpr-DC pulsed with GP33 (closed symbols) as target cells and spleen cells from acutely LCMV infected C57BL / 6 (C, E) or PKO (E, F) mice as effectors. Unpulsed 51Cr-labeled DC served as controls (open symbols).
The susceptibility of DC to perforin and / or Fas-mediated killing in vivo was assessed by injecting 5- and 6-carboxyfluorescein diacetate succimidyl ester (CFSE)-labeled DC s. c. into the footpad of naive or acutely LCMV-infected recipient mice and following CFSE-positive DC in the draining popliteal lymph node by immunohistochemistry. H8-DC injected into the footpad homed to the T cell zone of the popliteal lymph node and persisted for > 36 h in naive C57BL / 6 mice (Fig. 5 A and C). Only few H8-DC were found in the popliteal lymph nodes of acutely LCMV-infected mice after 36 h (Fig. 5 B and C). In contrast, homing of B6-DC to the popliteal lymph node in naive and LCMV-infected mice was comparable (Fig. 5 C). Furthermore, persistence of H8-DC in the draining lymph node was also reduced in mice primed 8 days before with H8-DC, although to a lesser extent (Fig. 5 C). Elimination of H8-DC in acutely LCMV-infected C57BL / 6 mice was rapid, because also after 18 h post injection only few H8-DC were found in the draining lymph node (Fig. 5 D). Importantly, H8-DC and GP33-pulsed lpr-DC did not persist in acutely infected PKO mice (Fig. 5 D), indicating that perforin-deficient CTL can eliminate DC in vivo, even in the absence of Fas expression.
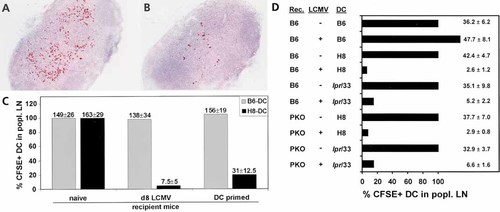
Mechanisms of DC killing in vivo. CFSE-labeled (A) B6-DC or (B) H8-DC were injected s. c. into acutely LCMV-infected C57BL / 6 mice. The draining popliteal lymph nodes were removed after 36 h and CFSE-positive DC were detected by immunohistochemistry. (C) Quantification of B6-DC (grey bars) and H8-DC (black bars) persistence in naive C57BL / 6 control mice and C57BL / 6 mice that had been either infected 8 days previously with 200 pfu LCMV or immunized with 2 × 105 H8-DC. The mean number of DC per section in naive mice was calculated and set 100 %. The absolute DC counts per section (mean ± SEM) are shown on the respective column. (D) Perforin and Fas dependence of DC elimination. CSFE-labeled B6-DC, H8-DC or GP33-pulsed lpr-DC were injected s. c. into the footpad of naive or acutely LCMV-infected C57BL / 6 or PKO mice. Eighteen hours later, the popliteal lymph nodes were removed and the presence of DC was evaluated as described above.
3 Discussion
T cell-dependent mechanisms regulating DC activation and survival are most likely crucial for the outcome of primary and secondary immune responses. The major findings of this study are first, that activation of CTL by DC and the subsequent down-regulation does not dependent of perforin, and second, that elimination of DC during cognate interaction with CTL in vivo is largely independent of perforin and / or Fas / FasL interactions. It has been suggested that in addition to its immediate antiviral 17, 26 and antitumor 18 effector function, perforin may be crucial for homeostatic regulation in the CD8 T cell compartment 27, 28. Potential negative feedback mechanisms include perforin-dependent lysis of professional APC 20, 21 or altered activation-induced cell death 19, 29. In contrast to these previous studies, there was no obvious immunoregulatory function of perforin detectable in the in vivo interaction of DC with CTL. Perforin-competent and -deficient CTL expanded equally well after adoptive transfer of GP33-presenting DC and both populations showed similar kinetics in the contraction phase (compare Figs. 1 B and 4 B). Furthermore, the absence of perforin did not influence the secondary CTL response after booster immunization with DC. It is possible that the previously observed negative feedback regulation via perforin in vivo is restricted to very strong stimuli, such as superantigen stimulation 29, virus infection 29, or severe graft-versus-host disease 19 and that during the relatively weak and transient CTL activation via DC other molecules compensate for the potential regulatory function of perforin. It is important to note that the absence of perforin, Fas or TNFR alone has also only a marginal effect on the elimination of lymphocytes by CTL in vivo 30, further supporting the notion killing of lymphocytes and myeloid cells is a multifactorial process.
The Fas / FasL immunoregulatory pathway is involved in the homeostatic control of the lymphoid system 31. However, despite relatively high Fas expression, DC lack susceptibility to Fas-mediated apoptosis by Th cells 32 and CTL, as shown in this report. Although CTL-mediated killing of DC in vitro was mainly mediated via perforin, in vivo elimination of DC via CTL neither depended on perforin nor on Fas / FasL interaction. Since the contribution of perforin and Fas in the elimination of DC via CTL in vivo appears to be minimal or absent, it is likely that perforin and Fas contribute to the control of DC persistence in concert with other effector mechanisms. Regulation of DC survival on multiple levels would represent an important fail-safe mechanism to prevent exaggerated immune responses.
Th cells regulate initial DC activation, for example via CD40 / CD40L membrane interactions 9, 11 or soluble mediators such as TNF 33. However, T cell-derived cytokines, such as IL-10 34 or TGFβ 35 may inhibit DC function or induce apoptosis of mature DC 11. Therefore, loss of DC in the late stages of cognate Th / DC interactions 13 or after strong nonspecific immune stimulation 36 may be due to massive release of such suppressive cytokines. It is likely that soluble mediators also contribute to the elimination of DC by cytotoxic T cells (37 and in this report). The presented results suggest that these "alternative" cytotoxic pathways are crucial in the regulation of DC homeostasis. It will be important, particularly for clinical applications, to identify these additional cytotoxic effector mechanisms and to elaborate strategies to manipulate selectively DC persistence in vivo. CTL-mediated killing of DC via one antigenic determinant may prevent priming of an immune response against a second antigen presented by the same DC 37. However, as shown in our study, DC are able to efficiently restimulate CTL responses despite the presence of memory CTL. It appears therefore, that priming of naive CTL responses requires a prolonged presentation of antigen via DC whereas the relatively short time window for reactivation of memory CTL via DC is sufficient and necessary to maintain protective immunity against viral infections 38 or to mediate autoimmune disease 39.
The high potency of DC to induce T cell immunity is currently exploited in clinical trials to immunize patients with tumors of hematologic or solid origin 40 – 42. The application of the tetramer technology proved to be advantageous to exactly follow the course of primary and secondary peptide-specific, oligoconal CTL responses after DC priming. Our findings that DC-induced CTL responses are rapidly down-regulated because DC are eliminated by effector CTL impinges on the design of DC-based immunotherapeutical approaches. First, initial optimal CTL activation DC should be achieved before cytotoxic effectors T cells gain the ability to eliminate the antigen-presenting DC. The tetramer technology is probably the method of choice to help to establish optimal protocols in humans. Second, since CTL activity depends on the presence and appropriate presentation of antigen in secondary lymphoid organs 43, reactivation is required to maintain efficient CTL responses. Importantly, the restoration of memory CTL activity is best achieved when the antigen is directly introduced via DC 38.
Taken together, this study delineates general mechanisms involved in initiation and maintenance of CTL responses via DC and provides relevant information how to further optimize DC-based adaptive immunotherapy.
4 Materials and methods
4.1 Mice
C57BL / 6 mice were obtained from the Institut für Labortierkunde (University of Zurich, Switzerland). B6.MRL-Faslpr mice were obtained from The Jackson Laboratories (Bar Harbor, ME).Transgenic mice expressing the LCMV GP33 epitope in all tissues (H8 mice) 44 and mice lacking perforin (PKO mice) 17 have been previously described. Experiments were carried out with age-(6 – 8 weeks) and sex-matched animals.
4.2 Viruses, peptides and cell lines
LCMV WE strain, originally obtained from Dr. F. Lehmann-Grube (Hamburg, Germany), was propagated on L929 cells and plaqued as previously described 45. LCMV-GP peptide KAVYNFATM (GP33) was purchased from Neosystem (Strasbourg, France).
4.3 Preparation of DC
Our method of generation of DC from bone marrow cultures has been previously described 14. For peptide pulsing, DC were resuspended in RPMI / 5 % FCS at 106 / ml and incubated with GP33 (10– 6 M) at 37 °C for 60 min. DC were resuspended at 4 × 105 / ml in BSS and injected in a volume of 0.5 ml i. v. into the tail vein. DC suspensions showed a purity of 60 – 90 % as determined by FACS analysis using anti-CD11c staining (Pharmingen). Fas-expression of DC was determined by two color flow cytometry using anti-CD11c-FITC together with anti-Fas-PE (all Pharmingen). Cell suspensions were analyzed on a FACScan flow cytometer (Becton Dickinson).
4.4 Cytotoxicity assay
Effector cell suspensions were prepared from spleens of infected C57BL / 6 or PKO mice on day 7 after infection. DC were pulsed with LCMV GP33 (10– 6 M, 1.5 h at 37 °C) and used in a standard 5-h 51Cr-release assay. Unlabeled DC served as controls. The supernatant of the cytotoxicity cultures was counted in a Cobra II Gamma Counter (Canberra Packard). Spontaneous release was always below 20 %.
4.5 Construction of tetrameric class I-peptide complexes and flow cytometry
MHC class I (H2-Db) tetramers complexed with GP33 were produced as previously described 23. Briefly, recombinant H2-Db and human β2-microglobulin molecules were expressed in E. coli (the plasmids were kindly provided by John Altman, Emory University, Atlanta). Biotinylated H2-Db peptide complexes were purified using an Aekta Explorer 10 chromatography system (Pharmacia, Sweden) and tetramerized by addition of streptavidin-PE (Molecular Probes, Eugene, OR).
At the indicated time points after DC immunization, single-cell suspensions were prepared from spleen and aliquots of 5 × 105 cells or three drops of blood were stained using 50 μl of a solution containing tetrameric class I-peptide complexes at 37 °C for 10 min followed by staining with anti-CD8-FITC (Pharmingen) at 4 °C for 20 min. Erythrocytes in blood samples were lysed with FACS lysis solution (Becton Dickinson) and the cells were analyzed on a FACScan flow cytometer (Becton Dickinson) after gating on viable lymphocytes.
4.6 Immunohistology
CFSE (Molecular Probes) was used to label DC 14. CFSE-labeled DC (2 × 105 – 3 × 105) were injected s. c. into the left and right footpad of naive or pre-immunized recipient mice (three mice per group). Draining popliteal lymph nodes were removed after 18 or 36 h post injection, immersed in HBSS and snap-frozen in liquid nitrogen. Histological procedures were performed as described 46 using rabbit anti-fluorescein antibody (Dako) to detect CFSE-positive cells. Two to three sections from each lymph node were evaluated and the mean number of DC per section was calculated.
Acknowledgements
We thank Lenka Vlk and Anne Henzelin for expert technical assistance and Norbert Wey and Ida Schmieder for excellent photographical work. This work was supported by the Swiss National Science Foundation, and the Kanton Zürich.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




