Production of IL-1 receptor antagonist by hepatocytes is regulated as an acute-phase protein in vivo
Abstract
IL-1 receptor antagonist (IL-1Ra) is produced by isolated human hepatocytes with characteristics of an acute-phase protein. There are multiple IL-1Ra peptides, one secreted (sIL-1Ra) and three intracellular (icIL-1Ra1, 2, 3). sIL-1Ra, but not icIL-1Ra1, mRNA is transcribed by cultured human hepatocytes. In this study, we examined in vivo production of IL-1Ra by the liver in mice in two experimental models of acute-phase response, systemic lipopolysaccharide (LPS) administration and local turpentine injection. Liver sIL-1Ra expression was up-regulated in response to both types of stimulation. After LPS injection, the hepatic production of sIL-1Ra correlated with the increase in plasma IL-1Ra levels. In addition, the total amount of IL-1Ra present in the liver after LPS injection was six- and tenfold higher than in the lung and spleen. As assessed by in situ hybridization, sIL-1Ra, but not icIL-1Ra, mRNA was produced by hepatocytes in vivo after LPS injection. Using IL-6– / – mice, we demonstrated that in turpentine-induced inflammation production of IL-1Ra mRNA by the liver is regulated by IL-6. In contrast, local production of IL-1Ra is independent of IL-6. Taken together, these results indicate that IL-1Ra is produced by the liver as an acute-phase protein in vivo.
Abbreviations:
-
- APP:
-
Acute-phase protein
-
- IL-1Ra:
-
IL-1 receptor antagonist
-
- ic:
-
Intracellular
-
- s:
-
Secreted
-
- SAA:
-
Serum amyloid A
-
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
1 Introduction
The acute-phase response includes a large number of changes that accompany various inflammatory and non-inflammatory conditions such as infections, immune-mediated diseases, tumors, and trauma. These changes include variations in the plasma concentrations of some proteins, defined as acute-phase proteins (APP), and a large number of other metabolic and biochemical modifications. The APP are primarily produced by the liver in response to different cytokines. These proteins may have different biologic functions and can contribute to both initiation and resolution of inflammatory responses, as well as to subsequent repair processes (reviewed in 1).
IL-1 receptor antagonist (IL-1Ra) is a member of the IL-1 family that binds to IL-1R but does not induce any intracellular responses. IL-1Ra prevents the binding of IL-1 to its cell surface receptors, competitively inhibiting the biological effects of IL-1 on target cells 2. IL-1Ra refers to different peptides, one secreted (sIL-1Ra) and three intracellular (icIL-1Ra1,2,3). sIL-1Ra, icIL-1Ra1 and icIL-1Ra2 are the products of three different mRNA 3 – 5, whereas icIL-1Ra3 is synthesized by alternative translation initiation from both sIL-1Ra and icIL-1Ra1 mRNA in vitro 6.
Several studies have described that serum levels of IL-1Ra are elevated in patients with inflammatory conditions 7 – 9, suggesting that this cytokine behaves as an APP. Recently, we demonstrated that human hepatocytes in primary culture and HepG2 hepatoma cells produce sIL-1Ra and icIL-1Ra3 following stimulation with IL-1β and IL-6, with characteristics of a classical APP 10. Consistent with these data, we showed that sIL-1Ra mRNA production is stimulated in the liver following administration of LPS to mice 11. However, the relative contribution of the liver to circulating levels of IL-1Ra following LPS injection and in the context of other experimental models of inflammation is currently unknown.
The studies described herein show that hepatocytes are a major in vivo source of sIL-1Ra in response to LPS, thus contributing significantly to the elevation in circulating levels of IL-1Ra. sIL-1Ra mRNA is also produced by the liver in response to a distant inflammatory stimulation in a manner similar to serum amyloid A (SAA), a classical APP. Furthermore, using gene knockout mice, we showed that IL-6 is necessary to stimulate IL-1Ra mRNA expression in the liver, but not in locally inflamed tissues.
2 Results
2.1 Hepatocytes are a major source of circulating IL-1Ra in response to LPS
We recently demonstrated that sIL-1Ra mRNA is produced in different organs following systemic administration of LPS to mice 11. In order to examine the relative importance of the liver as a source of IL-1Ra in the circulation, C57BL / 6 mice were injected with LPS. The results show that circulating levels of IL-1Ra were elevated in mice 4 h after injection with LPS (9.1 ± 1.8 ng / ml, four mice), whereas IL-1Ra remained undetectable in the plasma of mice injected with saline (< 0.3 ng / ml). sIL-1Ra mRNA was not present in the lung, liver and spleen of mice injected with saline, but its production was strongly up-regulated after LPS administration (Fig. 1). In contrast, icIL-1Ra1 mRNA was not detected in any of the examined organs.
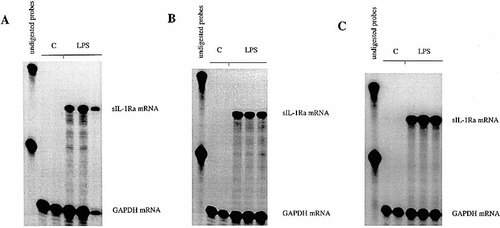
Production of IL-1Ra mRNA by the liver, spleen and lung in response to LPS. C57BL / 6 wild-type mice were injected i. p. with either LPS (n = 3) or pyrogen-free saline (n = 2) and killed after 4 h. Total RNA was extracted from the liver (A), spleen (B), and lung (C). Total RNA (30 μg) were hybridized with a 32P-labeled riboprobe that recognizes sIL-1Ra and icIL-1Ra1 mRNA as two different protected size fragments. A riboprobe for GAPDH mRNA was hybridized simultaneously as a control. Following RNase treatment with RNase A and RNase T1 the protected RNA fragments were electrophoresed in a denaturing polyacrylamide gel. The gels were dried and autoradiographies were immediately performed.
To compare the relative amounts of IL-1Ra synthesized in each organ in response to LPS, we determined the production of total IL-1Ra protein by the liver, spleen and lung. The results show that the total content of IL-1Ra in the liver was six- and tenfold higher than in the lung and spleen, respectively (Table 1). This result indicates for the first time that the liver is the major source of IL-1Ra following LPS injection in vivo.
Different cells within the liver such as macrophages and fibroblast-like cells may produce IL-1Ra in response to LPS 12, 13. By in situ hybridization, we determined the cellular source of IL-1Ra in the liver and which IL-1Ra isoforms were produced. As shown in Fig. 2, a positive signal for sIL-1Ra, but not icIL-1Ra1, mRNA was observed in parenchymal hepatocytes of mice injected with LPS. In contrast, no signal was detected with control sense probes or in the liver of mice injected with saline (data not shown). These results demonstrate that hepatocytes contribute to the circulating levels of IL-1Ra following in vivo stimulation by LPS.
|
|
Liver |
Spleen |
Lung |
|---|---|---|---|
|
IL-1Ra [ng / ml] |
56.1 ± 5.38 |
49.3 ± 5.93 |
53.7 ± 9.8 |
|
IL-1Ra [ng / mg tissue] |
0.224 ± 0.02 |
0.197 ± 0.02 |
0.214 ± 0.04 |
|
Organ weight [mg] |
986.7 ± 13 |
110 ± 5.77 |
180 ± 10 |
|
Total IL-1Ra content [ng] |
221.4 ± 21.5* |
21.9 ± 3.77 |
38.9 ± 8.19 |
- a) Each organ was homogenized 1 : 4 (w / v) in PBS-Tween-20. IL-1Ra levels were determined by ELISA. The amount of IL-1Ra for each organ was then calculated per mg of tissue. Total IL-1Ra content represents the values obtained after correction according to the weight of each organ. The values represent the mean ± SEM of three different mice.
- * p < 0.01 as compared with the two other organs, as determined by Student's t-test.
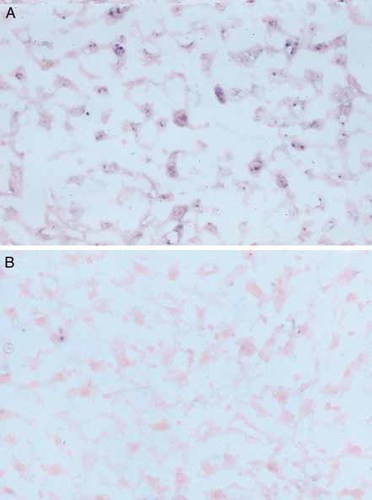
Expression of sIL-1Ra mRNA by hepatocytes in response to endotoxin in vivo. LPS was injected i. p. and the mice were killed after 4 h. Frozen tissue sections from the liver were prepared and in situ hybridization was performed using a digoxigenin-labeled antisense riboprobe complementary to either murine sIL-1Ra (A) or icIL-1Ra1 mRNA (B). The presence of positive hybridization was detected by the addition of alkaline phosphatase-conjugated anti-digoxigenin antibodies followed by NBT / BCIP substrate. The slides were counterstained with nuclear fast red. The presence of either IL-1Ra isoform mRNA is demonstrated by purple staining.
The presence of the different IL-1Ra isoforms can also be distinguished by Western blot analysis, as shown in Fig. 3 A. Cell lysates of resident peritoneal macrophages and of RAW 264.7 murine macrophage cells stimulated with LPS (lanes 1 and 3) contained both 18-kDa icIL-1Ra1 and 16-kDa icIL-1Ra3, a recently characterized low molecular mass intracellular isoform. However, only icIL-1Ra1 but not icIL-1Ra3 was constitutively present in skin extracts (lane 2). As recently described 14, the cell lysates of LPS-stimulated peritoneal macrophages contained two additional bands (lane 1): a higher molecular mass band corresponding to pro-sIL-1Ra and a very faint band of 17 kDa corresponding to mature sIL-1Ra (detected only on the original autoradiograph). Western blot analysis was performed on proteins extracted from the liver and spleen of mice injected with LPS. Three different bands were present in extracts of the two organs (Fig. 3 B, lanes 2 and 3). A weak middle band migrated at the same level as recombinant 17 kDa murine sIL-1Ra and represented unglycosylated mature sIL-1Ra. The presence of the lower and the higher molecular mass bands is consistent with 16-kDa icIL-1Ra3 and pro-sIL-1Ra, respectively. As opposed to RAW 264.7 cells and proteins extracted from the skin (Fig. 3 B, lanes 1 and 4), icIL-1Ra1 protein was not detected in extracts of the spleen and liver, thus further supporting the findings at the mRNA level.
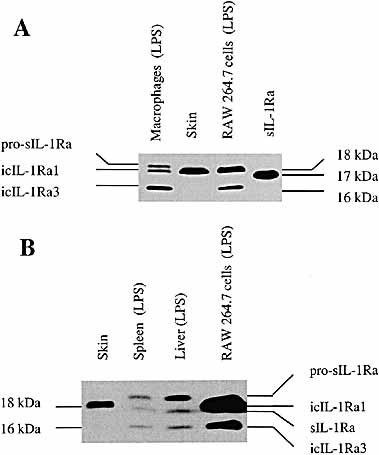
Western blot analysis of IL-1Ra isoform expression. (A) Total proteins (20 μg) from LPS-stimulated resident peritoneal macrophages, skin extracts, and LPS-stimulated RAW 264.7 murine macrophages were electrophoresed in a 17.5 % polyacrylamide gel, followed by electrophoretic transfer to a PVDF membrane. Western blot analysis was performed using polyclonal goat anti-murine IL-1Ra antibodies. Recombinant murine sIL-1Ra was also included as a control. (B) Skin, spleen, liver extracts, and LPS-stimulated RAW 264.7 cell lysate were examined by Western blot analysis, as described above.
2.2 Production of sIL-1Ra mRNA by the liver in response to turpentine-induced local inflammation
Plasma concentrations of IL-1β, IL-6, IL-1Ra and SAA as well as IL-1Ra and SAA1 mRNA levels in the liver were determined at different time-points after local injection of turpentine. In contrast to mice treated with LPS, circulating IL-1Ra and IL-1β levels remained undetectable after turpentine-induced inflammation 11. Plasma levels of IL-6 increased with a peak occurring 24 h after the injection of turpentine (Fig. 4 A). However, the levels of IL-6 were much lower than those observed in response to LPS 11. Plasma levels of SAA were also elevated and remained high up to 48 h after stimulation (Fig. 4 B). Production of sIL-1Ra and SAA1 mRNA by the liver were also up-regulated in response to local turpentine-induced inflammation (Fig. 5). In contrast to IL-1Ra, neither IL-6 nor IL-1β mRNA were present in the liver of mice injected locally with either saline or turpentine (data not shown). Thus, although sIL-1Ra mRNA was present in the liver of mice with local turpentine inflammation, no IL-1Ra protein could be detected in the circulation.
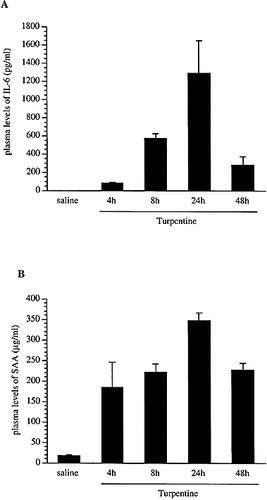
Circulating levels of IL-6 and SAA in mice after s. c. injection of turpentine. Blood of C57BL / 6 mice injected with either turpentine or saline was collected at diferent time points. Plasma levels of IL-6 (A) and SAA (B) were determined by ELISA. The values are the mean ± SEM of three mice. These results are representative of at least three experiments.
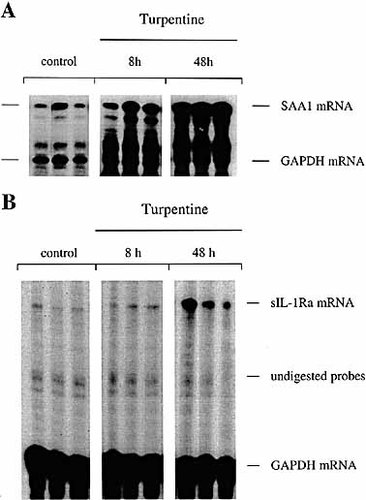
Liver SAA1 and IL-1Ra mRNA levels in mice after s. c. injection of turpentine. Livers of C57BL / 6 mice injected with either turpentine or saline were collected at 8 h and 48 h. Quantitative expression of SAA1, IL-1Ra and GAPDH mRNA were assessed by RNase protection assay. Liver total RNA (50 μg) were hybridized with a 32P-labeled riboprobe complementary to SAA1 mRNA (A) or to a probe that recognizes sIL-1Ra and icIL-1Ra1 mRNA as two different protected size fragments (B). A probe for GAPDH mRNA was hybridized simultaneously as a control. The RNA protected fragments were electrophoresed in a denaturing polyacrylamide gel. The gels were dried and autoradiography was immediately performed. Expression of SAA1 was very strong and the gels were exposed for 3 h, whereas the gels for IL-1Ra were exposed for 18 h. Identification of the bands was previously performed by hybridizing total RNA with either riboprobe separately.
IL-6 is known to play a major role in the induction of APP by the liver. To investigate the role of IL-6 in production of sIL-1Ra and SAA by the liver, IL-6– / – and IL-6+ / + littermates were injected locally with turpentine or saline. Steady-state levels of sIL-1Ra mRNA remained unchanged in the livers of IL-6– / – mice after the injection of turpentine, whereas the levels were significantly up-regulated in IL-6+ / + mice (Fig. 6 A). The levels of hepatic SAA1 mRNA also were significantly lower in IL-6– / – than IL-6+ / + mice after turpentine injection as well as in controls injected with saline solution (Fig. 6 B). Consistent with this result, plasma levels of SAA were elevated in IL-6+ / + mice, but remained undetectable in IL-6– / – mice following turpentine injection (data not shown). These results indicate that IL-6 is required for hepatic production of both IL-1Ra and SAA1. In addition, basal production of SAA but not of IL-1Ra is dependent on IL-6 (Table 2).
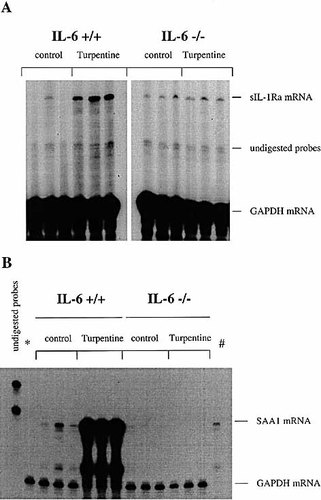
Turpentine-induced acute-phase response in IL-6+ / + versus IL-6– / – mice. IL-6+ / + and IL-6– / – mice were injected s. c. with either turpentine or saline. After 48 h, livers were collected for determination of IL-1Ra mRNA and SAA1 mRNA levels by RNase protection assay. Liver total RNA (50 μg) were hybridized with a probe for either SAA1 or IL-1Ra mRNA and digested with RNase A and RNase T1. A probe for GAPDH was hybridized simultaneously as a control. The RNA protected fragments were electrophoresed in a denaturing polyacrylamide gel. The gels were dried and autoradiography was immediately performed. (A) represents sIL-1Ra mRNA in IL-6+ / + and IL-6– / – mice 48 h after saline or turpentine injection. (B) represents SAA1 mRNA in IL-6+ / + and IL-6– / – mice 48 h after saline or turpentine injection. Liver total RNA of a IL-6+ / + mouse injected with saline was also hybridized with riboprobes complementary to either GAPDH (*) or SAA1 (#) mRNA separately.
|
|
IL-6+ / + |
IL-6− / − |
||
|---|---|---|---|---|
|
|
Control |
Turpentine |
Control |
Turpentine |
|
IL-1Ra / GAPDH |
2.6 ± 0.7 |
20.5 ± 1.5 |
2.9 ± 0.4 |
2.7 ± 0.94* |
|
SAA1 / GAPDH |
174 ± 77.5 |
4305 ± 430 |
7.4 ± 3.57* |
4.13 ± 0.38* |
- a) IL-6+ / + and IL-6− / − mice were injected with saline (control) or turpentine and killed after 48 h. Levels of IL-1Ra, SAA1 and GAPDH mRNA were examined by RNase protection assay. Results represent sIL-1Ra / GAPDH mRNA or SAA1 / GAPDH mRNA ratio × 103. The values are the mean ± SEM of three mice.
- * p < 0.05 as compared with IL-6+ / + mice, as determined by Student's t-test.
2.3 Production of IL-1Ra at the site of inflammation after local injection of turpentine
The production of IL-1Ra in the local tissue in turpentine-induced inflammation was next examined and compared with the levels of IL-1β. In contrast to hepatocytes, both sIL-1Ra and icIL-1Ra1 mRNA were produced in inflamed tissues (Fig. 7 A). The result of the Western blot analysis confirmed that each IL-1Ra protein isoform was present in the inflamed tissues and also increased between 8 h and 48 h (Fig. 7 B). Local IL-1β mRNA and protein production was also up-regulated following turpentine injection. However, in contrast to IL-1Ra, local levels of IL-1β mRNA peaked at 8 h and decreased after 48 h (Fig. 8). Production of IL-6 mRNA exhibited a similar kinetics as IL-1β (Fig. 8). The IL-1Ra / IL-1β protein ratio in the inflamed tissue increased from 10 : 1 at 8 h to over 1000 : 1 at 48 h (data not shown).
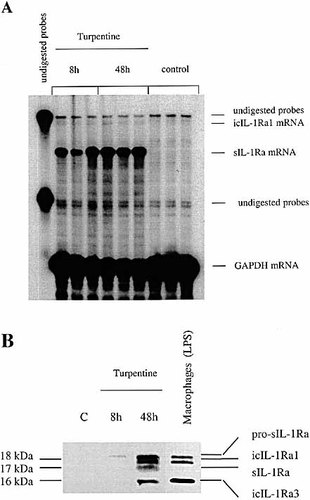
Production of IL-1Ra isoforms at the site of inflammation in mice injected with turpentine. C57BL / 6 mice were injected s. c. with either turpentine or saline and local tissues at the site of injection were collected at 8 h and 48 h. Quantitative expression of IL-1Ra mRNA isoforms was assessed by RNase protection assay using a probe that distinguishes sIL-1Ra and icIL-1Ra1 mRNA as two different protected size fragments. Local tissue RNA (10 μg) was hybridized simultaneously with 32P-labeled riboprobes for IL-1Ra and GAPDH mRNA and digested with RNase A and RNase T1. The RNA protected fragments were electrophoresed in a denaturing polyacrylamide gel. The gels were dried and autoradiography was immediately performed (A). Production of IL-1Ra isoforms was also examined at the protein level by Western blot analysis (B). Protein from local tissues (20 μg) collected at different time points was electrophoresed in an SDS-17.5 % polycrylamide gel and electrotransferred to a PVDF membrane. Western blot analysis was performed using polyclonal goat anti-mouse IL-1Ra antibodies. Cell lysate of LPS-stimulated resident peritoneal macrophages was added as control.
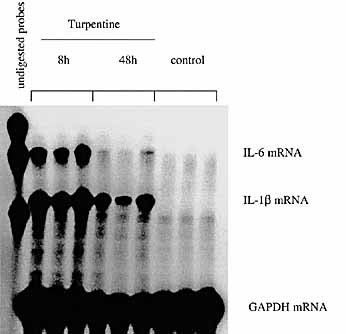
Local IL-1β and IL-6 production and the balance between IL-1Ra and IL-1β in the course of turpentine-induced inflammation. C57BL / 6 mice were injected s. c. with either turpentine or saline and the local tissues at the site of injection were collected at 8 h and 48 h. Quantitative expression of IL-1β and IL-6 mRNA was assessed by RNase protection assay. Total RNA (10 μg) were hybridized simultaneously with 32P-labeled riboprobes complementary to IL-1β, IL-6 and GAPDH and digested with RNase A and RNase T1.
To determine whether turpentine-induced IL-1Ra production was regulated in a similar manner in the liver and in inflamed tissues, local IL-1Ra mRNA levels were also measured in IL-6– / – and in IL-6+ / + mice. As opposed to the liver, local production of IL-1Ra after turpentine injection was not significantly different in IL-6– / – and IL-6+ / + mice (Fig. 9). Local tissue levels of IL-1Ra protein were normalized to total protein concentrations. In accordance with the results at the mRNA level, the concentrations of IL-1Ra protein were not significantly different in IL-6– / – and IL-6+ / + mice (72 ± 12 vs. 76 ± 11 pg / μg total protein, p > 0.05).
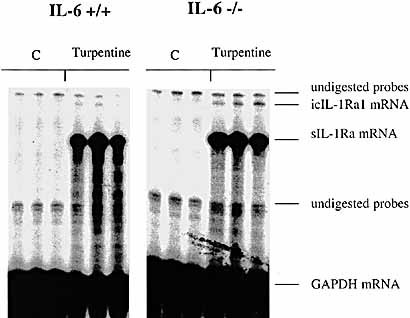
Local tissue IL-1Ra mRNA levels in IL-6+ / + versus IL-6– / – mice. C57BL / 6 IL-6+ / + and IL-6– / – mice were injected s. c. with either turpentine or saline and local tissues at the site of injection were collected at 48 h. Quantitative expression of IL-1Ra mRNA isoforms was assessed by RNase protection assay using a probe that distinguishes sIL-1Ra and icIL-1Ra1 mRNA as two different protected size fragments. Local tissue RNA (10 μg) were hybridized simultaneously with 32P-labeled riboprobes for IL-1Ra and GAPDH mRNA and digested with RNase A and RNase T1. The RNA protected fragments were electrophoresed in a denaturing polyacrylamide gel. The gels were dried and autoradiography was immediately performed.
2.4 icIL-1Ra3 is an in vivo product ofsIL-1Ra mRNA but not of icIL-1Ra1 mRNA
Recently, 16-kDa icIL-1Ra3 has been characterized as a smaller molecular mass isoform of IL-1Ra. Using an in vitro translation assay, icIL-1Ra3 was demonstrated to be produced by alternative translation initation from both sIL-1Ra and icIL-1Ra1 mRNA 6. To examine the production of icIL-1Ra3 in vivo, we used transgenic mice overexpressing either human sIL-1Ra or icIL-1Ra1. We previously showed that IL-1Ra is not detected in normal tissues except for the constitutive presence of icIL-1Ra1 in the skin and intestine 14. As shown in Fig. 10 A, both glycosylated sIL-1Ra and icIL-1Ra3 were present in different tissues of mice possessing the sIL-1Ra transgene and in the lysates of murine macrophage RAW 264.7 cells transfected with the transgenic construct containing human sIL-1Ra. In contrast, only icIL-1Ra1 and not icIL-1Ra3 was detected in mice overexpressing icIL-1Ra1 mRNA (Fig. 10 B). Similar results were obtained in the different transgenic mouse lines. This result indicates that icIL-1Ra3 is primarly produced from sIL-1Ra mRNA in vivo.
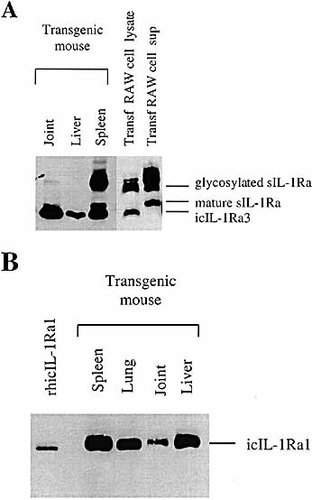
Western blot analysis of tissues from transgenic mice overexpressing either human sIL-1Ra or icIL-1Ra1. Transgenic mice overexpressing human sIL-1Ra (A) or icIL-1Ra1 (B) were generated as described in Sect. 4.1. Proteins from different tissues (20 μg) were electrophoresed in an SDS-17.5 % polyacrylamide gel and electrotransfered to a PVDF membrane. Western blot analysis was performed using a mouse anti-human IL-1Ra mAb that recognizes all the different isoforms. Recombinant human icIL-1Ra1, lysate and supernatant of RAW 264.7 cells (Transf RAW cell) transfected with human sIL-1Ra were included as controls.
3 Discussion
Previously published studies from our laboratory showed that sIL-1Ra is produced by cultured human hepatocytes and HepG2 hepatoma cells in vitro 10. In addition, LPS injection in mice led to sIL-1Ra mRNA production in the liver, spleen and lung 11. Data described herein confirm that sIL-1Ra, but not icIL-1Ra1, mRNA is produced by the liver in response to LPS stimulation in vivo. Furthermore, the extent of sIL-1Ra protein synthesized by the liver indicates that this organ contributes significantly to the elevated circulating levels of IL-1Ra observed following LPS administration. By in situ hybridization, we demonstrated that hepatocytes are the cellular source of sIL-1Ra mRNA production in the liver. After s. c. injection of turpentine, an experimental model of local tissue damage, liver sIL-1Ra mRNA levels were up-regulated in a manner similar to SAA1 mRNA, a classical APP. Furthermore, using IL-6 gene knockout mice, we showed that in vivo production of sIL-1Ra mRNA by hepatocytes is dependent on the presence of IL-6 as a classical APP. IL-1Ra was also synthesized in the inflamed tissues in response to turpentine. However, in contrast to the liver, IL-6 had no effect on local production of IL-1Ra.
Previous studies in rodents demonstrated that infiltrating inflammatory cells, including neutrophils and macrophages, are present at early time points at the site of turpentine injection 15, 16. These cells contribute to the production of IL-1β and IL-6, which play a major role in the acute-phase response 17, 18. Human and murine phagocytes, including neutrophils and macrophages, are able to produce large amounts of IL-1Ra in vitro and in vivo following different types of stimulation 12, 14, 19, 20. Oral infection of mice with Yersinia enterocolitica resulted in the production of IL-1Ra by neutrophils in Peyer's patches and in peripheral blood. In this model, IL-6 was required for the expression of IL-1Ra. In contrast, production of IL-1Ra by neutrophils in response to LPS is partially independent of IL-6 20. Our results obtained with IL-6– / – mice indicate that IL-6 does not contribute to production of IL-1Ra in the local tissue following turpentine injection. These observations suggest that the importance of IL-6 in IL-1Ra production by phagocytes is likely to vary with the type of stimulation.
The term IL-1Ra refers to different peptides produced from the same gene. One isoform (17-kDa sIL-1Ra) is glycosylated and secreted with a molecular mass that varies between 22 and 25 kDa 21. The three other IL-1Ra isoforms do not possess a leader sequence and therefore remain intracellular (icIL-1Ra1, 2, 3). sIL-1Ra and icIL-1Ra1 are derived from the same gene but have two different first exons, with two separate promoters and mRNA 22, 23. The icIL-1Ra1 protein possesses seven additional amino acids in the N-terminal region as compared with mature sIL-1Ra and has a molecular mass of 18 kDa 3. The sequence of icIL-1Ra2 mRNA contains an additional exon located between the first exons for icIL-1Ra1 and sIL-1Ra and which codes for 21 amino acids, thus producing a predicted 26-kDa protein 4. Recently, another intracellular IL-1Ra variant has been described, primarily in hepatocytes and neutrophils (16-kDa icIL-1Ra3) 6. This latter isoform is produced by alternative translation initiation. Using transgenic mice overexpressing either human sIL-1Ra or icIL-1Ra1 mRNA, we demonstrated that in vivo synthesis of icIL-1Ra3 is derived only from sIL-1Ra mRNA.
The physiologic function of IL-1Ra has recently been demonstrated using IL-1Ra knockout mice and blocking antibodies. IL-1Ra– / – mice are more susceptible to LPS-induced death than their wild-type littermates 24. Our data demonstrate that the liver is a major source of IL-1Ra production following LPS administration. Production of IL-1Ra by hepatocytes was also up-regulated in bacteria-induced hepatitis 25. In addition, using neutralizing antibodies against IL-1Ra, it was demonstrated that synthesis of IL-1Ra by the liver influenced survival in this experimental model of hepatitis 25. Taken together, these findings suggest that production of IL-1Ra by hepatocytes plays a significant role in host defense.
In contrast, hepatic production of IL-1Ra in response to turpentine-induced tissue damage was not sufficient to increase the levels of IL-1Ra in the circulation. Therefore, it is unlikely that liver-derived IL-1Ra participates in modulation of local inflammatory conditions in the mouse. Similar results were also observed in murine collagen-induced arthritis (unpublished data, C. Gabay et al.). In contrast, production of IL-1Ra was strongly up-regulated at the site of inflammation, with its levels further increasing at later time points after the administration of turpentine. Local IL-1β concentrations peaked at an early time point in turpentine-induced inflammation, but returned to near baseline levels by 48 h. These changes in the balance between agonist and antagonist are likely to contribute to the local resolution of acute inflammatory reactions. Consistent with these findings, the physiologic role of the balance of IL-1 and IL-1Ra in the magnitude of turpentine-induced inflammation was recently demonstrated using mice overexpressing IL-1Ra and IL-1Ra knockout mice 26.
4 Materials and methods
4.1 Animals and treatments
IL-6– / – and IL-6+ / + C57BL / 6 control mice were purchased from Jackson Laboratory (Bar Harbor, ME). Transgenic mice overexpressing either human sIL-1Ra or icIL-1Ra1 under the control of a β-actin-CMV promoter were generated as follows. Human sIL-1Ra and icIL-1Ra1 coding regions were subcloned into pCAGGSβ. High levels of protein expression were obtained with this vector in transgenic mice 27. The fragments containing the IL-1Ra sequence, the promoter, and the polyA signal regions were gel purified and injected into fertilized eggs obtained after crossing FVB females with DBA / 1 males, as described 28. The presence of the transgene was identified by PCR in founder mice and their offspring, and expression of human IL-1Ra isoforms was then examined in the transgenic offspring.
Six- to eight-week old C57BL / 6 mice were used to examine systemic inflammation. LPS (Escherichia coli 055 : B5, Difco, Detroit, MI) was injected i. p. at a dose of 100 μg, as previously described 11. To create a model of local tissue inflammation, turpentine was injected s. c. at a dose of 100 μl in each hindlimb, as previously described 29. All procedures using mice were approved by the University of Colorado Health Sciences Center Committee on Care and Use of Animals.
4.2 Determination of plasma levels of cytokines and SAA
Plasma and tissue levels of IL-1β and IL-6 were measured using ELISA kits specific for murine cytokines (Endogen, Inc, Cambridge, MA). Murine IL-1Ra levels were measured using a sandwich ELISA, as previously described 11. The detection limits for IL-1β, IL-6 and IL-1Ra were 10, 30, and 300 pg / ml, respectively. Plasma levels of SAA were determined using a direct ELISA 30. The SAA ELISA sensitivity was 15 μg / ml.
4.3 RNA isolation and RNase protection assays
Total RNA from the different tissues was extracted with Triazol (Life Technologies, Gaithersburg, MD). Riboprobes complementary to murine IL-1Ra, IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were created as recently described 31. The riboprobe for IL-1Ra is able to distinguish between the two murine IL-1Ra mRNA isoforms as different protected size products following RNase treatment. Riboprobes for IL-6 and SAA1 mRNA were created using the following primers: 5′IL-6, GCA TGA ATT CTT AAT TAC ACA TGT TCT CTG; 3prime;IL-6, GCA TAA GCT TGT TCT TCA TGT ACT CCA GG; 5′SAA, GCA TGA ATT CAA CTC AGA CAA ATA CTT CCA TGC TCG; and 3prime;SAA, GCA TAA GCT TCT GAG CTA ATA GGA GGA CGC TCA GT. The PCR products were cleaved with EcoRI and HindIII, gel purified, and cloned into pBluescript SK + (Stratagene, San Diego, CA). The plasmids containing the probes were linearized with EcoRI and transcribed with T7 RNA polymerase and 32P-labeled CTP. The RNase protection assays were performed as recently described 10.
4.5 In situ hybridization
Mice were injected i. p. with either LPS or saline and killed by CO2 asphyxiation. The liver was dissected and immediately frozen in OCT embedding matrix on dry ice and isopentane, then stored at − 80 °C until use. Sections (5 μm) were cut in a cryostat and mounted on Superfrost-plus slides (Eric Scientific Company, Portsmouth, NH). The in situ hybridization studies were performed as recently described 14.
4.6 Protein preparation and Western blot analysis
For Western blot analysis, total proteins from different tissues were prepared using Triazol, as recently described 14. Concentration of total proteins was determined using a protein assay kit (Bio-Rad). For IL-1Ra and IL-1β ELISA, organs were weighed and homogenized in PBS-0.1 % Tween-20 in a 1 : 4 dilution (weight : volume). The Western blot analysis was performed using a horseradish peroxidase-labeled goat anti-murine IL-1Ra antibody that recognizes all the described IL-1Ra isoforms. For Western blot analysis on protein extracts from transgenic mice expressing human IL-1Ra, a mouse anti-human IL-1Ra mAb was used 10.
Acknowledgements
We would like to thank Dr. W. A. Franklin and K. E. Fox (Department of Pathology, University of Colorado Health Sciences Center, Denver, CO) for assisting with the in situ hybridization studies. This work was supported by National Institutes of Health Grant AR40135 (to W.P.A.) and by Swiss Science Foundation Grants 3231-054954.98 and 3200-054955.98 (to C.G.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




