Organization of plasma membrane functional rafts upon T cell activation
Abstract
Raft microdomains have been shown to play a key role in T cell activation. We found that in human T lymphocytes the formation of functional rafts at the plasma membrane was induced by T cell priming. In resting T cells from peripheral blood Lck and the raft glycosphingolipid GM1 resided in intracellular membranes. T cell activation induced synthesis of GM1 and effector cells showed very high levels of this lipid, which became predominantly plasma membrane associated. TCR triggering also induced targeting of the cytosolic Lck to the plasma membrane. Thus, effector cells acquire an improved signaling machinery by increasing the amount of rafts at the plasma membrane. The fact that, when compared with naive T cells, memory T cells showed higher GM1 levels suggests that raft lipid synthesis may be developmentally regulated and tune T cell responsiveness.
Abbreviation:
-
- CT-B:
-
Cholera toxin-B
1 Introduction
Cell membranes are composed of a complex mixture of cholesterol and various glycerolphospholipids and sphingolipids. Glycerolphospholipids and sphingolipids exhibit distinct biophysical properties and behave in a different way when forming a monolayer, with glycerolphospholipids showing tendency to adopt a mobile fluid phase and sphingolipids showing a more tigthly packed organization 1. Simons and Ikonen 2 formulated a general "raft hypothesis" that predicts that in all cell types attractive forces between sphingolipids and cholesterol mediate the formation of lateral lipid clusters in an unsaturated glycerolphospholipid environment. According to this hypothesis, rafts serve as platforms were particular molecules are enriched while others are excluded.
A variety of signaling molecules are accumulated in raft domains, including the two Src family kinases Lck and Fyn, both implicated in T cell activation, the T cell linker molecule LAT, monomeric and heterotrimeric G proteins, G-coupled protein receptors and molecules involved in Ca2 + responses 3, 4. Posttranslational addition of lipids (myristylation, palmitoylation and farnesylation) is a critical requirement for the targeting of many of these molecules to the membrane as well as for their functions 5.
When clustered by antibodies, many glycosylphosphatidylinositol (GPI)-anchored proteins have been shown to transduce intracellular signals leading to tyrosine phosphorylation, Ca2 + flux or cell proliferation 6. This signaling depends on the raft-associated kinases and can be suppressed by reduction of the membrane cholesterol level or by changing the membrane lipid composition, indicating that integrity of raft domains is required 7.
In T cells it has been shown that engagement of TCR induces its recruitment to rafts and that raft integrity is required for efficient T cell activation 8, 9. Upon TCR triggering, supramolecular signaling complexes containing hyperphosphorylated ζ chains, activated ZAP-70 and many other signaling molecules are accumulated in raft domains. These events, as well as T cell activation, can be suppressed by disrupting raft structure, indicating that membrane compartmentation is a prerequisite for TCR signal transduction. It has been recently shown that CD28 engagement promotes an impressive redistribution of rafts at the TCR contact site, thus amplifying and sustaining TCR-induced signaling 10. Thus, if the recruitment of TCR into rafts may induce its triggering and may start the signaling cascade, the active recruitment and clustering of raft microdomains to the site of TCR triggering may represent a general mechanism by which co-stimulation can amplify the signaling process 11.
While the outcome of naive T cell activation is strongly influenced by CD28 co-stimulation, effector T cells exhibit a lower dependence on co-stimulation and a faster response than naive cells 12, 13. Recently, it has been shown that effector and memory T cells have a more efficient TCR signaling machinery than naive T cells. In murine naive cytotoxic T cells only few CD8 molecules are associated with Lck and the kinase is homogeneously distributed inside the cell, while in vivo priming of naive T cells induces the targeting of Lck to the CD8 coreceptor in the cell membrane 14.
The present study was undertaken to determine whether T cell activation results in reorganization of rafts at the cell membrane. We found that in vitro activation of human resting T cells induced rapid targeting of the cytosolic Lck and GM1 to the plasma membrane. Moreover, activation of resting T cells induced synthesis of the raft lipid GM1 which is highly expressed in effector cells.
2 Results and discussion
Raft microdomains are known to be enriched for glycosphingolipids, and the ganglioside GM1, which is recognized by cholera toxin, has been used in several studies as a raft marker. We analyzed the expression of GM1 in human resting peripheral blood CD4+ T cells and activated CD4+ T cells by FACS analysis and immunoblotting. Activated T cells always showed higher levels of GM1 expression than resting T cells, while expression of the CD3 complex was unaffected (Fig. 1 A, B). The higher level of GM1 in activated T cells was due to de novo synthesis induced by T cell activation, as shown by the kinetics of GM1 up-regulation and its inhibition by fumonisin B1, an inhibitor of sphingosine (sphinganine) N-acetyltransferase (ceramide synthase) (Fig. 1 C). The inhibition of GM1 synthesis by fumonesin B1 was not due do inhibition of T cell activation, as demonstrated by the fact that control and treated cells produced the same amount of IL-2 (not shown).
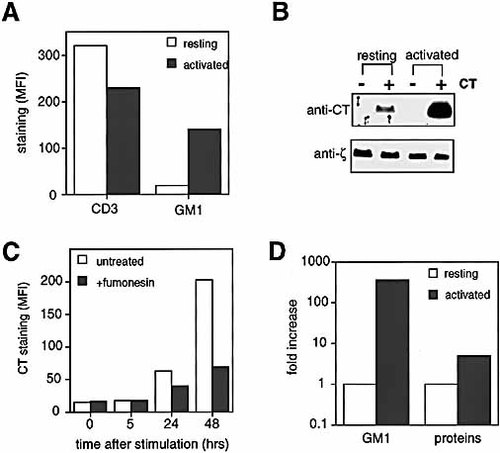
Up-regulation of the raft marker GM1 upon T cell activation. (A) Surface anti-CD3ϵ (TR66-FITC) and anti-GM1 (CT-B-FITC) staining in CD4+ peripheral blood T cells either resting or 3 days after PHA activation. (B) Anti-CT-B and anti-ζ chain immunoblotting in resting and activated T cells that were pre-incubated with (+) or without (−) 10 μg / ml CT-B. The bands from three different experiments were quantified by densitometry, and the result obtained showed a ∼ 10-fold increase in CT-B amount in activated versus resting T cells. (C) GM1 up-regulation is due to de novo synthesis. CD4+ resting T cells were activated in the presence or in the absence of fumonisin B1, and stained with CT-B-FITC at different times. (D) The GM1 ELISA showed a ∼ 350-fold increase in total GM1 amount in activated versus resting T cells. This higher difference compared to the FACS results is due to the different sensitivity of the methods. The following values were obtained: 2 pg GM1 / 106 resting T cells, 750 pg GM1 / 106 activated T cells; 9.7 μg protein / 106 resting T cells, 48.8 μg protein / 106 activated T cells. All the results are representative of at least three experiments.
To quantify the increase in GM1 levels in activated as compared to resting T cells, we performed GM1 ELISA. In all the experiments, activated T cells showed ∼ 350-fold increase in total GM1 amount as compared to resting T cells (Fig. 1 D). This impressive difference was not due to the bigger size of activated T cells, as shown by the fact that the total protein content was only ∼ 5 times higher in activated than in resting T cells (Fig. 1 D).
These results show that resting T cells have very low levels of the raft marker GM1 and that T cell activation induces synthesis of this lipid which is then expressed at very high levels in the membrane of activated / effector cells.
To study localization of rafts in T cells, we performed GM1 intracellular staining of naive and activated T cells, and analyzed the cells by confocal microscopy. As shown in Fig. 2 A, while in naive T cells the FITC-cholera toxin-B (CT-B) staining was diffuse inside the cell showing a clear intracelular distribution of GM1, in activated T cells the staining was restricted to the membrane, indicating that all the synthesized GM1 is not stored inside the cells, but directly targeted to the plasma membrane. Thus, while in resting T cells rafts are mainly intracellular and present only at low levels on the cell surface, in effector T cells they are present at higher levels and only on the cell surface. The Src-family kinase Lck is developmentally regulated in murine CD8 T cells 14. While in naive cytotoxic T cells Lck has a cytosolic distribution and is weakly associated to the coreceptor, in effector and memory CD8 T cells, the kinase is targeted to the membrane and associates with the coreceptor. To investigate whether this is true also in the case of human CD4+ T cells, we performed an anti-Lck staining of naive and activated T lymphocytes, and analyzed the cells by confocal microscopy. We found that in naive cells the Lck staining was cytoplasmic, while the kinase was predominantly plasma membrane-associated in cells activated by PHA (not shown). The translocation of Lck from the intracellular compartment to the membrane was followed at the microscope by looking at cells stimulated by anti-CD3 + anti-CD28-coated beads (Fig. 2 B). The fraction of Lck which was not plasma membrane associated was however raft associated (Fig. 3), suggesting that in naive T cells vesicles containing pre-formed rafts are retained in the cytoplasm and exported to the plasma membrane upon TCR triggering. We also found higher levels of Lck in activated versus resting T cells. This result fits with the higher expression of rafts observed in activated cells.
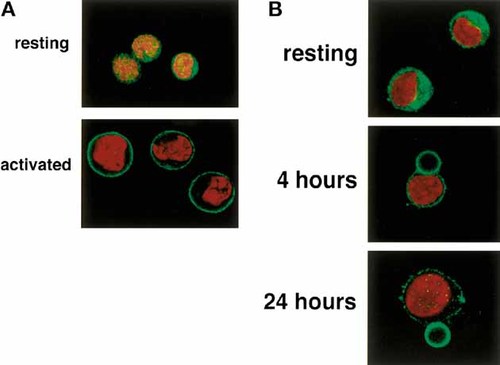
Cytosolic localization of rafts in naive T cells. (A) CD4+ naive and activated / effector T cells were permeabilized and stained with CT-B-FITC (green). (B) CD4+ naive T cells were incubated with beads coated with anti-CD3 + anti-CD28 antibodies for different times and then stained with an anti-lck (MOL171, IgG1) followed by FITC-labeled goat anti-mouse IgG1 (green). Nuclei were stained with propidium iodide (red). The results are representative of five different experiments.
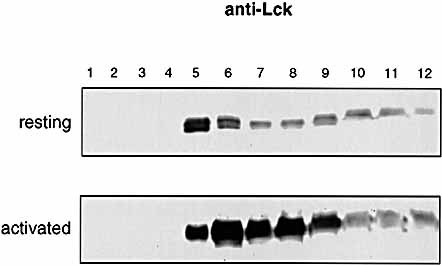
Lck is raft-associated in resting and activated T cells. CD4+ resting and activated T cells were homogenized with Mes-buffered saline containing 1 % Triton X-100 and subjected to sucrose gradient centrifugation as described in Sect. 3.6. Gradients were fractionated, and 100-μl aliquots of each fraction were subjected to SDS-PAGE followed by Western blotting with anti-Lck. Fraction 1 represents the top of the gradient.
Together, these results show that in human naive T cells, raft microdomains are not organized at the cell plasma membrane, but retained in the cytoplasm. Upon T cell stimulation, these domains are targeted to the membrane and assembled to create signaling compartments. Moreover, T cell priming induces neo-synthesis of raft components, allowing effector cells to be more responsive than naive T cells. The fact that the up-regulation of GM1 in activated / effector T cells is related to the signaling capacity of these cells was proved by the higher co-stimulation offered by GM1 cross-linking to activated than to naive T cells (not shown).
Finally, we tried to investigate whether, among the resting peripheral blood T cells, there was a difference in the GM1 levels of naive (CD45RAhigh, CD45ROlow) and memory cells (CD45ROhigh, CD45RAlow). We sorted these populations and found that memory cells showed constantly higher levels of GM1 staining than naive cells (Fig. 4). However, the GM1 staining in resting memory cells was always much lower than in activated / effector cells, indicating that rafts are regulated during the different stages of T cell life.
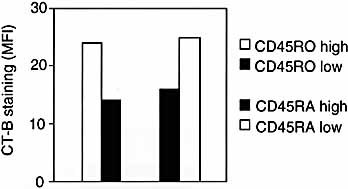
Higher level of GM1 in memory than in naive T cells. CD4+ resting T cells were stained with an anti-CD45RO or with an anti-CD45RA antibody. Four different populations of cells were sorted [CD45ROhigh (memory) and CD45ROlow (naive); CD45RAhigh (naive) and CD45ROlow (memory)], stained with CT-B-FITC and analyzed by FACS. Comparable results were obtained in three experiments.
The rapid interaction between triggered receptors and signaling molecules requires availability of components that depends on their concentration and distribution. The organization of the plasma membrane in distinct lipid microdomains which concentrate specific molecules represents a strategy to assemble or separate receptors and signaling molecules. Here we showed that the availability of the signaling components can vary in different cells or under different conditions, because the local concentration of the various components can be developmentally regulated. Naive T cells have few raft domains organized on the membrane, and in order to be activated they need CD28 co-stimulation which amplifies the TCR signaling by recruiting more rafts to the triggered TCR. Upon activation, the T cell signaling machinery is optimized, with the de novo synthesis of GM1 and Lck, and the organization of signaling microdomains at the plasma membrane. Indeed, effector T cells respond to lower doses of antigen than naive cells and do not require the CD28-mediated recruitment of rafts. In this way, by up-regulating or down-regulating the local concentration of signaling molecules, the T cell can modulate its responsiveness to antigens during different phases of its life.
3 Materials and methods
3.1 Cells
Human peripheral blood CD4+ T cells, naive CD45RA+ and memory CD45RO+ cells were sorted by negative selection, using a fluorescence-activated cell sorter (FACS Vantage). Resting cells were either maintained in culture or activated with PHA (Murex Diagnostics) for 3 days. In some experiments, cells were stimulated with polystyrene latex microspheres (Polysciences Inc.) coated with anti-CD3 (TR66) plus anti-CD28 (CD28.1; provided by D. Olive). In other experiments, T cells were stimulated in tissue culture wells that had been coated with anti-CD3 at different concentrations or anti-CD3 plus CT-B (Sigma) as described 10. Thymidine incorporation was measured after 72 h.
3.2 FACS analysis
Resting and activated cells were stained with FITC-labeled CT-B (Sigma) or with a FITC-labeled anti-CD3 (UCHT1, IgG1; Immunotech) and analyzed on a FACScalibur (Becton and Dickinson System). In some experiments, resting T cells were treated with fumonisin B1 before activation.
3.3 Anti-GM1 immunoblotting
Resting and activated T cells (106 cells) were incubated for 30 min at 4 °C with 10 μg / ml CT-B, washed and lysed for 30 min on ice in 1 % NP40 lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA in the presence of inhibitors of proteases and phosphatases: 10 μg / ml leupeptin, 10 μg / ml aprotinin, 1 mM pefabloc-sc, 50 mM NaF, 10 mM Na4P2O7 and 1 mM NaVO4. The proteins were resolved by SDS-12 % polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes. Blots were incubated with anti-ζ (mouse; UBI, GB) or anti-CT-B (rabbit; Sigma) antibodies, extensively washed and, after incubation with horseradish peroxidase (HRP)-labeled goat-anti-rabbit-IgG or HRP-labeled goat anti-mouse (Amersham International, Amersham, GB), developed with the enhanced chemiluminescence detection system (Amersham International).
3.4 GM1 ELISA
Gangliosides were extracted according to the chloroform-methanol-water method of Svennerholm and Fredman 15, which was optimized using a chloroform-methanol-aqueous ratio of 4 : 8 : 3. Specific GM1 detection in the ganglioside fraction was then determined by ELISA 16, using CT-B followed by an anti-CT-B antibody (rabbit; Sigma) and HRP-labeled goat anti-rabbit IgG. Monosialoganglioside GM1 (Sigma) was used as standard.
3.5 Confocal microscopy
Cells were immobilized on poly-L-lysine-coated slides, fixed in 2 % paraformaldehyde and permeabilized in 0.1 % Triton-X 100 before staining with FITC-CT-B or with a mouse mAb specific for Lck (MOL171, Pharmingen) followed by a FITC-labeled goat anti-mouse IgG1 (Southern Biotechnology Associates), as described 14. The nuclei were stained with propidium iodide.
3.6 Isolation of lipid rafts
Membrane rafts were isolated as previously described 17. Briefly, resting and activated CD4+ T cells (0.7 × 109) were homogenized with MES-buffered saline (25 mM MES, pH 6.5, 150 mM NaCl) containing 1 % Triton X-100 (v / v) and subjected to sucrose gradient centrifugation at 39,000 rpm for 16 – 20 h in a in SW41 rotor (Beckman Instruments). From the top of each gradient, 1 ml gradient fractions were collected to yield a total of 12 fractions. An equal volume from each gradient fraction was separated by SDS-PAGE (10 % acrylamide) and subjected to immunoblot analysis [anti-lck IgG (Santa Cruz Biotechnology) diluted 1 : 200; peroxidase-conjugated secondary antibody (Bio-Rad lab. Hercules, CA) diluted 1 : 3,000].
Acknowledgements
We thank Dr. Thomas Harder for helpful discussions and Prof. Rajewsky for critical reading of the manuscript. The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche Ltd., Basel, Switzerland. A. V. is supported by EMBO.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




