The cytoskeleton-associated TCR ζ chain is constitutively phosphorylated in the absence of an active p56lck form
Abstract
The TCR recognizes peptide-MHC complexes and transmits activation signals leading to cellular responses. We have previously characterized two TCR populations expressed on the T cell surface; one is linked to the cytoskeleton via a detergent-insoluble cytoskeleton-associated ζ (cska-ζ) chain, while the other is detergent soluble and not linked to the cytoskeleton. The cska-ζ form displays unique properties: it is constitutively phosphorylated, does not undergo hyperphosphorylation upon TCR stimulation as opposed to its non-cytoskeleton-associated counterpart (non-cska-ζ) and it maintains a molecular mass of 16 kDa. It is well established that p56lck and possibly p59fyn are responsible for the generation of the 21 / 23-kDa phosphorylated detergent-soluble ζ form. We now demonstrate that the posphorylation of cska-ζ does not require the activity of p56lck. We also show that although Lck does not phosphorylate cska-ζ in vivo, it retains the capacity to phosphorylate cska-ζ in vitro. Moreover, differences in ζ-associated kinase activity were detected for non-cska-ζ and cska-ζ. Our results indicating that different kinases phosphorylate the two ζ forms are consistent with a growing consensus that each TCR form may regulate distinct cellular functions.
Abbreviations:
-
- PTK:
-
Protein tyrosine kinase
-
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
-
- NEpHGE:
-
Non-equilibrium pH gradient electrophoresis
-
- INS:
-
Detergent insoluble
-
- SOL:
-
Soluble
-
- PI:
-
Isoelectric point
1 Introduction
The TCR is a multisubunit complex composed of the α / β heterodimer that is involved in antigen-MHC recognition and binding, the invariant CD3 γ, δ and ϵ chains, and the ζ / ζ homodimer that couples antigen recognition to various intracellular signal transduction pathways 1, 2.
Since none of the TCR subunits possess intrinsic kinase activity, antigen-induced TCR-mediated signaling depends upon a plethora of intracellular signaling molecules that associate with the receptor. The Src-family protein tyrosine kinase (PTK) p59fyn associates with the TCR ζ chain in vivo, both in non-activated and activated T cells 3, 4. Upon TCR stimulation, Src-kinase activity mediated by p56lck and possibly by p59fyn induces the in vivo tyrosine phosphorylation of the ζ and ϵ chains within immune receptor tyrosine-based activation motifs (ITAM). Consequently, ZAP-70, a Syk-family PTK 5, is recruited to the TCR and associates with the ζ chain. This interaction brings ZAP-70 to the proximity of p56lck. The latter then phosphorylates ZAP-70, thus inducing its activation, which subsequently leads to the phosphorylation of various substrates including LAT, SLP-76, PLCγ1, Vav, Cbl and others. Ultimately, the activation signals are transmitted into the nucleus resulting in cytokine production and cell proliferation 1, 2.
An increasingly diverse number of intracellular molecules have been shown to interact with the TCR and play a role in signaling. For example, the TCR ζ chain binds to catalytic molecules including p59fyn 3, 4, ZAP-70 5 and CD45 6, and also to a number of non-catalytic intracellular molecules, including Shc 7 and Grb2 8. Such interactions link the TCR via the ζ chain to the Ras signaling pathways. These molecules may also link the TCR to the cytoskeleton through the SH3 domains possessed by many intracytoplasmic adapter molecules, which are able to interact with proline-rich proteins known to localize to the cytoskeleton 9. Indeed, stimulation of T cells via the TCR has been shown to induce actin polymerization which depends on the recruitment of SLP-76, Vav and Nck by LAT. These new protein-protein interactions lead to the activation of the kinase PAK, as well as the GTPases Rho and Rac, which regulate actin assembly into stress fibers and induce membrane ruffles and lamellipodia, respectively 2. Data from other receptor systems suggest a role for the cytoskeleton in the transmission of signals from cell surface receptors to the cell interior (reviewed in 10).
We have previously demonstrated that 20 – 40 % of the cell surface-expressed TCR are linked to the actin-based cytoskeleton via a cytoskeleton-associated TCR ζ chain (cska-ζ) and thus are detergent insoluble 11 – 13. It has also been shown that ζ chain co-immunoprecipitates with monomeric globular actin 14. The physiological significance of the cska-ζ and its associated receptor complex has not been fully defined. However, a number of important features distinguish cska-ζ from its detergent-soluble non-cska-ζ counterpart, one of which is related to the phosphorylation pattern. While in non-activated T cells the non-cska-ζ chain is generally non-phosphorylated and migrates in SDS-PAGE with a molecular mass of 16 kDa, upon TCR-mediated activation, it undergoes hyperphosphorylation and displays a retarded migration pattern with a molecular mass of 21 / 23 kDa. However, in both non-activated, "rested" T cells, as well as in cells that have undergone TCR stimulation, a constant level of the cska-ζ form is constitutively phosphorylated. Despite this phosphorylation, cska-ζ retains its usual migration pattern in SDS-PAGE as a 16-kDa protein 12.
In the present study, we utilize various strategies to provide experimental data demonstrating that the activity of p56lck is not required for phosphorylation of cska-ζ. The results presented herein support a hypothesis for distinct functional roles of the non-cska-ζ and cska-ζ forms and their associated TCR.
2 Results and discussion
2.1 Phosphorylated ζ forms with different isoelectric points are localized to the cytoskeleton
Previous studies have demonstrated that the cska-ζ chain is phosphorylated in both non-activated 11 and activated T cells 12, 14, 15. Moreover, differences in the apparent molecular mass of phosphorylated cska-ζ (16 kDa) and the phosphorylated non-cska-ζ chains (21 kDa and 23 kDa) strongly suggest that the ζ forms may be phosphorylated at different sites and / or may differ in the number of their phosphorylated tyrosine residues. Indeed, Kersh et al. 16 have used sequence-specific anti-phosphotyrosine antibodies to delineate the phosphorylated tyrosine residues of the p21 and p23 non-cska-ζ chains. While the p21 form has only three phosphorylated tyrosines, each localized to a different ITAM, the p23 form has six phosphorylated tyrosine residues, with two in each of the three ζ-ITAM. A different mode of phosphorylation for these non-cska-ζ forms as well as for cska-ζ might have important functional ramifications. It has been previously shown that differences in phosphorylation affect interactions with various intracellular signaling molecules. For example, ZAP-70 binds to ζ via its two SH2 domains that recognize tandemly phosphorylated tyrosines in the same ITAM 17, while Fyn has the ability to bind to a ζ ITAM phosphorylated on a single tyrosine residue 18. Moreover, a recent study shows that partially phosphorylated TCR ζ molecules in either resting T cells or T cells activated with antagonist ligands can inhibit T cell activation 19.
Accordingly, we aimed to determine whether differently phosphorylated ζ forms exist within the cytoskeletal fraction of non-activated T cells by using the two-dimensional non-equilibrium pH gradient electrophoresis (NEpHGE) / SDS-PAGE analysis. Detergent-insoluble pellets were boiled in sample buffer and sonicated to extract detergent-insoluble (INS) proteins. The extracted phosphorylated and non-phosphorylated cska-ζ forms were immunoprecipitated with anti-ζ antibodies. While this was the most efficient procedure for immunoprecipitation, the poor yield of the precipitated proteins observed (Fig. 1 A) rendered this method of analysis not feasible for subsequent analysis. We therefore analyzed total INS lysates rather than immunoprecipitated proteins. To separate proteins from the cytoskeletal fraction of T cells, on the basis of their isoelectric points (PI) in two-dimensional NEpHGE / SDS-PAGE, we extracted the proteins from the INS fraction with urea and ampholines (A. Ben-Zeev, pesonal communication). Total lysates [INS and soluble (SOL)] were separated on NEpHGE / SDS-PAGE. As shown in Fig. 1 B, ζ chain from the SOL fraction of freshly isolated lymphocytes following separation by NEpHGE / SDS-PAGE revealed several ζ forms displaying both a lower PI and increasing apparent molecular mass (see lower panel). These forms result from differential phosphorylation as determined by anti-phosphotyrosine antibodies (data not shown). The different phosphorylation pattern changes the charge of the protein and its migration in the first dimension and induces a conformation that migrates more slowly in SDS-PAGE (second dimension). However, since immunoblotting with anti-phosphotyrosine antibodies was not sufficiently sensitive to observe phosphorylated cska-ζ by two-dimensional NEpHGE / SDS-PAGE analysis, it was necessary to first label splenocytes with [32P] orthophosphate in vivo. Our results revealed (Fig. 1 B, upper panel) a pattern of cytoskeleton-localized, phosphorylated proteins of varying molecular mass. Upon immunoblotting with anti-ζ antibodies, only three closely migrating bands of 16 kDa could be discened, each with a slightly different PI. The ζ-band with the lowest PI overlaps precisely with the heavily phosphorylated band observed upon exposure. This band is likely phosphorylated on more tyrosine residues than the other two ζ bands which exhibited a higher PI. In accord with our previous results 12, unlike the non-cska-ζ chain, cska-ζ maintains its apparent molecular mass of 16 kDa even when phosphorylated on tyrosine residues. Although we 11, 12 and others 14, 15 have previously shown that cska-ζ is phosphorylated on tyrosine residues, from these experiments we cannot rule out the possibility that cska-ζ might also be phosphorylated on serine and / or threonine residues. These results demonstrate that cska-ζ chain is phosphorylated in non-activated normal murine lymphocyts, and that there are apparently at least two forms of differently phosphorylated cska-ζ, which maintain a molecular mass of 16 kDa.
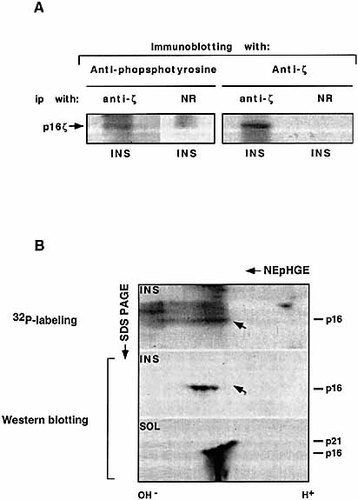
(A) Immunoprecipitation of tyrosine-phosphorylated cska-ζ chain with anti-ζ antibodies from the INS fraction. Normal splenocytes (1 × 108 cells) were lysed and separated into SOL and INS fractions. The INS proteins were extracted by sonication and immunoprecipitated using anti-ζ antibodies or normal rabbit serum (NR) as control. The samples were resolved by one-dimensional / reducing SDS-PAGE and subjected to Western blot analysis using anti-ζ and anti-phosphotyrosine anibodies. (B) Three cska-ζ chain isoforms with different PI indicate varying levels of phosphorylation. Normal splenocytes (2 × 108 cells) labeled with [32P] orthophosphate were lysed and separated into SOL and INS fractions. Proteins from each fraction (3.3 × 107 cells) were resolved by two-dimensional NEpHGE / SDS-PAGE, transferred to nitrocellulose, exposed to phosphorImager or X-ray films, and later subjected to immunoblottting with anti-ζ antibodies.
2.2 Cska-ζ does not require p56lck activity to undergo phosphorylation in vivo
The different mode of phosphorylation exhibited by cska-ζ raised the question as to whether p56lck, which plays a crucial role in the generation of the phosphorylated p21 / 23 non-cska-ζ form 1, 2, is also involved in the phosphorylation of cska-ζ. To resolve this issue, we took advantage of the p56lck-inactive JCaM1.6 cell line 20 and its parental (p56lck-active) Jurkat human T cell leukemic line. The relative levels of ζ chain localized to the SOL and cytoskeletal fractions were compared using non-activated and activated cells from each line (Fig. 2, insets). The ratio of cska-ζ to non-cska-ζ was similar in both cell lines, indicating that an active p56lck form is not obligatory for the localization of ζ chain to the cytoskeleton, at least in JCaM1.6 cells.
We next compared the phosphorylation levels of the two ζ forms (Fig. 2). The non-activated Jurkat cells maintained a basal level of constitutively phosphorylated cska-ζ similar to that previously observed in normal splenocytes 12. However, unlike normal splenocytes, the SOL fraction of Jurkat cells displayed high levels of constitutively phosphorylated p21 / 23 non-cska-ζ forms which dramatically increased upon activation via the TCR. However, the basal level of phosphorylated cska-ζ in these cells remained virtually unchanged even after TCR-mediated activation.
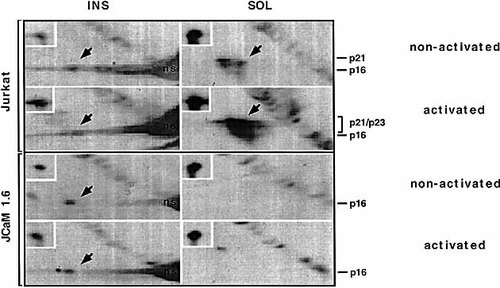
Cska-ζ chain is phopshorylated in JCaM1.6 cells lacking an active Lck PTK. Non-activated or activated Jurkat JCaM1.6 cells were lysed and separated in to SOL and INS fractions. Samples (SOL; 2.5 × 107 and INS; 1 × 108 cells) were resolved by two-dimensional non-reducing / reducing SDS-PAGE and Western blot analysis. Phosphorylated and non-phosphorylated ζ chains were detected as described in Fig. 1. Arrows indicate the positions of the phosphorylated ζ forms and insets indicate levels of the ζ chain.
We then asked whether an active p56lck form is required for the generation of phosphorylated 16-kDa cska-ζ, similar to its requirement for the generation of the phosphorylated p21 / 23 non-cska-ζ forms. As depicted in Fig. 2, non-activated JCaM1.6 cells displayed an undetectable level of p21 / 23 phosphorylated non-cska-ζ chain. Moreover, phosphorylation of non-cska-ζ was severely impaired upon TCR-mediated stimulation. This is in agreement with previously published observations 20 and residual levels of p21 / 23 non-cska-ζ chain were likely due to the compensatory effect of other kinases, such as p59fyn. In contrast, the absence of an active p56lck form in these cells did not prevent phosphorylation of the 16-kDa cska-ζ form.
To provide further evidence suggesting that p56lck is not involved in the phosphorylation of cska-ζ, we compared normal splenocytes isolated from C57BL / 6 mice to those isolated from knockout mice lacking expression of an active p56lck form. The normal C57BL / 6 splenocytes exhibited properties similar to those previously observed in other strains: ratios of cska-ζ / non-cska-ζ were similar, the constitutively phosphorylated 16-kDa cska-ζ form was unique to the INS fraction, and the 21 / 23-kDa ζ form was localized entirely to the SOL fraction (Fig. 3 A; top panel). p56lck could not be detected at all by Western blotting analysis in splenocytes of the p56lck-inactive mice (Fig. 3 B). However, splenocytes from these mice still contained basal levels of phosphorylated 16-kDa cska-ζ chain (Fig. 3 A; lower panel) The relatively low levels of ζ chain depicted in p56lck-inactive splenocytes compared to normal mice (Fig. 3) are in accordance with the low T cell / B cell ratio previously observed 21. These results provide further evidence that p56lck is not involved, directly or indirectly, in the in vivo phosphorylation of cska-ζ.

Cska-ζ is phosphorylated in splenocytes isolated from mice lacking an active Lck form. Splenocytes from normal C57BL / 6 mice or from mice lacking an active Lck were lysed, and fractionation and detection of phosphorylated proteins was done as described in Fig. 1. (A) shows levels of phosphorylated SOL ζ chain (3 × 107 cells) and cska-ζ chain (INS; 3.7 × 107 cells) in normal (top) and Lck-inactive mice (bottom). Total levels of ζ chain (SOL and INS) were detected with anti-ζ antibodies and are depicted below the results with anti-phosphotyrosine. (B) illustrates the level of Lck in splenocytes (2 × 107) isolated from normal mice and from Lck-inactive mice.
Complementary analysis was performed using T cells isolated from the spleen of lpr mice. These T cells are known to display enhanced tyrosine phosphorylation levels of the non-cska-ζ chain 22, most likely due to enhanced p56lck and p59fyn kinase activities 23. As shown in Fig. 4, in the SOL fraction of non-activated lpr splenocytes enhanced tyrosine phosphorylation was observed when compared to normal non-activated splenocytes. This was reflected by the elevated levels of the p21 / 23 ζ chain form. These results indicate that basal levels of PTK activity are indeed elevated in the lpr cells. In contrast, levels of phosphorylated and non-phosphorylated 16-kDa cska-ζ (INS) were unchanged in the lpr splenocytes and resembled phosphorylation levels of cska-ζ in normal splenocytes. Again, these results indicate that phosphorylation levels of cska-ζ are independent of p56lck kinase activity and most likely also of the p59fyn activity.
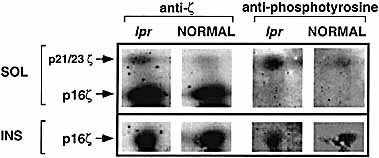
Enhanced tyrosine kinase activity of p56lck and p59fyn does not affect phosphorylation and protein levels of cska-ζ. Splenocytes were harvested from lpr and control mice (age and strain matched). Lysis of cells (5 × 107), fractionation and detection of ζ and phosphorylated ζ protein levels were done as described in Fig. 1.
2.3 The cska-ζ chain serves as a potential in vitro substrate for p56lck
Our results show that while p56lck is required for the phosphorylation of non-cska-ζ chain, it is not essential for the in vivo phosphorylation of the cska-ζ chain. One compelling question that arose is why p56lck does not phosphorylate cska-ζ in vivo. We suggested two possible general mechanisms: (1) cska-ζ is in a conformation that prohibits binding and / or in vivo phosphorylation by p56lck; (2) a "compartmentalization effect", where an active form of p56lck is not accessible to cska-ζ in vivo, perhaps as a result of differential subcellular localization to separate microdomains. To provide evidence that might support either possibility, we sought to determine whether cska-ζ has the potential to serve as a substrate for p56lck in vitro.
To this aim, we designed a reconstituted in vitro kinase assay where ζ chain is first immunoprecipitated from either the SOL or the DNasel-treated cytoskeletal fraction (INS; see Sect. 4.5). Some of the ζ immunoprecipitates were then "reconstituted" with immunoprecipitates containing p56lck (Fig. 5). The samples were subsequently subjected to in vitro kinase assays, and phosphorylated proteins were analyzed. Under these experimental conditions, the anti-ζ mAb precipitated non-cska-ζ along with a kinase(s), as reflected by a level of kinase activity observed in vitro even without "reconstitution" with exogenous p56lck (Fig. 4 A; upper left panel). We then endeavoured to combine and "reconstitute" the immunoprecipitates of the SOL non-cska-ζ chain with immunoprecipitates of Lck. The results show an increase in the phosphorylation of ζ (Fig. 5 A; upper right panel), demonstrating that the "reconstitution system" of combining immunoprecipitates that the "reconstitution system" of combining immunoprecipitates of both kinase (p56lck) and substrate (ζ) could be utilized. We then determined whether the cska-ζ chain could also undergo phosphorylation upon in vitro "reconstitution" with p56lck. Under the conditions used, little or no co-associated kinase activity was found in the cska-ζ immunoprecipitates, and therefore basal phosphorylation levels of the cska-ζ chain in vitro were not observed (Fig. 5 A; lower left panel). However, upon in vitro "reconstitution" with p56lck, cska-ζ underwent substantial phosphorylation and in some of the experiments performed, several phosphorylated forms of cska-ζ could be obtained (Fig. 5 A; lower right panel). In several cases, the CD3 subunits that co-immunoprecipitated with cska-ζ also served as substrates for p56lck in the in vitro "reconstitution" assays (Fig. 5 A; lower right panel). Control experiments were performed for non-cska-ζ (data not shown) and for cska-ζ (Fig. 5 B). In the latter, cska-ζ was "reconstituted" with immunoprecipitates using protein A-Sepharose beads precoated with normal rabbit serum, and was devoid of any kinase activity which phosphorylates cska-ζ (Fig. 5 B; upper right panel). Immunoprecipitations of p56lck under the conditions specified did not "drag along" ζ chain (data not shown), indicating that the phosphorylation of ζ was indeed due to the "reconstitution" with the p56lck immunoprecipitate. These results show that while p56lck is not responsible for the phosphorylation of cska-ζ in vivo, it retains the capacity to phosphorylate cska-ζ in in vitro kinase assays. This hints that cska-ζ is not phosphorylated by p56lck in vivo due to the physical separation between kinase and substrate. In support of such a possibility is the study by Rodgers and Rose 24 demonstrating that the localization of Lck to glycolipid-enriched membrane domains induces a physical separation between phosphatase (CD45) and substrate (Lck). In this case, Lck is hyperphosphorylated and displays weakened kinase activity, an effect also exhibited for detergent-soluble Lck in T cells lacking CD45 24.
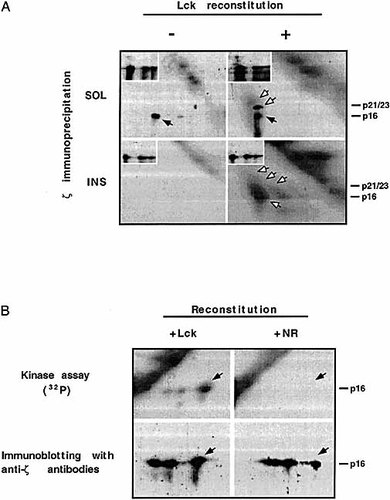
Lck can potentiate the phosphorylation of cska-ζ in vitro as determined by "reconstituted" kinase assays. (A) Splenocytes were "rested", lysed and ζ chain was immunoprecipitated from the SOL fraction of 2.5 × 107 cells and subjected to in vitro kinase assays [SOL(−)]. The cska-ζ chain was immunoprecipitated from the INS of 1 × 108 cells after DNaseI treatment and subjected to in vitro kinase assays [INS(−)]. In parallel, immunoprecipitates of Lck from the soluble fractions of 2 × 107 cells were mixed with anti-ζ immunoprecipitates of the SOL and INS fractions. These mixtures were then subjected to in vitro kinase assays [SOL(+) and INS(+)]. (B) Immunoprecipitations were done with normal rabbit serum (+ NR), in parallel to Lck (+ Lck) anti-serum, and mixed with immunoprecipitated cska-ζ. The reactions were terminated by boiling in the presence of SDS and samples were processed as described in Fig. 1. Nitrocellulose filters were exposed to x-ray film or PhosphorImager. Both in A (insets) and B (bottom panel), nitrocellulose filters were subjected to immunoblotting with anti-ζ antibodies. Arrows in (A) indicate basal phosphorylated ζ chain (black) and ζ phosphorylation dependent on exogenous Lck (white). Arrows in (B) denote positions of the phosphorylated cska-ζ form (upper panel) and total levels of cska-ζ chain (lower panel).
It is conceivable that "compartmentalization" or physical separation of TCR subunits from specific intracellular molecules may be utilized by T cells to regulate signal transduction pathways. Several reports provide strong evidence that ζ and the TCR localize to detergent-resistant "rafts" 25. Most of the studies suggest that TCR localization to the "rafts" appears following TCR stimulation 26 – 29. Results are conflicting regarding the appearance of the TCR within "rafts" in resting T cells. However, unlike the non-cska- and cska-ζ forms, the raft-associated ζ chain appears to localize to these domains alone, without the other TCR subunits. It is noteworthy that while detergent insolubility is common to both raft- and cytoskeleton-associated proteins, the insolubility of the former is considered to be unaffected by inhibitors of the cytoskeleton 30. Moreover, while the cholesterol-sequestering agent saponin dissociates ζ from its "rafts" localization 27, it is not known to promote dissociation of cytoskeleton-linked proteins. Since we 11 and others 14 have demonstrated that the detegent insolubility of ζ chain depends on an intact actin microfilament system, it is likely that there are two distinct detergent-insoluble pools, only one of which localizes to the cytoskeleton and lacks an active form of Lck. However, these two detergent-insoluble ζ forms may not be mutually exclusive. A recent study by Harder and Simons 28 shows that T cell rafts accumulate filamentous actin and tyrosine-phosphorylated proteins. Another study by Moran and Miceli 29 demonstrates that cholesterol depletion in T cells not only impairs raft formation, but also interferes with the association between ζ and the cytoskeleton.
2.4 The cska-ζ-associated kinase(s) requires different conditions for optimal in vitro activity than the kinases associated with non cska-ζ chain
The results presented herein show that p56lck is not required for the phosphorylation of cska-ζ. Moreover, treatment of cells with the Src-family selective tyrosine kinase inhibitor PP1 31 did not affect levels of phosphorylated cska-ζ (data not shown).
The apparent involvement of a different kinase(s) in the phosphorylation of cska-ζ suggests a difference in function of the two TCR populations. We attempted to precipitate kinase activity with cska-ζ by immunoprecipitating non-cska- and cska-ζ from normal non-activated or activated T lymphocytes, and subjecting them to in vitro kinase assays. These immunoprecipitates were washed according to condition A (see Sect. 4.7). As indicated in Fig. 6 A (upper panels), the non-cska-ζ chain was weakly phosphorylated in non-activated cells, but showed greatly enhanced phosphorylation upon activation. Another kinase-dependent measure of TCR-mediated activation, the appearance of the ubiquitinated ζ chain 32, could also be observed. The CD3 γ, δ and ϵ chains also served as substrates for the TCR-associated kinase(s). However, under these conditions, precipitations of cska-ζ failed to display kinase activity (as observed after long exposures) in either non-activated or activated T cells (Fig. 6 A; lower panels).
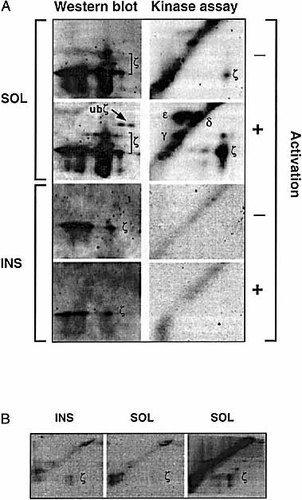
Kinase activity associated with cska-ζ can be detected under different conditions than that associated with the detergent-soluble ζ chain. Normal splenocytes were harvested and "rested" for 16 h. Non-activated cells (9 × 107; A and B) or cells activated with anti-CD3 antibodies (A), were washed, lysed and separated into SOL and INS fractions. The INS fraction was treated with DNAseI, and then both SOL and INS fractions was treated with DNASeI, and then both SOL and INS fractions were immunoprecipitated with anti-ζ mAb. In (A), immunoprecipitates were washed twice in "wash buffer" (containing 0.01 % Triton x-100; see Sect. 4.6) and once in kinase buffer. In (B), immunoprecipitates were either washed twice with "wash buffer" and once with "wash buffer" without EDTA (left and middle panels), or washed a forth time with water (right panel). Samples were then subjected to in vitro kinase assays and resolved by phosphorimager as in Fig. 5 (A, B), and / or immunoblotted with anti-ζ antibodies (A).
Since it is likely that a different kinase(s) is needed to phosphorylate the cska-ζ chain, we hypothesized that the conditions required for its optimal activity may be distinct from those needed by kinases associated with the non-cska-ζ form. We therefore performed kinase assays under a wide variety of conditions, in an attempt to observe in vitro phosphorylation of cska-ζ by its associated kinase(s) (Fig. 6 B and data not shown). Levels of phosphorylated cska-ζ could be observed when cska-ζ immunoprecipitates were washed under condition B (see Sect. 4.7) (Fig. 6 B; left panel). These results were also obtained after preclearing with a non-relevant antibody, suggesting that the cska-ζ-associated kinase activity is specific. Under these conditions, no ζ-associated kinase activity was detected in the SOL fraction (Fig. 6 B; central panel). However, when a wash with water was added (condition C, see Sect. 4.7), non-cska-ζ chain was readily phosphorylated by an associated kinase (Fig. 6 B; right panel), but cska-ζ did not undergo in vitro phosphorylation (data not shown). Thus, while cska-ζ is phosphorylated in vitro by a kinase that co-immunoprecipitates with it and is active in the presence of trace levels of Triton X-100, the non-cska-ζ-associated kinase is most active in vitro in the absence of detergent. Although the activity obtained in vitro is generally weak, these results further support our data indicating that different kinases are responsible for the phosphorylation of these two ζ forms. One possible explanation for the weak level of kinase activity in the cytoskeletal fraction is that the interaction between cska-ζ and its phosphorylating kinase may be a transient one.
A recent study has reported the involvement of lck in phosphorylating a form of ζ that localizes to the INS fraction 33. However, this lck-dependent phosphorylated, dimeric ζ form is induced upon TCR stimulation, similar to the non-cska ζ chain and a ζ form that associates with cholesterol and sphingomyelin-enriched "rafts". While we have been able to detect only the 16-kDa uniquely phosphorylated ζ form in the INS cytoskeletal fraction, it is clear that the reported INS ζ forms may have distinct functions and will need to undergo further investigation.
One way to elucidate a functional role for cska-ζ may be to identify molecules that might link the TCR to the actin microfilaments. Indeed, two recent studies 34, 35 provide strong evidence that the proto-oncogene Vav may well be such a linker protein. Determining both how zeta associates with the cytoskeleton, and identifying the kinase responsible for its phosphorylation should help unravel the role of cska-ζ.
3 Concluding remarks
We have demonstrated here that the 16-kDa cska-ζ form shows at least two varying degrees of phosphorylation in non-activated T cells. By complementary techniques, we have also established that unlike the phosphorylated non-cska-ζ chain, phosphorylation of cska-ζ is independent of p56lck activity. The eventual identification of this kinase may prove to be crucial in deciphering TCR-mediated signaling pathways, particularly those transmitted via the cytoskeleton.
4 Materials and methods
4.1 Animals
BALB / c and C57BL / 6 female mice were bred in our SPF facility. lpr mice were purchased from The Jackson Laboratory (Bar Harbor, MA). Mice lacking expression of an active Lck form were generated and generously provided by Dr. T.W. Mak 21.
4.2 Cells
Splenocytes were isolated from mice aged 12 – 16 weeks and "rested" overnight, unless otherwise indicated, at 37 °C in RPMI 1640. The human leukemic Jurkat C cell J.E6-1 and JCaM1.6 Lck-inactive lines 20 were grown in the same medium.
4.3 Antibodies
The 145-2C11 (2C11) and OKT3 mAb have been described 36, 37. Polyclonal rabbit anti-ζ antibodies were generated as described 38. The anti-ζ mAb H-146 39 was a kind gift from Dr. R. Kubo and Dr. D. Wiest. Anti-phosphotyrosine mAb (4G10) were obtained from Upstate Biotechnology.
4.4 Cell activation
Normal splenocytes were harvested after being incubated overnight at 37 °C ("rested"), washed and resuspended in full media at a concentration of 1 × 107 cells per ml. Cells were stimulated by anti-CD3ϵ antibodies (2C11 ascites diluted 1 : 250) for the time indicated. Jurkat and JCaM1.6 cell lines were activated by incubation with OKT3 ascites (1 : 100) for 2 min.
4.5 Cell lysis and separation of SOL and INS cytoskeletal fractions
Cell pellets were lysed with MES lysis buffer, as previously described 12. Following lysis, the detergent-soluble fraction was isolated and the supernatant was designated as "SOL". The detergent-insoluble pellet was washed and designated as "INS". For analysis of the total insoluble fraction, proteins were extracted by incubation at 95 °C in sample buffer containing 4 % SDS. When in vitro actin microfilament depolymerization of the cytoskeletal fraction was required, the INS pellet was treated with DNasel as previously described 12. Unless otherwise indicated, proteins from each fraction were resolved by two-dimensional non-reducing / reducing SDS-PAGE on 12 % and 13 %, gels, respectively.
4.6 In vivo labeling of cells with [32P] orthophosphate and two-dimensionsal NEpHGE / SDS-PAGE analysis
Freshly isolated splenocytes were "phosphate-starved" and then suspended at 2 × 107 cells / ml in phosphate-free RPMI containing 7 % dialyzed FCS and 0.3 mCi [32P] orthophosphate / ml. Cells were labeled at 37 °C for 4 h, washed, lysed and separated into SOL and cytoskeleton-enriched INS fractions, as described above. Proteins were eluted from the INS pellet by the addition of NEpHGE sample buffer (containing 57 % urea w / v, 4 % ampholines pH 3.5 – 10, 0.2 % NP40 and 3 % 2-ME) followed by mechanical agitation. The solubilized proteins were then loaded onto NEpHGE capillary tubes and separated at 15 mA and 0.3 W / tube until the voltage reached 500 V. Samples were then separated for another 5 h at 500 V (15 mA and 0.3 W / tube). Tube gels were then equilibrated for 30 min in 0.5 M Tris pH 6.8 containing 0.1 % SDS and 0.5 % DTT (w / v), and resolved on 10 – 20 % gradient SDS-PAGE. All gels were transferred to nitrocellulose, exposed to PhosphorImager or X-ray films, and in some cases immunoblotting analysis was performed.
4.7 Immunoprecipitations and kinase assays
For immunoprecipitations, the INS pellets were either treated in vitro with DNasel (Boehringer Mannheim), as previously described 12 or sonicated (3 min, 4 °C) and eluted (30 min, 4 °C) in a buffer containing 20 mM Tris pH 8, 150 mM NaCl, 0.1 % SDS, 0.1 % Triton X-100, 0.5 % deoxycholic acid, 40 mM DTT, protease and phosphatase inhibitors. The extracted INS proteins and the SOL lysates were equilibrated to 150 mM NaCl and incubated with antibody-coated protein A-Sepharaose beads (Pharmacia) for 3 h at 4 °C. Immunoprecipitates were either washed and separated on one-dimensional, reducing SDS-PAGE or treated according to condition A (i. e. washed twice a buffer containing 0.1 % Triton X-100, and then once in kinase buffer containing 100 mM NaCl, 25 mM Hepes pH 7.5, 5 mM MgCl2 and 5 mM MnCl2), condition B (i. e. washed twice with a buffer containing 0.1 % Triton X-100, and then once in wash buffer lacking EDTA in the phosphatase inhibitors), or condition C (i. e. washed twice with a buffer containing 0.1 % Triton X-100, and then once on deionized water) and subjected to a kinase reactin (in kinase buffer) with the addition of 1 μCi [γ-32P] -ATP / reaction. Reconstitution studies were performed using two sets of immunoprecipitations performed in parallel: (1) non-cska-ζ and cska-ζ were immunoprecipitated as described above; (2) Lck was immunoprecipitated from non-activated splenocytes using protein A-Sepharose beads precoated with rabbit serum containing anti-Lck antibodies. A control immunoprecipitation was done using protein A-Sepharose beads alone or protein A-Sepharose beads coated with preimmune normal rabbit serum. After the immunoprecipitation, the beads were washed and immunoprecipitates from each group were mixed together. The mixtures were then subjected to in vitro kinase assays as described above. The reaction was terminated by the addition of sample buffer containing SDS and boiling. The samples were then subjected to two-dimensional non-reducing / reducing SDS-PAGE. Separated proteins were transferred to nitrocellulose filters and either exposed to films or PhosphorImager. The filters were subsequently immunoblotted with the designated antibodies.
Acknowledgements
We are indebted to Dr. L. Samelson for his fruitful discussions and for providing us with anti-Lck antibodies and to Avri Ben-Zaev for his most helpful advice on the NEpHGE analysis of cytoskeletal fractions. We thank Dr. T. W. Mak and Dr. S. Phyte for generously providing us with mice lacking an active Lck kinase. We are grateful to Dr. R. Kubo and Dr. D. Wiest for the H-146 mAb. We appreciate the efforts of Prof. E. Yefenof for his critical reading of the manuscript. We also thank Mika Caplan for her efforts on our behalf. We gratefully acknowledge the support of the Society for Research Associates of the Lautenberg Center, the United States-Israel Binational Science Foundation, the Concern Foundation of Los Angles and the Abisch-Frankel Foundation for the Life Sciences.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




