Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H
Abstract
To understand immune evasion mechanisms of Borrelia burgdorferi we compared serum-resistant B. afzelii and serum-sensitive B. garinii isolates for their capacity toacquire human complement regulators. Here we demonstrate that the two borrelial genospecies show different binding of the two important human complement regulators, FHL-1/reconectin and Factor H. All serum-resistant B. afzelii isolates bound FHL-1/reconectin and also Factor H, and all analyzed serum-sensitive B. garinii isolates showed no or a significantly lower binding activity. Using recombinant deletion mutants, the binding domains were localized to the C terminus of FHL-1/reconectin to short consensus repeats 5–7. The borrelial binding proteins were located in the surface of the bacteria as demonstrated by immunofluorescence staining of intact, serum-exposed bacteria and by enrichment of outer membrane proteins. The surface-attached complement regulators maintained complement regulatory activity as demonstrated in a cofactor assay. By ligand blotting two different borrelial binding proteins were identified that were responsible for the surface attachment of FHL-1/reconectin and Factor H. These borrelial complement regulators acquiring surface proteins (CRASP) were further characterized as either CRASP-1, a 27.5-kDa molecule which preferentially binds FHL-1/reconectin and which was present in all serum-resistant borreliae, or CRASP-2, a 20/21-kDa protein which interacts preferentially with Factor H and the expression of which was more restricted, being detected in four of the six isolates analyzed. In summary, we describe a new immune evasion mechanism of B. burgdorferi, as these bacteria acquire human complement regulators to control complement activation on their surface and to prevent formation of toxic activation products.
Abbreviations:
-
- SCR:
-
Short consensus repeats
-
- VBS:
-
Veronal-buffered saline
-
- rprotein:
-
Recombinant protein
-
- OspC:
-
Outer surface protein C
1 Introduction
The innate immune system is essential for the elimination of microorganisms 1, 2 and the complement system is a particularly important and effective part ofthis important defense system. As the complement system is immediately and directly activated upon entrance of a pathogen, control of its activation is of great importance. One strategy of microorganisms has recently attracted particular interest, the ability of many human pathogens to acquire human fluid-phase complement regulators. Binding of the soluble human complement regulators and adhesion proteins such as FHL-1/reconectin and complement Factor H has been demonstrated for Streptococcus pyogenes 4, S. pneumoniae 5 Neisseria meningitidis 6, N. gonorrhoeae 7, Echinococcus granulosus 8, Yersinia enterocolitica 9 and also the human immunodeficiency virus 10. Binding of these regulators allows control of complement activation directly at the surface of the pathogen and results in protection and enhanced survival 11, 12.
FHL-1/reconectin and Factor H are two central human complement regulators that control early steps in the complement activation cascade. Both proteins are structurally related and are encoded by the same gene, and their transcripts are derived by alternative processing of a nuclear RNA transcript 15, 16. The two plasma proteins are exclusively composed of individually folding protein domains, which are termed short consensus repeats (SCR). The 42-kDa FHL-1/reconectin protein is composed of 7 SCR and the 150-kDa Factor H glycoprotein is composed of 20 SCR domains. The SCR of FHL-1/reconectin are identical to the N-terminal domain of Factor H, but the protein has an unique C-terminal extension of four amino acids. As the major regulatory domains of the complement regulators are located in the N-terminal SCR 1–4, both proteins share the same complement regulatory functions: they control C3b formation and stability by acting as cofactor for Factor I-mediated degradation of C3b and the decay of the C3 convertase 17–19.
Borrelia burgdorferi sensu lato complex, including B. burgdorferi sensu stricto (s.s.), B. garinii and B. afzelii, and other closely related Borreliae are the causative agents of Lyme disease or Lyme borreliosis 20. Spirochetes are transmitted to the human host during the blood meal of infected ticks by invasion of a small number of organism into the dermis of the human host. The initial skin infection is often accompanied by a local skin rash (erythema migrans), which appears to be self-limiting in most cases. Untreated Lyme disease can develop into a chronic, multisystemic disorder by hematogenous dissemination of the pathogen, which may affect the joints, heart, central nervous system and skin, leading to manifestations such as Lyme arthritis, carditis, neuroborreliosis, or acrodermatitis chronica atrophicans 20.
The molecular mechanisms of the interaction of B. burgdorferi with the innate immune system, in particular with the complement system, and the mechanisms of immune evasion are the focus of extensive research. Borreliae can activate the alternative and also the classical complement pathway in the absence of Borrelia-specific antibodies 21–24. There is, however, a striking heterogeneity of B. burgdorferi isolates with regard to their complement resistance 21–24. Several isolates, mainly members of the genospecies B. afzelii resist the bactericidal activity of non-immune human serum (NHS), whereass isolates of the genospecies B. garinii are killed effectively by the complement cascade 21–24. These studies demonstrate the existence of both serum-resistant and serum-sensitive isolates of B. burgdorferi.
To gain a better understanding of the mechanism of this complement resistance, we have characterized serum-sensitive and serum-resistant B. burgdorferi isolates for their capacity to acquire human complement regulators. Here we demonstrate that the two groups show different binding of the two important human complement regulators FHL-1/reconectin and Factor H. Binding of FHL-1/reconectin correlates directly with the serum sensitivity observed after incubation with NHS. All complement-resistant B. afzelii isolates bound FHL-1/reconectin and also Factor H and all serum-sensitive B. garinii isolates analyzed showed a dramatically reduced binding activity. Using recombinant deletion mutants the binding domains were localized within the FHL-1/reconectin protein. Apparently the interacting region of the protein is located within the SCR domains 5–7. In addition, we identified two borrelial proteins of 27.5 kDa and of a 20/21 kDa in the outer membrane of various serum-resistant B. afzelii isolates, which were termed complement regulators acquiring surface proteins CRASP-1 and CRASP-2 and which exhibited different binding characteristics for FHL-1/reconectin and Factor H.
2 Results
2.1 Binding of complement regulators to different B. burgdorferi isolates
To determine how Borreliae control complement activity on their surface, we inverstigated whether serum-resistant and serum-sensitive isolates differ in their binding capacities for human complement regulators. Serum-resistant and serum-sensitive isolates were incubated in NHS (derived from non-immune, non-vaccinated individuals). After extensive washing bound proteins were eluted, separated by SDS-PAGE and analyzed by Western blotting for binding of the two central human complement regulators FHL-1/reconectin and Factor H. The results demonstrate that all analyzed serum-resistant isolates (EB1, EB2, FEM1-C1, FEM1-D15, PKo, and FEM2) bound both complement regulators, while none of the analyzed serum-sensitive isolates (PBr, PSth, G1, G2 and A87SB) bound these plasma proteins at a comparable intensity (Fig. 1). FHL-1/reconectin was detected as a 42-kDa and Factor H as a 150-kDa protein. Binding was considered specific as the majority of the bound protein was detected in the elute fraction and only a minor fraction was present in the wash fraction. Binding of these regulators was concentration dependent; for FHL-1/reconectin, binding was detected when 108 or 109 cells were incubated in 750 μ l NHS, although with the serum-sensitive isolate PSth no binding was observed using the same number of cell (data not shown).
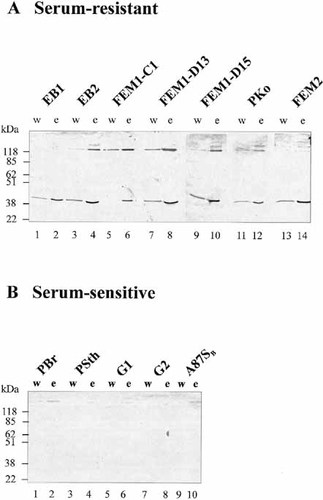
Binding of human complement regulators FHL-1/reconectin and Factor H by different B. burgdorferi isolates. (A) Serum-resistant B. afzelii isolates (EB1, EB2, FEM1-C1, FEM1-D15, PKo and FEM2) were incubated in NHS-EDTA. Borreliae were extensively washed and bound proteins were eluted using 0.1 M glycine, pH 2.0. Both the wash (w) and the elute fractions (e) obtained from each isolate were separated by SDS-PAGE, transferred to nylon membranes and probed with rabbit antiserum recognizing FHL-1/reconectin and Factor H. (B) Serum-sensitive B. garinii isolates (PBr, PSth, G1, G2 and A87SB) incubated in NHS were thoroughly washed with PBS and bound proteins were eluted and separated by SDS-PAGE as described in (A). Using these strains no binding of the two human complement regulators was detectable. The mobility of the size markers is indicated on the left.
2.2 Adsorption from human serum
To determine the adsorption of the human complement regulators from serum in more detail, we compared the concentration of the two regulators in human plasma to that in the wash and elute fraction (Fig. 2). Using the serum-resistant isolate FEM1-D15 and antisera specific for FHL-1/reconectin and Factor H, a large accumulation of the FHL-1/reconectin protein was detected on the surface of Borreliae (Fig. 2A, compare lanes 1 and 3). In addition, an antiserum that detects several members of the Factor H protein family, i.e. FHR-1α, FHR-1β, FHR-2α and FHR-2 31, revealed binding of these four proteins to isolate FEM1-D15 and higher levels of the two FHR-2 isoforms on the Borreliae surfaces as compared to human serum (Fig. 2A; lanes 5 and 6). In contrast, the serum-sensitive isolate PSth did not bind any of the regulators or additional members of the Factor H protein family (Fig. 2B).
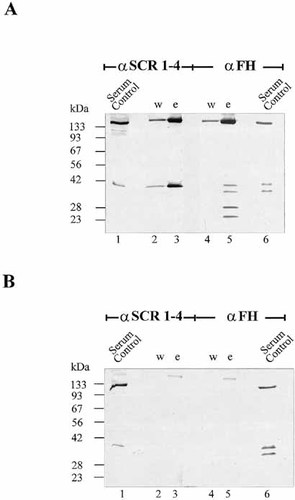
Adsorption of FHL-1/reconectin and Factor H from human serum by B. burgdorferi. (A) NHS (serum), as well as wash (w) and elute fractions (e) obtained from the serum-resistant B. afzelii isolate FEM1-D15 were separated by SDS-PAGE. After transfer to nitrocellulose proteins were identified by Western blotting using antiserum specific for FHL-1/reconectin and Factor H (αSCR1–4) (lanes 1–3). The specific antiserum detects the 42-kDa FHL-1/reconectin and the 150-kDa Factor H protein in NHS (lane 1) and the same proteins in the elute fractions of FEM1-D15 (lane 3). In addition an antiserum raised against Factor H (αFH) that detects Factor H as well as additional members of the Factor H protein family was used (lanes 4–6). (B) Similar fractions were obtained from the serum-sensitive B. garinii isolate PSth and analyzed by anti-SCR 1–4 (lanes 1–3) and anti-Factor H antiserum (lanes 4–6). The mobility of the size markers is indicated on the left.
2.3 Identification of other cell bound proteins
Having demonstrated specific binding and enrichment of complement regulators from NHS, we wanted to determine whether plasma proteins in general were absorbed from the Borreliae isolates, or whether borrelial outer surface proteins were removed from the bacterial surface under these conditions. The wash and elute fractions of serum-resistant and serum-sensitive isolates were obtained after incubation in PBS and NHS, and the samples were separated by SDS-PAGE and analyzed by silver staining. More proteins were detected in the elute fraction of the serum-sensitive isolate PSth compared to the serum-resistant isolate FEM1-D15 (Fig. 3A; compare lanes 4 and 8). For a direct comparison of the mobility, purified Factor H (lane 9) and recombinant FHL-1/reconectin (lane 10) were separated in the same gel. This analysis indicates that adsorption of human plasma proteins is specific, but also that several proteins are released from the bacterial surface during the conditions used for elution. In particular, the small number and low amounts of protein detected in the serum-resistant isolate FEM1-D15, which strongly absorbs both FHL-1/reconectin and Factor H from human serum (Fig. 3B), highlights the specificity of the adsorption. The slower mobility of the plasma form of FHL-1/reconectin to the recombinant protein (Fig. 3A and 4B) is explained by the histidine tag of 3.15 kDa that is attached to the recombinant form.
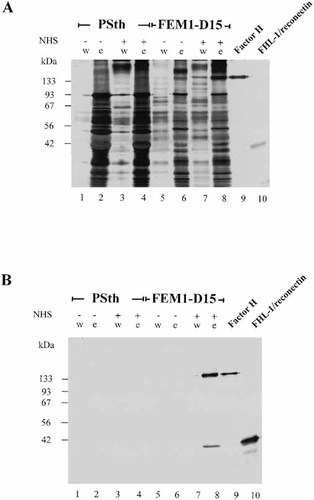
Characterization of eluted proteins by silver staining. Serum-sensitive isolate PSth, as well as serum-resistant isolate FEM1-D15 were either used directly or were incubated in NHS. The various wash and elute fractions, as well as purified Factor H and FHL-1/reconectin were separated by SDS-PAGE and analyzed by silver staining (A) or by Western blotting (B). As a comparison the 150-kDa Factor H protein (lane 9) and a recombinant FHL-1/reconectin protein (lane 10) were separated in the same gel. The mobility of the size markers is indicated on the left.
2.4 Localization of the binding domain of FHL-1/reconectin to B. burgdorferi
Recombinantly expressed FHL-1/reconectin protein was assayed for binding to serum-resistant isolate FEM1-D15 and compared to serum-sensitive isolate PSth. As found with the serum form, the recombinant FHL-1/reconectin also bound specifically to the serum-resistant isolate FEM1-D15, but not to the serum-sensitive PSth (Fig. 4A, lane 6 and Fig. 4B lane 6).
Localization of the domain required for binding of FHL-1/reconectin to B. burgdorferi. (A) Serum-resistant isolate FEM1-D15 was incubated with recombinant FHL-1/reconectin (lane 6), the indicated deletion mutants of this protein (lanes 1–5), as well as human serum (NHS) (lane 7). The elute fractions were separated by SDS-PAGE and analyzed by Western blotting. (B) Serum-sensitive isolate PSth was incubated with recombinant FHL-1/reconectin (lane 6), and the indicated deletion mutants (lanes 1–5). The elute fractions were separated by SDS-PAGE and analyzed by Western blotting. The mobility of the size markers is indicated on the left.
FHL-1/reconectin is composed of seven individually folding SCR domains 15. Binding of selected deletion mutants of FHL-1/reconectin, which have individual SCR domains sequentially deleted from the C terminus, to serum-resistant Borreliae allowed the interacting domain(s) to be localized. Again binding to serum-resistant isolate FEM1-D15 was analyzed and compared to that of the serum-sensitive isolate PSth. In addition to recombinant FHL-1/reconectin deletion mutants consisting of SCR 1–6, SCR 1–5 bound to serum-resistant isolate FEM1-D15, but not to serum-sensitive isolate PSth (Fig. 4A, B). No binding was observed for deletion mutant, which include SCR 1–4, or even further deletions. These data indicate the existence of two interacting sites on the host FHL-1/reconectin protein. Thus, the human immune regulator may either bind to two different borrelial proteins or has two distinct sites which interact with a single borrelial protein.
2.5 Binding of FHL-1/reconectin to intact Borreliae
Immunofluorescence staining of intact, unfixed cells revealed binding of FHL-1/reconectin to serum-resistant FEM1-D15. After incubation with recombinant FHL-1/ reconectin, the human immune regulator was distributed over the entire cells, suggesting that the binding borrelial proteins were equally expressed on the surface (Fig. 5A). This staining pattern is considered specific as it was not detected with serum-sensitive PSth cells (Fig. 5B). To demonstrate intactness of the bacterial cells and to exclude that staining resulted from damaged membranes, Borreliae were incubated with an antibody specific for the surface-expressed outer membrane protein C (OspC), which resulted in uniformly labeled cells (Fig. 5C, D). Intactness of the bacteria was further confirmed by demonstrating lack of reactivity for the cytosolic HSP70 protein (data not shown). The staining pattern revealed a different distribution of the FHL-1/Factor H binding protein(s), which shows a spotted/granular pattern and a uniform distribution of OspC.
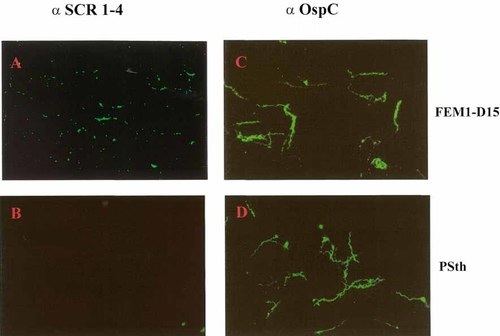
Detection of FHL-1/reconectin on the surface of intact borrelial cells. Serum-resistant FEM1-D15 and serum-sensitive PSth isolates were incubated with recombinant FHL-1/reconectin. Bound proteins were detected by immunofluorescence microscopy after incubation of isolate FEM1-D15 (A) and PSth (B) with an antiserum specific for FHL-1/reconectin. Surface-exposed borrelial proteins were detected by incubation of isolate FEM1-D15 (C) and PSth (D) with mAb specific for OspC. ×1,000.
2.6 Cell bound FHL-1/reconectin displays cofactor activity
To determine whether FHL-1/reconectin bound to the surface of Borreliae displays complement regulatory activity, we analyzed the C3b inactivating capacity of surface-attached and of eluted proteins following incubation of serum-resistant isolate FEM1-D15 and serum-sensitive isolate PSth in NHS or with the recombinant FHL-1/reconectin protein. Upon incubation with human serum or recombinant FHL-1/reconectin cofactor activity for Factor I-mediated cleavage of C3b was observed in serum-resistant isolate (FEM1-D15), whereas no or only a negligible conversion occurred in serum-sensitive isolate (PSth). Significant functional activity of absorbed FHL-1/reconectin and Factor H could be demonstrated on the microbial surface (Fig. 6A) or after elution of the proteins from the bacterial surfaces (Fig. 6B). Borrelia treated in veronal-buffered saline (VBS) and their elutes revealed no cofactor activity. No activity was observed when Borreliae were incubated in a medium containing an unrelated recombinant protein (rprotein) expressed in the same system. These results demonstrate that acquisition of FHL-1/reconectin to the surface of Borreliae enhances their complement control capacity. That a protease activity is responsible for the C3b-inactivating activity can be excluded, as inactivation was observed after incubation in NHS or recombinant FHL-1/reconectin protein, but not after treatment of the same genospecies in VBS or upon incubation with an unrelated protein synthesized in the same expression system (Fig. 6).
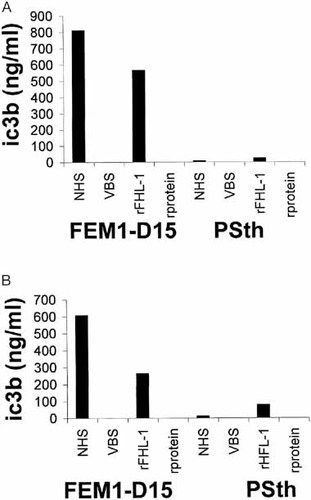
C3b-inactivating capacity of FHL-1/reconectin and Factor H. The functional activity of the absorbed human immune regulators was demonstrated for regulators directly adsorbed on the bacterial surface and for proteins eluted form the bacterial surface. After incubation of serum-resistant (FEM1-D15) and serum-sensitive (PSth) isolates with either NHS or with recombinant FHL-1 (rFHL-1) Borreliae were extensively washed; rprotein represents an unrelated recombinant protein expressed in the same insect expression system. (A) Cofactor activity of adsorbed FHL-1 on the cell surface was determined by incubation of Borreliae with C3b and Factor I. In (B) Factor I-mediated cleavage of C3b was measured after elution of the bound proteins from the bacterial surface. Borreliae incubated in VBS or unrelated rprotein expressed in the baculovirus expression system served as negative control.
2.7 Identification of the borrelial protein(s) binding FHL-1/reconectin or Factor H
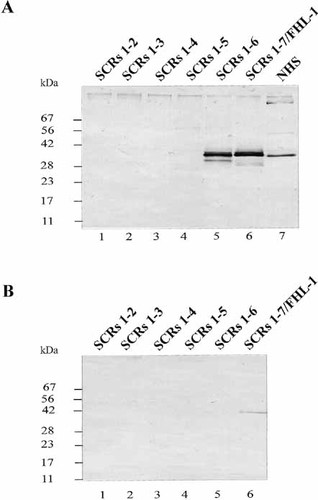
Having demonstrated binding of two human complement regulators to B. burgdorferi, we wanted to identify the bacterial protein(s) involved in this interaction. Cell extracts were generated from various serum-resistant B. afzelii isolates (EB1, EB2, FEM1-C1, FEM1-D15, PKo, and FEM2) and serum-sensitive B. garinii isolates (PBr, PSth, G1, G2, and A87SB) and used for ligand blotting. These extracts were separated on a 10% tricine gel, transferred to nitrocellulose membrane and incubated with recombinant FHL-1/reconectin. The blot was developed using a rabbit antiserum specific for SCR 1–4 of FHL-1/reconectin and Factor H (Fig. 7). All six serum-resistant B. afzelii isolates showed a dominant binding protein of approximately 27.5 kDa. The different mobilities of these binding proteins of the six serum-resistant B. afzelii extracts reveal the heterogeneity of the proteins. In addition, a second binding protein of lower mass was identified for EB2, FEM1-D15 and PKo. This protein of approximately 20 or 21 kDa also shows heterogeneity in size, and is either expressed at lower concentration on the borrelial surface or is bound by the immune regulators with apparently lower intensity. Expression of this smaller protein appears more restricted as it is only detected in three of the six serum-resistant isolates.
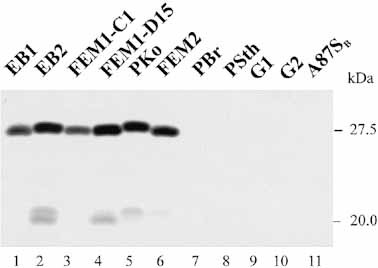
Identification of binding proteins for FHL-1/reconectin and Factor H in Borreliae. Whole cell extracts obtained from the indicated B. burgdorferi isolates were separated by 10% tricine gels and transferred to nitrocellulose. The membranes were incubated with recombinant FHL-1/reconectin and binding of the protein was visualized with antiserum specific for SCR 1–4 of FHL-1/reconectin and Factor H. The sizes of the binding proteins is indicated on the right.
2.8 Localization of FHL-1/reconectin binding domain(s)
To differentiate the two borrelial binding proteins and to characterize the domain of FHL-1/reconectin which binds to these proteins, the various recombinant deletion mutants were used for ligand blotting. This experiment showed different binding characteristics of the 27.5- and 20-kDa borrelial proteins of serum-resistant isolate FEM1-D15 for FHL-1/reconectin. The 27.5-kDa protein bound the intact protein SCR 1–7 and the deletion mutants containing SCR 1–6 and SCR 1–5 with weaker intensity, but did not bind deletion mutants SCR 1–4, SCR 1–3 and SCR 1–2 (Fig. 8). The 20-kDa borrelial protein bound the intact FHL-1/reconectin protein and the mutant SCR 1–6 weakly, but did not bind SCR 1–5. In summary, these experiments reveal the existence of two B. afzelii proteins of 27.5 and 20/21 kDa, which both bind the human complement regulator FHL-1/reconectin. However, these two proteins bind this human immune regulator with different affinities and interact with different domains.
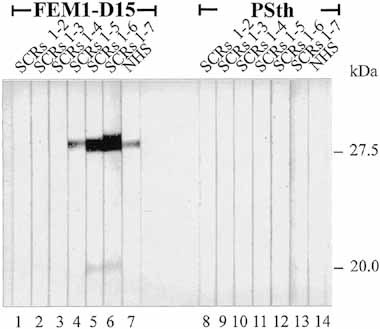
Identification of the FHL-1/reconectin binding domains. Whole cell extract of serum-resistant isolate FEM1-D15 (lanes 1–7) and of serum-sensitive isolate PSth (lanes 8–14) separated by 10% tricine gels were transferred to nitrocellulose and the membranes were incubated with the indicated deletion mutant SCR 1–2 (lane 1); SCR 1–3 (lane 2); SCR 1–4 (lanes 3, 10); SCR 1–5 (lanes 4, 11); SCR 1–6 (lanes 5, 12), intact recombinant FHL-1/reconectin (lanes 6, 13) and NHS (lanes 7, 14). Bound proteins were visualized using antiserum specific for FHL-1/reconectin and Factor H. The size of the binding molecules is derived from the mobility of the marker proteins.
2.9 Further characterization and localization of the binding proteins to the outer membrane
The mapping experiments describe above showed binding of both FHL-1/reconectin and Factor H to intact Borreliae and the existence of two borrelial binding proteins. To identify the binding preference of the two borrelial proteins for the two human immune regulators, due to the lack of a specific FHL-1/reconectin antibody, we used a differential staining approach. The reactivity of an antiserum specific for FHL-1/reconectin and Factor H was compared to that obtained with mAb VIG8, which is specific for the Factor H. mAb VIG8 revealed specific binding of Factor H to the 20-kDa protein (Fig. 9A, lane 2), while the polyclonal antiserum, which detects both immune regulators, identified both borrelial proteins as ligands (Fig. 9A, lane 1).
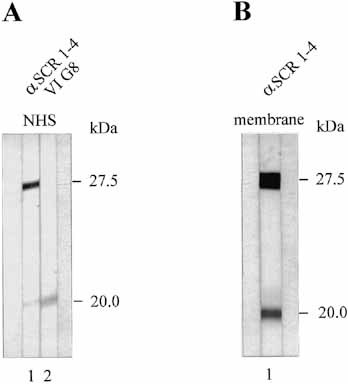
Further characterization and localization of the borrelial binding proteins. (A) Differential binding of FHL-1/reconectin and Factor H to the serum-resistant isolate FEM1-D15. Cell extracts were treated as described in Fig. 8. After incubation of the membranes with NHS the filters were developed with antiserum specific for SCR 1–4 of FHL-1/reconectin and Factor H (lane 1) or monoclonal antibody VIG8 which is specific for human Factor H (lane 2). (B) Localization of the binding. Purified outer membrane proteins of the serum-resistant FEM1 wild-type isolate were prepared and used for ligand blotting.
To confirm the surface localization of the two borrelial FHL-1/Factor H binding proteins, an outer membrane protein fraction was prepared from wild-type serum-resistant FEM1, separated on tricine gel and, after transfer to nitrocellulose, was used for ligand blotting. The strong intensity of the detection signal showed enrichment of the two binding proteins in this membrane extract (Fig. 9B).
3 Discussion
Here we describe a new immune evasion mechanism of B. burgdorferi; the bacteria acquire human complement regulators from serum to control complement activation on their surface and toprevent formation of toxic activation products.
The species B. burgdorferi s.s., B. garinii and B. afzelii are transmitted by infected ticks and are the causative agent of the multisystemic disorder termed Lyme disease or Lyme borreliosis 20. Upon entry into the human host, Borreliae are immediately exposed to the human complement system and to survive they must find means to inactivate this powerful host defense system. B. burgdorferi isolates differ in their capacity to resist cell lysis by human serum and, according to their serum susceptibility, they can be grouped into serum-resistant and serum-sensitive isolates. Initial experiments have shown that most of the serum-sensitive isolates belong to the genospecies B. garinii, while serum-resistant isolates were grouped to the genospecies B. afzelii 22–24. This resistance correlates directl with the control of complement activation, as shown by the lower number of complement activation products deposited on the surface of resistant compared to sensitive strains 22. Here the molecular mechanism(s) of serum resistance is shown for the first time to be due to the capacity to acquire the two human plasma regulators FHL-1/reconectin and Factor H.
Acquisition of these immune regulators results in enhanced complement regulatory activity and eventuates in immune evasion (Fig. 6). The characterization of the binding mechanisms and the localization of the interacting domains shows that two surface proteins located in the outer surface of serum-resistant isolates bind the human complement regulator FHL-1/reconectin at two distinct sites. In their bound configuration these human immune regulator maintain the complement regulatory activity (Fig. 6). All serum-resistant isolates containeda 27.5-kDa protein, that interacts particularly with FHL-1/reconectin, while an additional 20/21-kDa protein, that binds both Factor H and FHL-1, was present in four of the six serum-resistant B. afzelii isolates.
All six serum-resistant B. afzelii isolates analyzed expressed at least one surface protein that bound FHL-1/reconectin and Factor H, while none of the five analyzed serum-sensitive B. garinii isolates bound significant amounts of these human plasma proteins (Fig. 1). The use of recombinant deletion mutants of FHL-1/reconectin indicates the existence of a major interacting site, located within the C-terminal domains SCR 5–7 (Fig. 4). These data were also confirmed by immunofluorescence and affinity ligand blotting (Fig. 5 and 6).
The complement regulatory domain of FHL-1/reconectin is located within the N-terminal part of the protein, i.e. SCR 1–4 17–19. As FHL-1/reconectin attaches to the borrelial surface via its C terminus, the protein has the N-terminal complement regulatory domains oriented to the outside. Thus, upon binding to Borreliae, FHL-1/reconectincontrols C3b deposition and C3-convertase activity on or near the bacterial surface (Fig. 6). Degradation of C3b is specific, as (i) it is observed only in serum-resistant isolate FEM1-D15 and not in the serum-sensitive isolate PSth, (ii) it is observed upon incubation in NHS, but not in VBS, which excludes the possibility of C3b degradation by an unspecific surface-bound protease, (iii) incubation in NHS as well as in recombinant FHL-1/reconectin showed similar C3b-generating activities, and (iv) this activity is not observed after incubation with culture supernatant of Sf9 insect cell. The activity of FHL-1/reconectin on the borrelial surface reduces the absolute number of deposited and inactivated iC3 molecules dramatically, and this reduces phagocytosis of thesemicroorganisms. In summary, the data reported here show that the complement inhibitory function of FHL-1/reconectin (and of Factor H) is/are highly relevant for immune evasion of Borreliae. The identification and biochemical characterization of the corresponding proteins is, therefore, expected to identify new and important virulence factors of these bacteria.
In addition to B. burgdorferi, several other microorganisms such as S. pyogenes, S. pneumoniae, N. gonorrhoeae, Y. enterocolitica, E. granulosus and also the human immunodeficiency virus have been shown to bind human complement regulators such as FHL-1/reconectin and Factor H 4–10. In several cases the interacting bacterial proteins, which are located on the surface of these microorganisms, have been identified. For streptococci, the M protein, a major virulence factor expressed on the surface of the bacteria, and in N. gonorrhoeae the sialylated lipooligosaccharide (LOS) or porin, the major outer membrane protein 7, 13, have been identified as ligands for FHL-1/reconectin or Factor H. Apparently, the surface proteins of all microorganisms bind these host regulators via their C terminus, but attach the proteins at different sites. B. burgdorferi binds FHL-1/reconectin within domains SCR 5–7 (Fig. 4A), S. pyogenes binds the same protein preferentially at SCR 7 14 and LOS of N. gonorrhoeae binds Factor H inthe SCR 16–20 region. In contrast to streptococci, serum-resistant Borreliae express two surface proteins, which both bind FHL-1/reconectin, but apparently attach different C-terminal sites of this protein. Both the 27.5 and the 20-kDa borrelial proteins are unique, and the 27-kDa form represents the first bacterial proteins shown to bind to a domain within or close to SCR 5–7.
4 Materials and methods
4.1 Borreliae isolates and culture conditions
All Borreliae isolates used in this study were obtained from human biopsy material. Clonal FEM1-D15 and the OspC-lacking variant FEM1-C1 25 were derived from wild-type B. afzelii isolate FEM1 by twofold single-colony plating on solid medium as described 26. Serum-resistant B. afzelii EB1, EB2, FEM1-C1, FEM1-D15, PKo, and FEM2, serum-sensitive B. garinii PBr, PSth, G1, G2, and A87SB 24 were cultured until mid-log phase (5×107 cells/ml) at 33°C in modified Barbour-Stoenner-Kelly (BSK) medium 27. Samples of 1.8 ml were dispensed into screw-cap tubes (Nunc, Wiesbaden, Germany), frozen at –70°C, and used as stock cultures. Prior to use, afrozen suspension of spirochetes was thawed and inoculated into fresh BSK medium. The density of spirochetes was determined using dark-field microscopy and a Kova counting chamber (Hycor Biomedical, Garden Grove, CA).
4.2 Non-immune human serum
Sera (NHS) from twenty healthy human blood donors without a known history of a spirochetal infection were tested for the presence of IgG and IgM antibodies against B. burgdorferi by acommercial whole cell ELISA (Dade Behring, Marburg, Germany) and a immunoblot with recombinant proteins (Mikrogen, Martinsried, Germany). Only sera that proved negative in all assays were combined to form the NHS pool.
4.3 Expression of recombinant proteins
Recombinant proteins were expressed in insect cells infected with recombinant baculovirus. The cloning of various recombinant deletion constructs, expression and purification was performed as described 17, 28. Spodoptera frugiperda cells (Sf9) were grown at 28°C in monolayer cultures in protein-free expression medium (BioWhittaker, Verviers, Belgium) in the presence of streptomycin (100 μg/ml), penicillin (100 U/ml) and amphotericin B (250 ng/ml) (Life Technologies, Eggenstein, Germany). Adherent Sf9 cells were infected with recombinant virus using a multiplicity of infection of five. The culture supernatant was harvested after 9 days and used directly for adsorption experiments.
4.4 Serum adsorption experiments
Borreliae (5×108 cells) were grown to mid-log phase, harvested by centrifugation (5,000×g, 30 min, 4°C) and resuspended in 100 μl VBS (supplemented with 1 mM Mg2+, 0.15 mM Ca2+, 0.1% gelatin, pH 7.4). The cell suspension was then incubated in 750 μl NHS supplemented with 0.34 mM EDTA or in 1.4 ml supernatant of Sf9 insect cells infected with recombinant FHL-1/reconectin or the various recombinant deletion mutants (consisting of SCR 1–6, SCR 1–5, SCR 1–4, SCR 1–3, SCR 1–2) 17 for 1 h at room temperature with gentle agitation. After three washes with 0.15 M NaCl, 0.03 M phosphate, 0.02% sodium azide, pH 7.2 (PBSA) containing 0.05% Tween-20, the proteins bound to the Borreliae were eluted by incubation with 0.1 M glycine-HCl, pH 2.0, for 15 min. The bacterial cells were sedimented by centrifugation (14,000×g, 20 min, 4°C), and the proteins in the supernatant were analyzed by SDS-PAGE and Western blot experiments.
4.5 Isolation of borrelial outer membrane fractions
Fractions of the outer membranes of wild-type B. afzelii FEM1 were isolated as described previously 30.
4.6 SDS-PAGE and Western Blot analysis for detection of absorbed FHL-1/reconectin and Factor H
Wash and elute fractions obtained from the various Borreliae isolates were subjected to SDS-PAGE using 10% or 12% gels and, after transfer onto nitrocellulose membranes, proteins werevisualized by semidry blotting 17. The membranes were blocked with 5% (w/v) dried milk in PBS for 30 min and incubated overnight with polyclonal rabbit anti-Factor H SCR1–4 antibody (raised against the N-terminal SCR 1–4 of the protein) as described 19.
For the detection of binding proteins for FHL-1/reconectin and Factor H whole cell extracts of the indicated Borreliae isolates were generated by sonication using a Branson B-12 sonifier (Heinemann, Schwäbisch Gmünd, Germany) and separated by tricine-SDS-PAGE through 4% stacking and 10% separating gels as described previously 25. For Western blot analysis, proteins were transferred to nitrocellulose membranes as described above.
4.7 Immunofluorescence assay for detection of bound FHL-1/reconectin on intact borrelial cells
For indirect immunofluorescence assays with unfixed cells, Borreliae (1×108 cells) were grown to mid-log phase, harvested by centrifugation (5000×g, 30 min, 4°C), washed and resuspended in 200 μ l of culture supernatant of Sf9 insect cells containing recombinant FHL-1/reconectin. After incubation for 1 h at room temperature with gentle agitation the cell suspension was washed three times with PBS containing 1% BSA and incubated for 60 min with polyclonal rabbit anti-Factor H SCR1–4 antibody or mouse mAb 93–84/07 directed against OspC which was kindly provided by H. Peters (Dade Behring). Following three washes with PBS, the Borreliae were incubated for 60 min with a fluorescein-conjugated swine anti-rabbit IgG antibody or fluorescein-conjugated goat anti-mouse IgG antibody (Dako, Glostrup, Denmark) at a dilution of 1:50 in PBS containing 1% BSA, washed, and mounted for microscopy. Microscopy was performed with an Olympus CX40 fluorescence microscope at a magnification of ×1,000.
4.8 Functional assay for cofactor activity of FHL-1/reconectin and Factor H
Cofactor activity of and FHL-1/reconectin and Factor H was tested on borrelial surface as well as in elutes from the bacteria after adsorption of serum or recombinant FHL-1/reconectin by measuring Factor I-mediated conversion of C3b to iC3b. In brief, washed pellets of serum or FHL-1/reconectin treated Borreliae or elutes from pretreated Borreliae were incubated with optimal concentrations of purified C3b (Calbiochem, Bad Soden, Germany) and purified Factor I (Sigma, Deisenhofen, Germany) for 15 min at 37°C. Supernatants obtained by centrifugation of the bacteria as well as the elutes were tested for iC3b by ELISA. iC3b-ELISA was performed using neoepitope-specific mouse monoclonal anti-iC3b IgG as capture antibody and biotinylated rabbit ant-C3c IgG (Dako, Hamburg, Germany) as detector antibody. The reaction was visualized by the addition of streptavidin-peroxidase, followed by o-phenylenediamine/H2O2 as substrate. Purified iC3b (Calbiochem) was taken as standard. Control experiments included Borreliae preincubated with buffer instead of serum and with an unrelated recombinant protein expressed in the identical system. Elutes from serum-treated Borreliae were also tested directly for iC3b.
Acknowledgements
We thank Eva Kampen, Christa Hanssen-Hübner, Angelika Sames and Renate Rutz for skillful and expert technical assistance. This work was funded by the Deutsche Forschungsgemeinschaft DFG, Project Zi 342/5 and Br 446/11–1 and the Thüringer Ministerium für Wissenschaft, Forschung und Kultur.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




