Reconstitution of antigen presentation in HLA class I-negative cancer cells with peptide-β2m fusion molecules
The first two authors contributed equally to this work.
Abstract
Engineered MHC-peptide targets capable of inducing recognition by CTL may prove useful in designing vaccines for infectious disease and cancer. We tested whether peptides directly linked to β2-microglobulin (β2m) could complex with human HLA class I heavy chain, and could be recognized by human CTL, both as soluble reagents and as cell surface constituents. An HLA-A2-restricted peptide epitope was physically linked to the N terminus of human β2m. This fusion protein refolded efficiently in vitro with HLA-A2 heavy chain, and when multimerized, the resultant complexes ("fusamers") bound specifically to appropriate CTL clones. These fused peptide/MHC complexes were as efficient as standard tetrameric peptide/MHC complexes in recognizing antigen-specific CTL. When the fusion protein was delivered to target cells using a retroviral vector, these cells were recognized and killed by appropriate CTL clones. Efficient sensitization to CTL lysis was achieved in TAP-negative and β2m-negative cell lines, as well as in unmutated B cell lines, proving that such constructs may be effective in inducing CTL even when the MHC class I pathway has been disrupted. Specific peptides covalently linked to β2m and delivered via retroviral vectors may be useful reagents for in vivo priming of CTL against epitopes of clinical relevance.
Abbreviations:
-
- hβ2m:
-
Human β2-microglobulin
-
- MA:
-
Influenza A matrix peptide (residues 58–66, GILGFVFTL)
1 Introduction
MHC class I molecules are heterodimers which comprise a transmembrane heavy chain noncovalently associated with β2-microglobulin (β2m). These complexes display peptides from endogenously synthesized self or pathogen derived proteins (epitopes). The peptides are transported into the endoplasmic reticulum via a peptide dependent transporter and assembled with newly synthesized class I molecules 1. The light chain β2m is important for intracellular transport, peptide binding and conformational stability. The properly assembled complex is then transported to the cell surface and recognized by circulating T cells.
CTL responses may play an important role in antitumor activity 2. Many cancers have inefficient mechanisms of antigen presentation which allow their escape from CTL surveillance 3. The disruption may occur at the level of peptide transport into the endoplasmic reticulum which causes loss of HLA class I expression on the cell surface. Many tumors harbor mutations in their β2m gene leading to a lack of synthesis or a truncated protein 3. β2m gene alterations leading to total loss of HLA class I phenotype have been described in melanoma 4, colorectal tumors 5 and lymphomas 6.
It is of interest to design vaccines that help to stimulate an appropriate immune response against tumors. This can be achieved by gene therapy strategies such as delivery of cytokine genes (IL-2; IL-12) 7, 8, co-stimulatory molecule genes (B7.1) 9 or antigen presentation by dendritic cells 10.
Our aim was to design a vaccine that could overcome the antigen presentation defects of HLA class I-negative cancer cells and elicit CTL responses. We asked whether it was possible to "label" tumor cells with a viral epitope and whether an anti-viral immune response might be exploited to kill tumor cells.
We fused the light chain (β2m) of the HLA class I complex to the HLA-A2-restricted influenza matrix epitope 11. The linker design was facilitated by previous attempts to engineer multimeric proteins as single chain molecules. Subunits can be held together by the introduction of synthetic linkers. Such strategies have been used for antibodies, 12 MHC class I 13, 14 and MHC class II molecules 15.
Using data from the X-ray structure of an HLA-A2/peptide complex 16 we designed an appropriate linker connecting the N terminus of β2m with the C terminus of the antigenic peptide.
We decided to multimerize this β2m-fusion molecule into a staining reagent ("fusamers") to test its ability to form proper complexes capable of binding to antigen-specific CTL. β2m can be refolded in vitro together with the HLA class I heavy chain to form a stable HLA class I complex which can be multimerized as tetrameric complexes (tetramers) 17. They have proven invaluable as research tools both in studies of the activation of CTL 18, and in the characterization of antigen-specific CTL responses in viral diseases and tumors 2, 19, 20.
A peptide-β2m fusion molecule as part of a mouse MHC class I complex was recently described 21. These peptide-β2m fusion/MHC complexes could be recognized as targets for mouse CTL in vitro 21 and their stability was enhanced by linking the epitope to β2m 22.
Soluble mouse MHC class I and class II tetrameric complexes with covalently linked peptide 23, 24 showed that such complexes were stable and stained mouse T cells and hybridomas. Our data showed that human β2m fused to the influenza matrix epitope (MA 58–66) refolded into complexes with human HLA-A2 heavy chain and when multimerized as fusamers bound to influenza-specific human CTL clones as efficiently as MHC class I tetramers.
These observations encouraged us to test whether this β2m fusion protein could be used as a peptide delivery system in tumors once it was expressed endogenously by retroviral transduced cells. The virus was able to deliver the protein to antigen presenting cells and cell lines lacking either β2m or the TAP transporter system. This molecule was able to elicit very efficient CTL responses in vitro strengthening our earlier observations that human β2m covalently linked to a peptide epitope was able to assemble properly with the HLA-class I heavy chain. The endogenous expression of fusion molecules in tumor cells transduced by retroviral vectors could provide an alternative way of delivering peptides to tumors which could then be eradicated by an antiviral immune response.
2 Results
2.1 Construction and expression of peptide-β2m fusion molecule
We linked the influenza matrix epitope GILGFVFTL to the N terminus of β2m via a synthetic linker (Fig. 1A). The linker was designed by molecular modeling and comprised a Gly-Gly-Ser motif, with the first five amino acids glycines to avoid interference of bulky side chains with the alpha helixes of the binding groove (Fig. 1B). A linker of 15 amino acids could span the gap between the C terminus of the peptide and the N terminus of β2m. The fusion protein had a molecular mass increase of ca 2.6 kDa consistent with the addition of 24 amino acids to β2m (Fig. 1C).
2.2 Expression and purification of β2m fusion molecule and complex formation with HLA class I heavy chain
The fusion molecule was folded together with soluble HLA-A2 heavy chain with a biotinylation tag on the C terminus. To test whether the complex contained the fusion molecule and to rule out cleavage of the peptide during handling, we ran fractions of the complex peak on an SDS-PAGE. The proteins detected corresponded to the intact fusion molecule and the HLA-A2 heavy chain (data not shown). We superimposed the Superdex 75 column elution profiles of the classic peptide/HLA complex (ternary complex) and the peptide-β2m fusion/HLA complex (binary complex). Both complexes showed similar running behavior, indicating comparable size and structure (Fig. 1D).
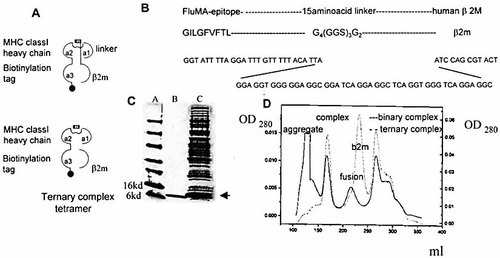
Construction, expression of peptide-β2m fusion protein and binary-complex formation with HLA-A2 heavy chain. (A) Schematic diagram of binary and ternary HLA class I complexes. (B) Cloning strategy for linking the influenza epitope (GILGFVFTL) to human β2m via a synthetic 15 amino acid linker. (C) Expression of fusion molecule: lane a: marker, lane b: β2m, lane c: peptide-β2m fusion protein as indicated by arrow. (D) Superimposed elution profiles of gel filtration runs (Superdex 75) for ternary complex and binary complex after refolding with HLA-A2 heavy chain.
2.3 Tetramerization of HLA class I complexes and staining of HLA-A2-restricted influenza matrix clone with tetramer and fusamer
The binary complex was biotinylated and multimerized with Streptavidin-PE as for the classic HLA/peptide tetrameric complexes (ternary complexes). The multivalent complexes of these binary and ternary MHC molecules are referred to as fusamers and tetramers. A CTL clone specific for the flu matrix epitope (58–66) stained with the fusamer (Fig. 2C, negative control Fig. 2A). An irrelevant melanoma-specific CTL clone, also restricted by HLA-A2, did not stain with this reagent (Fig. 2B). Stability of the fusamer was compared with stability of the tetramer by incubating both reagents at 37°C for 15 min, before staining CTL. The fusamer stained the flu clone as brightly as the tetramer (Fig. 2E, fusamer, 2F, tetramer, 2D, negative control). Hence under these experimental conditions, both reagents seemed to have similar stability at 37°C.
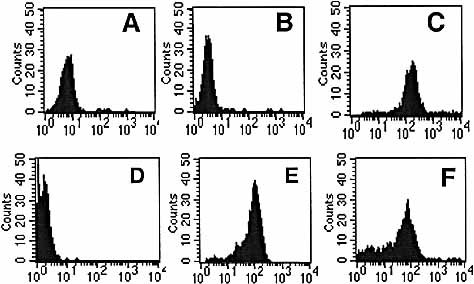
Staining of CTL clones with fusamer and tetramer. (A) HLA-A2-restricted influenza matrix epitope (MA58–66) specific CTL clone, unstained. (B) Irrelevant HLA-A2-restricted melanoma clone, stained with fusamer. (C) Influenza matrix clone, stained with fusamer. (D) Influenza matrix clone, unstained. (E) Influenza matrix clone, stained with fusamer. (F) Influenza matrix clone, stained with tetramer.
2.4 Design of the MA-β2m-pLXSN retroviral vector and analysis of transduced cells
The retroviral vector MA-β2m-pLXSN was constructed to express the peptide-β2m fusion protein where the immunodominant HLA-A2-restricted influenza peptide (58–66) is linked via a 15-amino acid linker to the N terminus of the human β2m (Fig. 3A). The β2m signal sequence was placed in front of the epitope to target the whole molecule to the endoplasmic reticulum. The virus was produced by transfection of the packaging cell line PA317 and the supernatant was harvested and used to transduce target cells.
TAP-deficient HLA-A2+ T2 cells were transduced with the retroviral vector MA-β2m-pLXSN, stained with the anti-HLA class I mAb W6/32 and analyzed by flow cytometry. An increase in MHC class I complex on the cell surface was observed (Fig. 3B), indicating that β2m-MA had complexed with the HLA class I heavy chain in a conformationally correct manner independent of the TAP transporter.
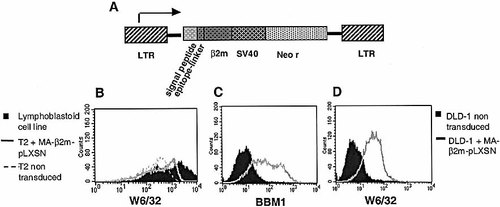
The MA-β2m-pLXSN retroviral vector and flow cytometry analysis of transduced cells. (A) Schematic representation of the retroviral construct. MA-β2m-pLXSN. (B) Flow cytometry analysis of transduced T2 cells. Non-transduced and transduced T2 cells were stained for HLA class I expression with the mAb W6/32. (C) Flow cytometry analysis of DLD-1 cells stained for β2m expression with the mAb BBM.1. (D) Flow cytometry analysis of transduced and non-transduced DLD-1 cells stained with the mAb W6/32.
A β2m deficient human colorectal adenocarcinoma cell line, DLD-1, was transduced with the retroviral vector and analyzed by flow cytometry for expression of cell surface β2m and MHC class I. As expected, an increase in cell surface expression of β2m was observed (Fig. 3C). Conformationally correct MHC class I complexes were also expressed (Fig. 3D).
2.5 Characterization of the expressed protein
Western blotting analysis was performed to test the expression of the fusion protein and to compare the amount of endogenous and retroviral proteins expressed in transduced cells. Cellular lysates from 1×106 T2 cells and transduced T2 cells were analyzed using the mAb BBM1 to detect the presence of the epitope-β2m fusion protein. The two forms of β2m were detected in the transduced T2 cells as the 12 kDa form and the 14 kDa fusion protein product of the fusion between hβ2m, the 15 aa linker and the 9 aa peptide (Fig. 4A). The amount of protein expressed by the retrovirus detected in this assay was about 20 times less than the amount of endogenous β2m as calculated from the intensity of the bands.
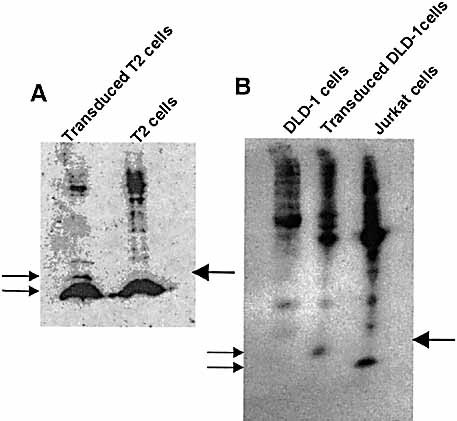
Detection of the peptide-β2m fusion protein in transduced cell lines. (A) Western blotting analysis of transduced T2 cells using the mAb BBM.1. (B) Immunoprecipitation of biotinylated surface protein with the mAb BBM.1 in DLD-1 cells. Cellular lysate from Jurkat cells was used as a positive control. The migration of the endogenous β2m and of the peptide-β2m fusion protein are indicated by the arrows on the left. The migration of the 15-kDa standard is shown on the right.
To test the integrity of the fusion protein on the cell surface, transduced DLD-1 cells were surface-labeled with biotin and the peptide-β2m fusion protein was immunoprecipitated. The results showed that the immunoprecipitated β2m from the transduced DLD-1 cell line was bigger than the endogenous β2m immunoprecipitated from a β2m positive cell line (Jurkat) used as control (Fig. 4B). The retroviral transduced protein corresponded to a 14 kDa protein. A quantitative analysis based on the intensity of the bands showed that the amount of recombinant β2m expressed was the same as in the control cell line.
2.6 Lysis of transduced cells by CTL
A 51chromium-release assay was performed using the transduced T2 cells expressing the peptide-β2m fusion protein. The assay was performed as described above, using a flu-matrix-specific CTL clone. The specific killing in absence of peptide was very high for the cells expressing the fusion protein (72%) comparable to the level of lysis obtained for the non-transduced cells pulsed with the peptide (70–80%) (Fig. 5A). The addition of peptide to the transduced cells did not alter significantly the percentage of specific lysis (70–80%).
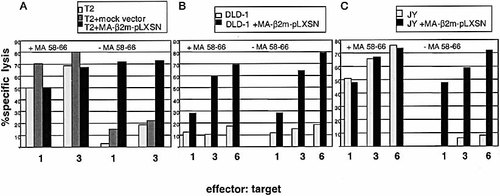
Generation of CTL targets in cell lines transduced with the retroviral vector MA-β2m-pLXSN. 51Chromium-release assays were performed on traduced and non-transduced cells using an influenza specific HLA-A2 restricted CTL clone. The assays were performed in the presence and absence of the peptide MA 58–66 as shown. Different effector:target ratios were used as indicated in the figure. (A) T2 cells transduced with a mock vector, T2 cells transduced with the MA-β2m-pLXSN vector and non-transduced T2 cells were used as target cells. (B) DLD-1 cells transduced with the MA-β2m-pLXSN vector and non-transduced DLD-1 were used as target cells. (C), JY cells transduced with the MA-β2m-pLXSN vector and non-transduced JY cells were used as target cells.
The values obtained for the T2 cells transduced with a mock virus were comparable to the values obtained for the non-transduced cells, demonstrating that the killing is specific for the peptide-β2m fusion protein.
The retroviral transduced DLD-1 cells were used as targets in a chromium-release assay to assess the ability of the fusion protein to form a functional class I complex in a β2m negative cell line. The results (Fig. 5B) showed a high level of specific lysis for the cells (78%) expressing the peptide-β2m fusion protein in both the absence and the presence of the peptide. The non-transduced cells showed only background lysis.
The human lymphoblastoid cell line JY was transduced with the retroviral vector MA-β2m-pLXSN and used in the assay as target cells (Fig. 5C). The specific killing activity with the transduced cells was as high as the normal control (non-transduced JY cells) where the peptide was added. On the contrary, the specific lysis in the absence of the peptide was around background levels for the non-transduced cell line while the transduced cell line showed values comparable with those obtained with the positive controls. In this assay, the fusion protein was able to form CTL recognition structures even in the presence of an endogenous HLA class I complex.
3 Discussion
The engineering of antigenic molecules may enable induction of specific immune responses. Fusion molecules in which the antigenic peptide is linked to human β2m were studied as soluble reagents and as cell surface constituents. These molecules were recognized by specific CTL, and as such are potentially useful tools to study and elicit specific CTL responses in humans.
We linked the immunodominant HLA-A2 restricted influenza epitope (MA 58–66) to human β2m to test its assembly with HLA class I heavy chain. Structural modeling data predicted a linker length of about 15 amino acids composed of glycines and serines as previously described 13. Covalent linkage of antigenic peptide to β2m resulted in functional HLA class I complexes as recently demonstrated for mouse MHC class I molecules 21. We showed that it was also possible to form functioning HLA class I complexes with human CTL epitopes. The peptide-β2m fusion protein folded to form a proper complex in vitro with human MHC class I heavy chain despite the extended peptide C-terminus and the addition of this potentially bulky linker. The binary complex behaved like the ternary complex on gel filtration chromatography indicating similarity in structure and size.
We multimerized the binary complex to make fusamers. Normally, these tetrameric complexes are made in vitro by refolding the HLA class I molecules with the appropriate peptide. Many peptides are poorly soluble, creating problems during refolding. The expression of soluble HLA class I complexes in which the antigenic peptide is provided as a fusion with β2m and multimerized to form fusamers may help overcome this problem. Moreover, the possibility to produce a fusion construct at the DNA level by cassetting the epitope as an oligonucleotide insert allows the fast production of different complexes without the synthesis of peptides as required for standard tetramers.
This reagent was used to stain human antigen-specific CTL. In our experiments where we compared the staining of an influenza-specific CTL clone, fusamers stained the cells as brightly as tetramers. Both reagents were stable for up to 8 h at 37°C (data not shown) but comparison of their physical melting points may prove more sensitive in detecting any increased stability of fusamers over tetramers. If any stability difference does emerge, fusamers may prove to be more useful tools for in vivo experiments.
Another advantage of peptide-β2m fusion complexes is their use in functional analysis of CTL. Standard HLA/ peptide complexes have the disadvantages that peptides derived from the complexes can easily contaminate the cultures, potentially resulting in indirect activation of T cells after binding to HLA on the surface of the target cells. In a very recent study looking into the fate of CTL after their encounter with antigen presenting cells, CTL were shown to die after killing their targets and the peptide-β2m fusion molecule was used to exclude the possibility that CTL were killing each other in a peptide specific fashion due to free peptide in the assay (Xiao-Ning Xu, unpublished data).
These results demonstrated that the fusion molecule as part of the HLA class I complex was recognized by CTL and may offer a very effective strategy to create strong CTL target structures since the peptide is not free to dissociate from the complex and has more contact points with the heavy chain. The stability of the peptide/HLA class I complex is fundamental in determining the strength of CTL responses 1. The fusion molecule was previously shown to be more stable than the peptide and it was also possible to load the fusion molecule onto antigen-presenting cells via exchange of β2m 21, 22. β2m seems to work by stabilizing the complex and enhancing the immune response 25.
These observations, together with our results encouraged us to use the peptide-β2m fusion molecule in different cell lines for endogenous loading of HLA class I molecules via retroviral vector gene delivery.
In TAP transporter-deficient cells (T2) the amount of HLA class I molecules present on the surface increased after retroviral transduction. The level of HLA class I on the cell surface is normally lower in these cells than in normal B cells. The presence of the peptide-β2m fusion molecule stabilized the complex and increased the level of HLA class I on the cell surface. This result confirmed the data shown previously for the H-2Db restricted NP-β2m fusion protein 21. The amount of expressed fusion protein was relatively low compared to the amount of endogenous β2m in this cell line. We do not know whether this is due to a poor transcription from the LTR promoter or to a low level of translation of the fusion protein. Despite the low level of protein expressed in T2 cells, it was possible to obtain a high level of specific killing when using these cells as target for an HLA-A2 restricted flu matrix specific CTL clone. This result indicated that a low amount of expression of this protein could create stable target structures and was sufficient for a very high level of CTL lysis.
We then considered the possibility of reconstituting HLA class I molecules on the surface of cells that are β2m deficient. The cell line DLD-1 is a human colon adenocarcinoma cell line that does not express β2m 26. After retroviral transduction, conformationally correct HLA class I molecules were expressed on the surface and recognized by a specific CTL clone, resulting in high levels of cell lysis. The ability of a single construct to create CTL targets in a β2m deficient cell line, bypassing the need for peptide, is unique to this kind of fusion protein. Interestingly, the amount of fusion protein expressed in this cell line was comparable to the amount of endogenous β2m expressed in a control cell line.
Human lymphoblastoid cells could also be sensitized to lysis by specific CTL using this retrovirus. The level of lysis for the transduced JY lymphoblastoid cell line was comparable to the level obtained for the same cells pulsed with the peptide. These results suggested that even in the presence of endogenous β2m, the fusion protein was able to compete and bind to the HLA class I heavy chain.
The integrity of the molecule on the cell surface was assessed by biotinylation of the surface proteins and immunoprecipitation of the peptide-β2m molecule with an anti-β2m antibody. The presence of the intact protein on the cell surface was also demonstrated for the fusion protein NP-β2m 21 and it may be relevant in case of in vivo delivery, where the peptide should not be presented by cells other than the targeted ones. This observation is consistent with generated target structures in the T2 cell line, where no peptide transport occurred.
When we cocultured peptide-β2m fusion expressing cells and non-transduced cells, no fusion protein was detected on the surface of the non-transduced cells analyzed by flow cytometry (data not shown). This showed that the fusion protein itself could not be shuffled from one cell to another.
Our results demonstrate that the peptide-β2m fusion molecule could elicit CTL responses when expressed endogenously by cells transduced with an MA-β2m retroviral vector. We showed that it was possible to reconstitute normal levels of MHC class I complexes on the surface of tumor cells that lack β2m and to reassemble CTL target structures. This approach could also generate a CTL response in tumor cells that do not lack HLA class I as shown in experiments with the lymphoblastoid cell line JY.
Many malignant tumors are characterized by the absence of β2m, due to chromosomal deletions or rearrangement 3, 4, 27. Also deficiency in the TAP transporter system 28 causes reduced expression of MHC class I and escape from immune surveillance. Peptide-β2m fusion molecules may be used in vivo to increase antigen presentation in tumors where peptides cannot be presented because they lack HLA class I complexes on the cell surface.
Several gene therapy strategies based on retroviral or adenoviral vectors 29 might be employed to improve an anti-tumor immune response. Autologous cancer cells can be genetically modified by ex vivo retroviral gene delivery to stimulate an efficient immune response 7. Retroviral transduction of tumors in vivo may be obtained by targeting the vector specifically to the tumor cells. Many cell-specific delivery systems have been studied based on the modification of the viral envelope protein. This protein is involved in recognition of cellular surface receptors and internalization of the virus. When the receptor-binding domain of the envelope protein is replaced by a ligand or a single chain antibody, specific cell surface receptors can be recognized 30, 31. Finally, the administration of ex vivo retroviral transduced antigen presenting cells expressing an antigenic protein could also give rise to a stable and efficient CTL response 32, 33.
Engineered MHC/peptide target structures may be exploited in vivo to stimulate a CTL response which is directed against a heterologous viral epitope. Tumor cells which lack HLA class I on the surface or even HLA class I positive tumor cells may be retrovirally transduced to express a viral epitope fused to β2m. The peptide-β2m fusion protein may be able to assemble with the HLA class I heavy chain and form CTL target structures on the cell surface which could be recognized by circulating CTL. This may lead to lysis of tumor cells "labeled" with the viral peptide, directing a viral immune response to an engineered cancer target.
4 Materials and methods
4.1 Cell lines, antibodies, bacterial strains and plasmids
T2 is a human lymphoblastoid cell line lacking HLA class I expression 34. JY is a human B lymphoblastoid cell line 35. DLD-1 is a human colorectal adenocarcinoma cell line that is defective in β2m expression 26. The anti-hβ2m mAb BBM.1 36 and the anti-HLA class I mAb W6/32 37 was a kind gift of Lisa Schimanski. The xenotropic cell line PG13 (American Type Culture Collection, Manassas, VA) was used for the production of recombinant viruses using the retroviral construct pLXSN (Clontech, Basingstoke, GB). The expression vector pGMT7 was kindly provided by Bent Jacobson. The bacterial strain used for recombinant proteins expression was BL21(DE3)plysS (Novagen, Nottingham, GB). Purified human β2m, extravidin peroxidase conjugate, extravidin-PE conjugated and 3,3prime;, 5,5′-tetramethylbenzidine (TMB) substrate were obtained from Sigma (Poole, GB).
4.2 Construction and expression of the β2m fusion molecule
The HLA-A2 restricted influenza matrix epitope (MA 58–66) was linked to human β2m by a 15 amino acid spacer (methionine residue added to the N terminus of the protein). The PCR was performed using 5′-primer OX297: 5′-GGG GGG CAT ATG GGT ATT TTA GGA TTT GTT TTT ACA TTA GGA GGT GGG GGA GGC GGA TCA GGA GGC TCA GGT GGG TCA GGA GGC ATC CAG CGT ACT CCA AAG ATT CAG G-3prime; and 3prime;-primer OX298: 5′-G ATA GTT AAG TGG GAT CGA GAC ATG TAA GCT TCC CCC-3prime;. The amplified fragment (NdeI-HindIII) was cloned into the expression vector pGMT7.
The retroviral vector MA-β2m-pLXSN was generated in a three step cloning strategy. The primers 5′ AAT TCG CCA CCA TGT CTC GCT CCG TGG CCT TAG CTG TGC TCG CGC TAC TCT CTC TTT CTG GCC TCG AGA CTA CTG-3prime; and 3prime;-GAT CCA GTA GTC TCG AGG CCA GAA AGA GAG AGT AGC GCG AGC ACA GCT AAG GCC ACG GAG CGA GAC ATG GTG GCG-5′ were annealed to originate the signal sequence and cloned as a EcoRI-BamHI fragment into the pLXSN construct. A XhoI site was present at the 3prime; end of the signal sequence.
A plasmid expressing β2m was used as template for PCR using the primer 5′-ATT ATT CTC GAG ATC ATC ATG GAT CCG GCG GAG GCG-3prime; encoding the 3prime; end of the linker and including the restriction site XhoI and BamHI and the primer 3prime;-TGCACAGATCTTTACATCTCCCGATCCCAT-5′ including a BglII site. This fragment was cloned into the XhoI-BglII sites of the retroviral vector pLXSN. Oligos encoding the epitope and part of the linker (5′-TCG AGG GCG GCA TCC TGG GCT TCG TGT TCA CCC TGG GCG GCG-3prime; and 5′-ATT TCG CCA CCA TGT CTC GCT CCT TGG CCT TAG CTG TGC TCG CGC TAC TCT CTC TTT CTG GCC TCG AGA CTA CTG-3prime;) were cloned into the XhoI and BamHI site.
4.3 Expression, purification and tetramerization of peptide-β2m fusion protein
The construct containing the HLA-A2 heavy chain and the biotinylation tag was constructed as described previously 17. The β2m fusion plasmid (pGMT7) was transformed into the bacterial strain BL21(DE3)plysS (for safety reasons better done in E.coli HMS 174) and expression induced at OD600= 0.5 with 0.5 mM IPTG. Bacteria were harvested after 5 h and 10 μl lysate analyzed by PAGE using 4–20% gels.
Soluble HLA-peptide tetramers and fusamers were generated as previously described 17. Recombinant hβ2m was used for the ternary complex while the peptide-β2m fusion protein was used for the binary complex. The refold was biotinylated and tetramers obtained by adding extravidin-PE at a molar ratio of 0.3:1 (extravidin:biotinylated protein) 17.
4.4 Retroviral production and cell transduction
The xenotropic packaging cell line PG13 was transfected with the retroviral constructs MA-β2m-pLXSN and pLXSN. After selection in G418 (800 μg/ml), supernatant from producing cells was used for transduction of target cells. The T2 cell line was transduced with both the vectors MA-β2m-pLXSN and the mock vector while DLD-1 and JY cells were transduced with the retroviral vector MA-β2m-pLXSN. Cells (5×106) were transduced using 5 ml of viral supernatant in presence of protamine (8 μg/ml) for 16 h and grown in selective medium containing 400 (T2 cells), 1,600 (DLD-1 cells) and 500 (JY cells) μg/ml of G418 for 2 weeks.
4.5 Flow cytometry
For the detection of β2m in retroviral transduced cells, 106 cells were washed in PBS/0.1% BSA/1% human serum and incubated with 1 μg of anti-β2m (BBM.1) mAb for 30' on ice. The cells were washed and incubated with a phycoerythrin conjugated secondary antibody for 30' on ice. Cells were washed twice and analyzed by flow cytometry. For the detection of HLA class I, 1 μg of anti-HLA class I mAb (W6/32) was used and the cells were stained as described.
For the tetramer staining, HLA-A2-restricted CTL clones specific for flu matrix protein (clone C3, 19) or the melanoma antigen melan-A (clone 3G3, 38) were stained with equivalent doses (0.5 μg) of the fusamer or the tetramer for 15 min at 37C. After the staining, the cells were washed twice and analyzed by flow cytometry.
4.6 Western blotting
Western blotting analysis was performed on the non-transduced and retroviral transduced cell lysate to detect the peptide-β2m fusion protein. Cells (5×106)) were pelleted, washed once in PBS and lysed in 60 ml of lysis buffer (20 mM Tris pH 7.6, 10 mM EDTA, 100 mM NaCl, 0.5% Nonidet P-40) for 20 min on ice, analyzed by SDS-PAGE and immunoblotted with the mAb anti-β2m BBM1. The intensity of the bands were quantified using FluorchemTM System (Alpha Innotech Corporation, San Leandro, CA)
4.7 51Chromium-release assay
The transduced cell lines were assayed as target cells in a chromium-release assay. Cells were labeled for 1 h at 37°C with 100 mCi/106 cells Na51Cr and pulsed with 5 mM of the synthetic peptide for 1 h before the addition of an HLA-A2-restricted flu-matrix-specific CTL clone. Following 4 h incubation at 37°C, 51Cr release was measured in a gamma counter. The specific lysis was calculated according to the formula 100 × (experimental cpm-background cpm)/(maximum cpm-background cpm). Background cpm values were determined incubating target cells alone and maximum values were determined by lysing the target cells in presence of 5% Triton x-100. All the experiments were performed in triplicate.
4.8 Biotin-labeling of surface proteins and immunoprecipitation of β2m
For biotinylation of surface proteins, 5×107 cells were incubated at 4°C for 1 h in 1 ml of PBS-5 mM biotin. The cells were washed once in PBS-glycine 5 mM, and twice in PBS and resuspended in 500 μl of lysis buffer (20 mM Tris pH 7.6, 10 mM EDTA, 100 mM NaCl, 0.5% Nonidet P-40). β2m was immunoprecipitated using anti-β2m BBM1 antibody (15 μg/ml) and analyzed by Western blotting with peroxidase-conjugated streptavidin (1:1000).
Acknowledgements
We thank Geraldine Gillespie, Jessica Wyer, Paul Klenerman for help and discussion. Gavin Screaton for help with cloning strategies and Bent Jacobsen for help with expression strategies. This work was supported by DTI-MRC link grant and British Biotechnology Limited.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




