NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46
Abstract
Interleukin-2 (IL-2)-activated polyclonal or clonal NK cells lysed autologous antigen presenting cells (APC) through the engagement of the natural cytotoxicity receptors (NCR) NKp30 and NKp46. NK cell-mediated cytolysis of APC correlated with the surface density of these NCR. Indeed, NK cell clones bearing low amounts of NKp30 and NKp46 did not lyse autologous APC, whereas NK cell clones with bright expression of these NCR efficiently killed autologous APC. Upon masking of NKp30 or NKp46 by specific monoclonal antibodies a strong reduction (by 50%) of APC lysis could be detected and the complete inhibition was achieved by the simultaneous masking of these NCR. Interestingly, NK cell-mediated APC lysis was impaired by the phosphatidylinositol 3-kinase (PI-3 K) inhibitors LY294002 or wortmannin. Similarly, these drugs strongly reduced NK cell activation triggered by NKp30 or NKp46 in a re-directed killing assay as well as the activation of Akt/PKB, substrate of PI-3 K, induced by the engagement of these receptors. Altogether, these findings strongly suggest that NCR are responsible for the killing of autologous APC through the activation of PI-3 K.
Abbreviations:
-
- DC:
-
Dendritic cells
-
- GAM:
-
Goat anti-mouse
-
- KIR:
-
Killer inhibitory receptor
-
- Mo:
-
Monocytes
-
- NCR:
-
Natural cytotoxicity receptor
-
- PI-3K:
-
Phosphatidylinositol 3-kinase
1 Introduction
Dendritic cells (DC) represent the specialized antigen presenting cells (APC) that are able to stimulate T lymphocytes to proliferate and to produce inflammatory or maturating cytokines after capture and processing of viral or bacterial antigens 1, 2. In turn, upon interaction with T lymphocytes DC produce several cytokines including IL-1, IL-12 and IL-18, which can amplify and modulate antigen specific T cell activation 1, 2. On the other hand, down-regulation of DC activities can lead to the inhibition of T cell-mediated immune responses 1, 2. Inhibition of DC may be consequent to the presence in the extracellular milieu of inhibitory cytokines as IL-10, which affect T cell functions. Alternatively, it may reflect killing of DC by cytolytic effect or cells. Indeed, it has recently been reported that autologous DC can be killed by IL-2-activated NK cells as well as CD8+ T lymphocytes 3–6. Such DC lysis has been proposed to be perforin-dependent 4.
It has been suggested that CD40L expressed on NK cells upon interaction with CD40, present on autologous DC, may be responsible for DC lysis. Thus, the blocking of this interaction achieved by the use of anti-CD40 specific monoclonal antibody (mAb) leads to a partial inhibition of NK cell-mediated lysis of autologous DC 3. However, the NK cell surface structures responsible for the activation of NK cell-mediated DC lysis are largely undefined.
Recently, NKp30, NKp44 and NKp46 NK cell specific surface molecules that trigger NK cell-mediated cytolysis have been characterized 7–11. These molecules have been termed natural cytotoxicity receptors (NCR) and are selectively expressed by resting and/or IL-2-activated NK cells. The disruption of NCR-ligand interaction by mAb-mediated masking inhibits the NK-cell-mediated cytotoxicity 9, 10. Since the reported inhibitory effect exerted by anti-CD40 mAb on DC lysis by autologous NK cells is only partial, we have considered the NCR as suitable candidates for mediating the lysis of autologous DC. Here, we demonstrate that NKp30 and NKp46 receptors are involved in NK cell-dependent lysis of autologous APC through the activation of phoshatidylinositol 3-kinase (PI-3 K).
2 Results
2.1 Different susceptibility of autologous APC to NK-cell-mediated lysis
We analyzed the cytolytic activity of polyclonal and clonal NK cell populations against autologous APC. To this aim, we used, as target cells, freshly isolated peripheral monocytes (Mo) or cell populations derived from Mo cultured with appropriate combination of cytokines which are known to induce their maturation towards DC. As shown in Table 1, peripheral Mo expressed high levels of CD14 antigen, lower amount of CD80 and CD86 while being CD1a and CD83 negative. After culture with GM-CSF, the CD14 molecule was down-regulated, whereas CD80 and CD86 antigen expression was up-regulated, and a fraction (15%) of cells was CD1a+. Cells derived from Mo cultured with GM-CSF and IL-4, or GM-CSF plus IL-4 and TNF-α were strongly CD1a+ and expressed high amount of CD80, CD86 and CD83 antigens. It is of note that CD83, HLA class I and HLA class II molecules were expressed at higher levels on DC derived upon culture with GM-CSF plus IL-4 and TNF-α compared to those obtained with GM-CSF plus IL-4 only (Table 1).
|
|
Surface expression of |
|||||||
|---|---|---|---|---|---|---|---|---|
|
|
Nil |
CD1a |
CD14 |
CD80 |
CD83 |
CD86 |
HLA-class I |
HLA-class II |
|
Mo Freshly isolated |
||||||||
|
% Positive cells |
2 ± 1 |
5 ± 2 |
95 ± 3 |
20 ± 5 |
2 ± 3 |
10 ± 5 |
95 ± 3 |
80 ± 5 |
|
MFI |
2 ± 1 |
25 ± 3 |
450 ± 35 |
20 ± 3 |
20 ± 2 |
24 ± 2 |
350 ± 25 |
320 ± 30 |
|
Mo cultured with: GM-CSF |
||||||||
|
% Positive cells MFI |
3 ± 2 |
15 ± 7 |
20 ± 10 |
35 ± 8 |
4 ± 4 |
23 ± 6 |
95 ± 2 |
85 ± 7 |
|
|
2 ± 1 |
25 ± 4 |
45 ± 7 |
20 ± 4 |
2 ± 1 |
3 ± 1 |
400 ± 25 |
330 ± 15 |
|
GM-CSF plus IL-4 |
||||||||
|
% Positive cells |
2 ± 1 |
95 ± 4 |
5 ± 3 |
70 ± 10 |
35 ± 8 |
80 ± 9 |
94 ± 2 |
95 ± 1 |
|
MFI |
4 ± 1 |
450 ± 23 |
20 ± 4 |
68 ± 5 |
30 ± 6 |
90 ± 7 |
450 ± 21 |
400 ± 15 |
|
GM-CSF plus IL-4 and TNF-α |
||||||||
|
% Positive cells |
2 ± 1 |
95 ± 2 |
2 ± 1 |
90 ± 5 |
70 ± 8 |
85 ± 6 |
94 ± 3 |
93 ± 3 |
|
MFI |
3 ± 2 |
550 ± 23 |
21 ± 2 |
120 ± 12 |
78 ± 9 |
140 ± 9 |
850 ± 23 |
500 ± 12 |
- a) Freshly isolated Mo or APC obtained from peripheral Mo cultured for 7 days with either GM-CSF (40 ng/ml) or GM-CSF (40 ng/ml) plus IL-4 (100 U/ml) or GM-CSF (40 ng/ml) plus IL-4 (100 U/ml) plus TNF-α (100 U/ml) (added on day 3) were analyzed on a FACSort (Becton Dickinson) after staining with specific mAb directed against the indicated surface molecules followed by PE-conjugated goatanti-isotype specific anti-mouse antiserum. Results are expressed as % of positive cells. Nil represents the negative control (cells stained with an unrelated mAb followed by the second PE-conjugated reagent) and as mean fluorescence intensity (MFI) of positive cells in arbitrary units (a.u.). Results are representative of four independent experiments.
Autologous NK cell populations were able to lyse freshly isolated Mo or Mo-derived DC cultured for 7 days with the above mentioned cytokines. As shown in Fig. 1 (panel B and C), the NK cell-mediated lysis of autologous APC was higher against DC obtained upon culture with GM-CSF, plus IL-4 and TNF-α, compared to APC obtained in the presence of GM-CSF or GM-CSF plus IL-4. On the other hand, resting NK cells were not able to kill autologous APC, although they efficiently lysed K562 tumor cell line (Fig. 1A).
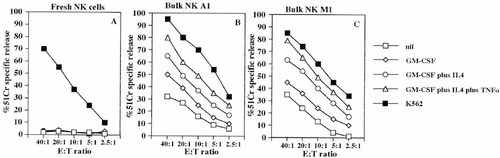
IL-2-activated polyclonal NK cell populations lyse autologous APC. Cytolytic activity of freshly isolated NK cells (A) or the polyclonal NK bulk populations A1 (B) and M1 (C) was analyzed in a 4 h 51Cr-release assay at the indicated E:T ratio against the following target cells: freshly isolated autologous Mo (nil) or DC derived from autologous Mo cultured for 7 days with GM-CSF or GM-CSF plus IL-4 or GM-CSF plus IL-4 plus TNF-α (added on day 3), or the NK-sensitive K562 cell line as indicated. Results are expressed as % of 51Cr specific release and are representative of ten different experiments using NK cells derived from ten healthy donors.
2.2 Surface expression of natural cytotoxic receptors (NCR) directly correlates with APC killing by autologous NK cell clones
A series of NK cell clones were also analyzed for their ability to lyse autologous APC. Two groups of NK cell clones could be identified on the basis of autologous APC lysis (6 clones for each group, out of 30 tested, are represented in Table 2). The first group strongly killed autologous APC (range from 54% to 87% at the E:T ratio of 5:1) while the second group did not (range from 1% to 5% at the E:T ratio of 5:1).
Recently, it has been shown that the surface density of NCR, represented by NKp30, NKp44 and NKp46 molecules, varies in different NK cell clones and it directly correlates with their cytolytic activity 10, 11. Thus, we analyzed the two groups of NK cell clones, displaying different cytotoxicity against autologous APC, for the expression of NCR. As shown in Table 2 the NK cell clones which efficiently lysed autologous APC expressed high levels of NKp30, NKp44 (not shown) and NKp46 (NCRbright) at the cell surface; by contrast, a low expression (tenfold lower) of NCR (NCRdull) was found on NK cell clones which did not kill APC. Lysis exerted by two clones (S24 NCRbright, panel A and C2.25 NCRdull, panel B) representative of the two groups, against APC derived with different combination of cytokines is shown in Fig. 2. Again, APC obtained by culturing Mo with GM-CSF plus IL4 and TNF-α (compared to Mo or DC obtained with GM-CSF or GM-CSF plus IL4) were more susceptible to NK cell-mediated lysis (Fig. 2).
|
|
Cytolytic activity against autologous |
|
|||
|---|---|---|---|---|---|
|
|
APC (E : T ratio)a) |
Surface expression (MFI) of |
|||
|
NK cell clone |
20 : 1 |
10 : 1 |
5 : 1 |
NKp30b) |
NKp46b) |
|
C1.12 (NCRdul1) |
15 |
10 |
5 |
45 |
48 |
|
S3.25 (NCRdull) |
12 |
7 |
3 |
35 |
37 |
|
A12.12 (NCRdull) |
6 |
3 |
1 |
24 |
32 |
|
F34.23 (NCRdull) |
10 |
5 |
3 |
34 |
28 |
|
D23.25 (NCRdull) |
15 |
7 |
4 |
54 |
23 |
|
M24.12 (NCRdull) |
12 |
6 |
2 |
23 |
34 |
|
M24.12 (NCbright) |
100 |
75 |
54 |
320 |
273 |
|
A6.50 (NCRbright) |
95 |
90 |
85 |
250 |
354 |
|
B4.25 (NCRbright) |
100 |
94 |
87 |
312 |
264 |
|
S23.6 (NCRbright) |
96 |
75 |
54 |
235 |
340 |
|
M21.6 (NCRbright) |
100 |
87 |
75 |
345 |
234 |
|
M12.3 (NCRbright) |
100 |
90 |
85 |
320 |
250 |
- a) The cytolytic activity exerted by a panel of NK cell clones against autologous APC derived from peripheral Mo cultured for 7 days with GM-CSF plus IL-4 was analyzed in a 4 h 51Cr-release assay at the indicated E:T ratio. Results are expressed as % of specific 51Cr release.
- b) The same NK cell clones were stained with the anti-NKp30 (7A6) or the anti NKp46 (BAB281) mAb followed by anti-isotype specific PE-conjugated goat antiserum and analyzed on a FACSort (Becton Dickinson). Results are expressed as mean fluorescence intensity (MFI) and are representative of 30 NK clones with a low (NCRdull) or a bright (NCRbright) expression of these two NCR.
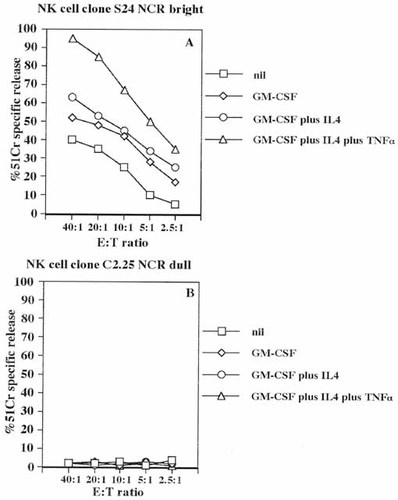
Lysis of autologous APC by NK cell clones correlates with the expression of NKp46 and NKp30 surface receptors. Cytolysis of the representative NK cell clone S24 expressing high levels of NCR (NCRbright NKp30: mean fluorescence intensity (MFI)= 246 arbitrary units (a.u.), NKp44: MFI=320a.u., NKp46: MFI= 220a.u. (A) or the NK cell clone C2.25 with low levels of NCR (NCRdull, NKp30: MFI = 45a.u., NKp44: MFI=34a.u., NKp46:MFI=45a.u.) (B) was analyzed in a 4 h 51Cr-release assay using as target cells autologous Mo freshly isolated from peripheral blood (nil) or APC derived from Mo cultured for 7 days with GM-CSF or GM-CSF plus IL4 or GM-CSF plus IL4 plus TNF-α (added on day 3) as indicated. Results are expressed as % of 51Cr specific release and are representative of ten different experiments using 30 NCRbright or 30 NCRdull NK cell clones.
2.3 Role of HLA-class I in NK cell-mediated lysis of APC
It is well established that HLA-class I expressed on target cells interacting with killer inhibitory receptors (KIR) for HLA expressed by NK cells could deliver an inhibitory signal, which in turn down-regulate NK cell-mediated cytolytic activity 13–15. To determine the role of HLA in NK cell-mediated lysis of autologous APC, we analyzed a panel of NK cell clones for their cytolytic activity against autologous targets in the presence of anti-HLA-class I-specific mAb. Fig. 3 shows results obtained using the 28.12 NCRbright (panel A) and the 66.25 NCRdull (panel B) NK cell clones.
The NCRbright 28.12 NK cell clone efficiently lysed autologous APC and this lysis was slightly (by 20%, panel A) increased by the addition of anti-HLA-class I mAb. At variance, the lysis of autologous PHA blasts exerted by the same NK cell clone was strongly augmented (by approximately tenfold) (panel A) when interaction between HLA-class I and KIR was abrogated by the addition of anti-HLA class I mAb to cytolytic assay. On the other hand, the NCRdull 66.25 NK cell clone did not lyse autologous APC, even in the presence of anti-HLA-class I mAb (Fig. 3, panel B). However, lysis of autologous PHA blasts exerted by the 66.25 NK cell clone was strongly increased (by tenfold) in the presence of anti-HLA class I mAb (Fig. 3B). Noteworthy, the percentage of specific lysis of autologous PHA blasts in the presence of anti-HLA-class I mAb exerted by these two NK cell clones was comparable (50% for the NCRbright 28.12 NK cell clone vs. 49% for the NCRdull 66.25 NK cell clone). Similar results were obtained using ten additional NK cell clones (five NCRbright and five NCRdull). That HLA-class I plays a minor role in NK cell mediated-APC lysis was further confirmed by additional experiments using bulk NK cell populations. As shown in Fig. 3 (panel C) lysis of APC was slightly increased (by 20%), at variance to that exerted against PHA blasts (panel D, increase of sixfold) in the presence of anti-HLA-class I mAb. Similar results were obtained by covering with specific mAb the CD94/NKG2A molecule (Fig. 3C and D) which represents the KIR with a broad specificity for HLA-class I alleles 13–15.
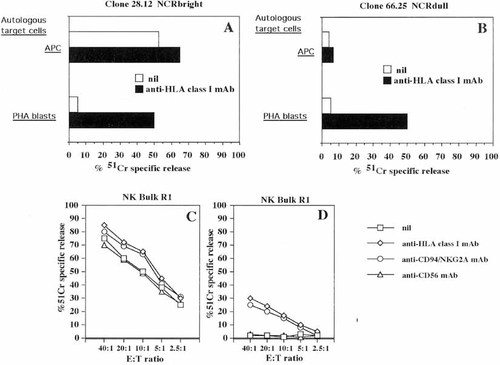
Role of HLA-class I in NK cell-mediated APC lysis. Cytolysis of the NCRbright 28.12 (A) or the NCRdull 66.25 (B) NK cell clones was analyzed in a 4 h 51Cr-release assay using as target cells autologous APC (E:T ratio 5:1) (DC obtained from peripheral Mo cultured for 7 days with GM-CSF plus IL-4) or autologous PHA blasts in the absence (nil) or in the presence of 5 μg/ml of anti-HLA-class I (A6136) mAb. Results are representative of 20 NCRbright or 20 NCRdull NK cell clones. Lysis exerted by the NK bulk population R1 against autologous APC cultured for 7 days with GM-CSF plus IL4 (C) or PHA blasts (D) in the absence (nil) or in the presence of either anti-HLA-class I (A6136) or anti-CD94/NKG2A (Y9) or anti-CD56 (NKH1A) mAb was analyzed in a 4 h 51Cr-release assay at the indicated E:T ratio. Results are expressed as % of 51Cr specific release and are representative of ten different experiments using NK cell populations derived from ten healthy donors.
2.4 NCR are involved in NK cell-mediated lysis of autologous APC
It has been shown that masking of NKp30 or NKp44 or NKp46 on NK cells, by using specific mAb, leads to the inhibition of cytolysis of several tumor targets as a consequence of the lack of interaction of NCR, expressed on NK cells, and their putative ligand(s) on target cells 10. Thus, we analyzed whether NCR are involved in the NK cell-mediated lysis of autologous APC. To this aim, cytolytic assays using NK cell clones and autologous APC as target cells were performed in the absence or in the presence of anti-NKp30 or anti-NKp44 or anti-NKp46 mAb alone or in combination. As shown in Fig. 4 (A and B), the addition of anti-NKp30 or anti-NKp46 mAb to the cytolytic assay consistently reduced, by approximately 50%, the lysis of autologous APC. In contrast, the addition of anti-NKp44 mAb led to a slight inhibitory effect (<15%). More importantly, a combination of anti-NKp30 and anti-NKp46 mAb led to a complete inhibition of APC lysis; at variance, the combination of anti-NKp44mAb with either anti-NKp30 or anti-NKp46 mAb did not increase the degree of inhibitory effect obtained using anti-NKp30 mAb or anti-NKp46 mAb alone.
It has been suggested that interaction between CD40L expressed on NK cells with CD40 present on DC may contribute to DC lysis. However, in our experiments, the addition of anti-CD40L and/or anti-CD40 mAb did not inhibit NK cell-mediated killing of APC (Fig. 4C). This was expected as all bulk NK cell populations (n=15) and clones (n=85) analyzed did not express detectable levels of CD40L (not shown).
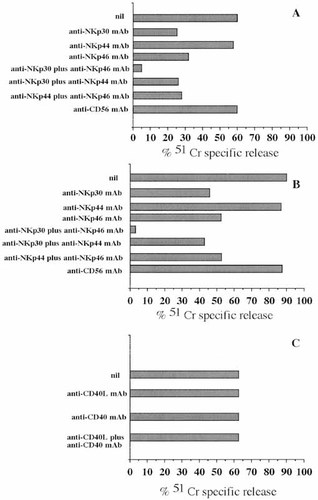
NKp30 and NKp46 molecules are involved in APC lysis mediated by autologous NK cells. The NK cell clone A2.12 expressing high levels of NKp30, NKp44 and NKp46 (NCRbright) was analyzed for cytolytic activity against autologous APC cultured with GM-CSF plus IL4 (A) or GM-CSF plus IL4 plus TNF-α (B) either in the absence (nil) or in the presence of saturating amounts (5 μg/ml) of anti-NKp30 (F252) or anti-NKp46 (KL247) or anti-NKp44 (KS38) or anti-NKp30 plus either anti-NKp46 (F252 plus KL247) or anti-NKp44 (F252 plus KS38) or anti-NKp44 plus anti-NKp46 (KS38 plus KL247) or anti-CD56 (NKH1A) mAb at the E:T ratio of 5:1. (C) APC (Mo cultured with GM-CSF plus IL4) lysis by clone A2.12 in the absence (nil) or presence of anti-CD40 (BE-1) or anti-CD40L (TRAP1) or anti-CD40 plus anti-CD40L (BE-1 plus TRAP-1) mAb at the E:T ratio of 5:1. Results are expressed as % of 51Cr specific release and are representative of ten experiments performed with different NK cell clones.
2.5 Phosphatidylinositol-3 kinase is involved in NCR-mediated lysis of autologous APC
NK cells lysis of autologous DC has been proposed to depend on calcium-mediated granule exocytosis, since total abrogation of cytotoxicity was observed in the presence of the calcium chelator EGTA 4. Thus, we further investigated the biochemical mechanism by which NK cell clones lyse autologous APC via NKp30 and NKp46. In this regard, the PI-3 K has been described to be involved in the exocytosis of granules from cytotoxic T lymphocytes 12. Thus, NK cell clones were treated with the PI-3 K inhibitors wortmannin or LY294002 and their cytolytic activity was measured.
As shown in Fig. 5, the NK cell-mediated lysis of autologous APC was strongly inhibited (up to 80%) by blocking PI-3 K with micromolar concentrations of LY294002 (Fig. 5A) or nanomolar concentrations of wortmannin (Fig. 5B).
A similar effect was observed on the NK cell activation induced by NKp30 or NKp46 in a re-directed killing assay using the FcγR+ P815 target cells (Fig. 5C). The involvement of PI-3 K in NCR-mediated signaling was further supported by the finding that the engagement of NKp30 or NKp46 molecules leads to the activation of Akt/PKB, which is a specific PI-3 K substrate (Fig. 5D). Altogether, these findings strongly suggest that the molecular mechanism underlying the cytolysis of autologous APC by NK cells is linked to the PI-3 K/Akt signal transduction pathway.
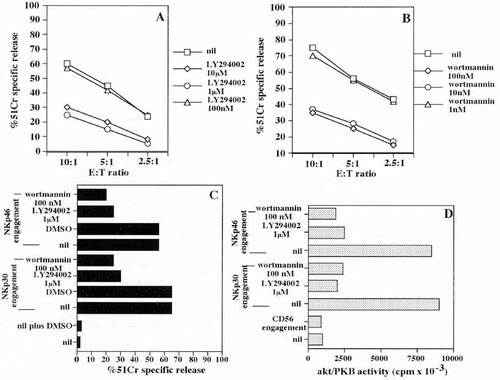
Lysis of autologous APC by NK cells is dependent on PI-3K activation. Cytolytic activity of the representative NK cell clone S23.25 (A) and S42.12 (B) expressing high levels of NKp46 and NKp30 surface receptors (NCRbright), were analyzed in the presence of the PI-3 K inhibitors LY294002 (A) or wortmannin (B) against autologous APC cultured with GM-CSF plus IL 4 for 7 days. (C) Cytolytic activity of the S23.25 NK cell clone was analyzed in a re-directed killing assay in the presence of either anti-NKp46 (BAB281) or anti-NKp30 (7A6) mAb, as indicated, using the FcγR+ murine mastocytoma P815. Cytolysis obtained in the absence of drugs (nil) or in the solvent of drugs (DMSO) is shown for comparison. Results are expressed as % of 51Cr specific release at the E:T ratio of 1:1 and are representative of six independent experiments using NK cell clones from six different healthy donors. (D) Akt/PKB activity in the presence or absence of the PI-3 K inhibitor LY294002 (1 μ M) or wortmannin (100 nM) after the engagement of the NKp30 or NKp46 or CD56 surface molecules achieved by incubating NK cells with saturating amounts of anti-NKp30 (7A6) or anti-NKp46 (BAB281) or anti-CD56 (TA181H12) mAb (5 μg/ml) followed by GAM. Nil: Akt/PKB activity in medium alone. Results are expressed as Akt/PKB activity (cpm × 10–3) evaluated after 3 min of stimulation and are the mean of three identical samples for each experimental condition.
3 Discussion
Here, we show that the NCR NKp30 and NKp46 can deliver a PI-3 K/Akt-dependent activating signal in NK cells, leading to the lysis of autologous APC.
Indeed, the killing of autologous APC correlates with the level of expression of the NKp30 and NKp46 at the NK cell surface and cytolysis is abrogated when the two NCR are masked. These findings would suggest that the natural ligands of NKp30 and NKp46 molecules are present on autologous APC. These cells were more susceptible to lysis when obtained after culture with GM-CSF plus IL4 and TNF-α in comparison with freshly isolated Mo or APC cultured with GM-CSF or GM-CSF plus IL 4. This would imply that the expression of NKp30 and NKp46 ligands on APC is up-regulated by TNF-α.
It has been shown, and we have confirmed here, that exposure to TNF-α strongly up-regulates HLA-class I molecules at the surface of APC. However, it is claimed that the expression of HLA-class I molecules inversely correlates with the susceptibility of target cells to NK cell-mediated lysis 13–15. Thus, TNF-α would down-regulate, rather than increase the susceptibility of APC to NK cell-mediated lysis as we found in our experiments. A possible explanation of these apparently conflicting results is that although HLA-class I molecules are up-regulated by TNF-α the increase of putative ligands of NKp30 and NKp46 is sufficient to overcome the inhibitory effect mediated by interaction of KIR with HLA-class I. Nevertheless, the finding that the addition of anti-HLA-class I mAb or anti-KIR mAb enhances the NK cell-mediated lysis of autologous APC cultured with TNF-α, suggests that a certain degree of inhibition due to KIR-HLA-class I interaction is still effective. Thus, one might hypothesize that during the interaction between NK cells and APC several activating and inhibitory molecules, expressed on either cell type, can be engaged and the final functional result is determined by the sum of opposing effects.
It has been recently suggested that the MHC class-I mediated inhibition can counteract NK-activating signals mediated by CD40L on NK cells upon its interaction with CD40 on DC 3. However, our NK cell clones and bulk populations did not express detectable levels of CD40L (not shown). In addition, anti-CD40 and/or anti-CD40L specific mAb did not inhibit the lysis of APC. Taken together, these findings suggest that NK cell clones, analyzed in this study, did not use CD40L as a costimulatory molecule capable of interacting with CD40 on APC. In addition, these data, together with those reported previously 3, support the idea that NK cell clones are quite heterogeneous and thus they could mediate different functional effects interacting with APC.
The NCR termed NKp44 apparently was not involved in APC lysis, as covering of NKp44 molecule with specific mAb on NKp44+ NK cell clones did not lead to the inhibition of APC killing. These data suggest that the NKp44 ligand is not present on autologous APC, while being expressed on certain tumor target cells 8, 11. This might contribute to render tumor cells more susceptible to NK cell-mediated lysis than APC.
Interestingly, we found that two inhibitors of PI-3 K, LY294002 and wortmannin, prevent the NK cell-mediated lysis of autologous APC. This is in keeping with the finding that PI-3 K is involved in the release of perforins and granzymes from cytolytic T lymphocytes 12. That perforins are involved in DC lysis by NK cells was also supported by the previously reported requirement of extracellular calcium for delivering the lethal hit 4. More importantly, the engagement of either NKp30 or NKp46 leads to the activation of PI-3 K and its specific substrate Akt/PKB. This suggests that ligation of NKp30 and NKp46 on NK cells by their natural ligands on APC leads to a PI-3 K-dependent perforin release and consequent lysis of target cells.
It is generally accepted that APC can trigger NK cell functions 2 and this is relevant in initiating innate anti-viral immune responses 16. We can speculate that NK cells eliminate DC during the early phases of viral infections to limit the spreading of viruses. This hypothesis is supported by the finding that NK cells can be isolated from influenza virus-infected lungs prior to the detection of virus specific T lymphocytes in mice 16. Moreover, it is well known that NK cells represent a first line of defense against the HIV-1 infection where they could participate in the clearance of the virus that, in presymptomatic stages of the disease, is stored in large quantities in follicular DC 17. Impairment of NK cell-mediated lysis of autologous HIV-1-infected DC might contribute to the following infection of CD4+ cells (lymphocytes and APC) leading to further progression of immunosuppression of full-blown AIDS 18. Finally, NK cells could affect DC maturation and life span within bone marrow and secondary lymphoid organs, thus providing an early regulation of adaptive immunity. Indeed, in keeping with others 3, we show that different cytokines modify the susceptibility of DC to NK cell-mediated lysis, possibly by modulating the expression of NCR ligands on DC, suggesting that the outcome of NK/DC interaction is dependent on the cytokine milieu.
In conclusion, in this report we define which surface structures are involved in the activation of NK cell-mediated APC lysis and we propose that this lysis is PI-3 K dependent. It remains to define both the natural ligand(s) of NKp30 and NKp46 and how their surface expression is regulated by the microenvironment in vivo, to better understand the physiological role of APC killing by autologous NK cells.
4 Materials and methods
4.1 Monoclonal antibodies and reagents
The anti-NKp30 (7A6, IgG1; F252, IgM) 9, the anti-NKp44 (Z231, IgG1; KS38, IgM) 8, 9, the anti-NKp46 (BAB281, IgG1; KL247, IgM) 7, 9, the anti-CD16 (VD4, IgG1; KD1, IgG2a) mAb 9 the anti-CD56 (TA181H12, IgG2a) mAb 19 and the anti-CD94/NKG2A (Y9, IgM; XA185, IgG1) were obtained as described 7, 9, 19. The anti-CD4 (Leu3a, IgG1) mAb, the anti-CD8 (Leu2a, IgG1) mAb were from Becton Dickinson (Palo Alto, CA). The anti-CD40L (TRAP1, IgG1) was from PharMingen (PharMingen International, San Diego, CA). The anti-CD3 mAb (UCHT-1, IgG1) was a kind gift from P.C.L. Beverly (Imperial Cancer Research Fund, London, GB). The anti-CD56 (NKH1A, IgM) was from Coulter Immunology (Hialeah, FL). The anti-CD40 (BE-1, IgG1) and the anti-CD40L (24–31, IgG1) mAb were from Ancell (Ancell Corporation, Bayport, MN). The specificity of anti-CD40L mAb was tested using 3T3 stable transfected with cDNA clone of CD40L (a kind gift of Dr S.Ferrini, IST-Genova). The affinity purified goat anti-mouse (GAM) anti-isotype specific antiserum was from Southern Biotechnology (Birmingham, AL). Purified GAM anti-Ig(H+L) was purchased from ICN (ICN Biomedicals, Inc., Aurora, OH). Recombinant (r) IL-4, granulocyte monocyte colny stimulating factor (GM-CSF) and TNF-α were from Pepro Tec (Pepro Tec EC, Ltd., London, GB). All cells used in our experiments were cultured in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% of fetal calf serum (FCS) (Sigma) and with glutamine and penicillin-streptomycin (Biochrom).
4.2 Indirect immunofluorescence
Single fluorescence staining was performed as described elsewhere 19. Briefly, aliquots of 105 cells were stained with the corresponding mAb followed by PE-conjugated anti-isotype specific GAM serum (Southern Biotechnology). Control aliquots were stained with the fluorescent reagent alone. Samples were analyzed on a flow cytometer (FACSort, Becton Dickinson) equipped with an argon ion laser exciting PE at 488 nm. Data were analyzed using Lysis II (version 1.1) program. Calibration was assessed with CALIBRITE particles (Becton Dickinson) using the AutoCOMP computer program (version 2.1.2).
4.3 Isolation and culture of polyclonal and clonal NK cell populations
Peripheral blood mononuclear cells (PBMC) from healthy volunteers were isolated by Ficoll-Hypaque gradient. Adherent cells were isolated after adhesion to plastic petri dishes for 2 h at 37°C.Nonadherent cells were incubated with anti-CD3, anti-CD4 and anti-CD8 mAb for 45 min at 4°C, washed and CD3-CD4-CD8-cells isolated after negative depletion using two rounds of immunomagnetic beads as described 15. The resulting cell population was 70% CD16+ but 99% CD3–CD4–CD8– and used in cytolytic assays as resting NK cells. Highly purified CD3– cells were stimulated with 10 μg/ml of PHA and then cultured in 96-well U-bottom microplates (Greiner Labortechnik, Nürtingen, Germany) with RPMI 1640 medium supplemented with 10% of FCS in the presence of 100 U/ml of recombinant (r) IL-2 (Proleukin, chiron Italia s.r.l.) in a final volume of 200 μl/well in the presence of 105/well irradiated allogeneic PBMC and 104/well 221 lymphoblastoid cell line. Under these culture conditions, by day 15 virtually all proliferating cells expressed CD16 and CD56 antigens. CD3– CD16+ clones wereobtained by culturing highly purified CD3–CD4–CD8– NK cells under limiting dilution condition as previously reported 19. All NK cell clones were analyzed for the expression of NCR by indirect immunofluorescence and only cell clones which were homogeneously positive for these receptors were further used in functional assays. The same NK bulk populations or clones were found CD40L negative by indirect immunofluorescence analysis using the two anti-CD40L mAb indicated in Sect. 4.1.
4.4 Isolation and culture of antigen-presenting cells
Mo were isolated from PBMC after adhesion to plastic petri dishes. Adherent cells expressed the CD14 antigen (75–85%) and were CD1a–. Adherent cells were then cultured with 40 ng/ml of GM-CSF either alone or in combination with 100 U/ml of recombinant IL-4 (Pepro Tec) 1, 20, 21. Adherent cells, cultured with only GM-CSF, down-regulated the expression of CD14 and were CD1a–, whereas cells cultured with GM-CSF plus IL-4 were CD1a+ CD14–. In some experiments 100 U/ml of TNF-α was added on day 3 of culture, in order to up-regulate the expression of HLA-class I and CD83 molecules. Mo were used in cytolytic assays on the same day of separation, while the other APC populations were used fromday 7 to day 10 of culture. During this period of time APC stably maintained the surface phenotype shown in Table 1 (Sect. 2).
4.5 Cytolytic assays
Cytolytic activity of CD3– CD16+ NK cell clones was tested in a 4 h 51Cr-release assay as previously described 19. Freshly isolated NK cells or polyclonal or clonal CD3–CD16+ NK cell populations were used as effector cells against different autologous APC populations or K562 erythroleukemia cell line or autologous PHA blastsat an effector target (E:T) ratio of 40:1 to 1:1, in a final volume of 200 μl of RPMI 1640 medium in V-bottom microwells. Masking experiments were performed by adding to the cytolytic assay anti-NKp30 (F252), or anti-NKp44 (KS38) or anti-NKp46 (KL247) mAb at 5 μg/ml in order to block interaction between NCR and their putative ligands expressed on APC. In some experiments, cytolytic activity of NK cell clones was analyzed in a re-directed killing assay using the FcγR+ murine mastocytoma cell line P815 in the presence of 5 μg/ml of activating mAb of IgG1 isotype directed against NCR molecules (anti-NKp30: 7A6; or anti-NKp44: Z231; or anti-NKp46: BAB281 mAb). The effect of the irreversible PI-3 K inhibitor wortmannin or the reversible PI-3 K inhibitor LY294002 on NK cell-mediated cytolytic activity was analyzed after treatment of NK cells with different concentrations (ranging from 10 μM to 1 nM) of either drug. The optimal concentration for each compound is indicated in Sect. 2. Cell viability was analyzed after incubation with the different drugs at various concentrations, or the appropriate dilution buffer (DMSO), and was always more than 98%.
4.6 Akt/PKB kinase assay
Akt activity in NK cell clones was tested, upon cross-linking of NKp30 or NKp46 achieved by pretreatment with specific mAb (7A6 or BAB281) followed by GAM. The experiments were performed in the absence or in the presence of the PI-3 K inhibitor LY294002, with the Akt/PKB assay kit, using the specific substrate and [γ-32P]ATP, after immunoprecipitation with the specific anti-Akt antibody (Upstate, Biotechnology Inc., Lake Placid, NY). Akt/PKB activity was analyzed at different time points (1, 3, 5 min) and data presented are referred to time 3 min and are the mean of triplicate samples.
Acknowledgements
This work was partially supported by grants from the Italian Association for Cancer Research (AIRC), Ministero della Sanità (PSN 2000–2002) and Istituto Superiore di Sanità (Special Project AIDS 1999–2000).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




