Abstract
Townes-Brocks syndrome (TBS) is a condition with imperforate anus, hand anomalies, and ear malformations with sensorineural hearing loss. Many cases are sporadic. Within and between families, the phenotype displays striking variability. Recently, the disease-causing gene for TBS was identified as SALL1, a zinc finger transcription factor. Here, we report a three-generation family with seven affected individuals who have a novel SALL1 mutation. Unique cardiac anomalies seen in this family include lethal truncus arteriosus in one patient and a lethal complicated defect, including pulmonary valve atresia, in a second patient. These severe cardiac anomalies have not previously been reported in a familial case of TBS. This family and a review of the literature indicate that cardiac evaluation is warranted in all individuals with this disorder. In addition, hypoplastic thumbs were seen in two individuals in this family and should, therefore, be considered a true feature of TBS. © 2001 Wiley-Liss, Inc.
INTRODUCTION
In 1972, Townes and Brocks described a family with a triad of malformations of the anus, hands, and ears in combination with deafness. Since that initial report, over 84 cases of Townes-Brocks syndrome (TBS) have expanded the phenotype and illustrate the great variability of this syndrome. Several patients have been reported with renal and genitourinary anomalies [Barakat et al., 1988; Cameron et al., 1991; de Vries-van der Weerd et al., 1988; Friedman et al., 1987; Ishikiriyama et al., 1996; Johnson et al., 1996; Kohlhase et al., 1998, 1999; Kurnit et al., 1978; Marlin et al., 1998; Newman et al., 1997; Rossmiller and Pasic, 1994; Yano et al., 1998]. Other rarer features have been reported including foot anomalies [Barakat et al., 1988; Cameron et al., 1991; de Vries-van der Weerd et al., 1988; Johnson et al., 1996; Konig et al., 1990; Kotzot et al., 1992; Marlin et al., 1998; Newman et al., 1997; O'Callaghan and Young, 1990; Rossmiller and Pasic, 1994; Townes and Brocks, 1972], skeletal findings excluding limb, such as scoliosis, extra ribs, and fused vertebra [Cameron et al., 1991; Ishikiriyama et al., 1996; Johnson et al., 1996; Marlin et al., 1998], palsy of the sixth and seventh cranial nerves [Cameron et al., 1991; Hersh et al., 1986; Konig et al., 1990], mental retardation [Cameron et al., 1991; Ishikiriyama et al., 1996; Johnson et al., 1996; Powell et al., 1995; Walpole and Hockey, 1982; Yano et al., 1998], and congenital heart defects [Barakat et al., 1988; Ferraz et al., 1989; Hersh et al., 1986; Johnson et al., 1996; Kohlhase et al., 1998; Konig et al., 1990; Monteiro de Pina Neto, 1984; Parent et al., 1994]. Previously, diagnosis of TBS has been complicated by the phenotypic overlap with oculoauriculovertebral spectrum and VATER syndrome. This extreme variability has raised questions over inclusion of certain features in the phenotype.
TBS is documented as an autosomal dominant condition in many families. Three reports described cytogenetic abnormalities of chromosome 16 in sporadic individuals [Friedman et al., 1987; Powell et al., 1995; Serville et al., 1993]. Recently, TBS was found to be caused by mutations within the SALL1 transcription factor gene at 16q12.1 in several affected individuals [Kohlhase et al., 1998, 1999]. The availability of this diagnostic test allows the ability to investigate the inclusion of certain malformations in the phenotypic spectrum of TBS. Here we report a three-generation family with seven affected individuals with a novel but typical SALL1 mutation who illustrate the clinical variability. Striking features in this family include lethal complex congenital heart disease, hypoplastic thumbs, and missing rib pair.
CLINICAL REPORT
Family History
The proband (IV-8) was an appropriate for gestational age male [3.335 g weight, 46.5 cm length, 34 cm occipito-frontal circumference (OFC)] delivered at 36 weeks gestation by premature spontaneous vaginal delivery to a 22-year-old G2P2 woman (III-5) affected with TBS. Findings on the infant (Figs. 1A and 2A) included anal stenosis with anterior placement, bilateral lowset ears with shortened, squared superior helices, bilateral preaxial polydactyly and triphalangeal thumbs, and coronal epispadias. Further evaluation demonstrated a right low-lying kidney and left urethral reflux. A patent ductus arteriosus (PDA) in the newborn period closed spontaneously. He also had right sensorineural hearing loss. Chromosome analysis was 46,XY normal male.
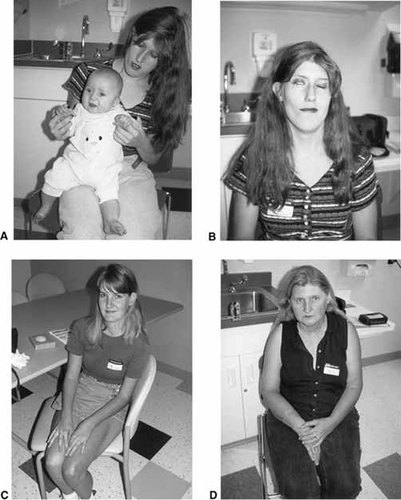
Affected family members. A: Proband (IV-8) at age five months and mother (III-5). B: Individual III-5. Note right 7th nerve paresis, facial asymmetry, and abnormal ear. C: Individual III-1. Note very mild presentation. D: Individual II-2. Note triphalangeal thumb.
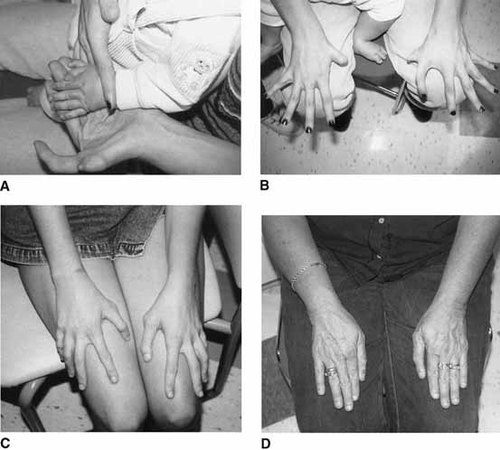
Hand malformations in family members. A: Proband (IV-8). Note preaxial polydactyly and triphalangeal thumb of left hand. B: Individual III-5. Note right hypoplastic thumb with absent distal phalanx and left preaxial polydactyly and triphalangeal thumb. C: Individual III-1. Note bilateral triphalangeal thumbs. D: Individual II-2. Note bilateral triphalangeal thumbs.
The mother (Figs. 1A, B and 2B) had anal stenosis with anterior placement of the anus, bilateral shortened superior ear helices, unilateral preauricular tag, bilateral sensorineural hearing loss, hypoplastic thumb with absent distal phalanx on the right, polydactyly and triphalangeal thumb on the left, bilateral hyperopia and Duane anomaly, microcephaly, facial asymmetry, right 7th nerve paresis, and a history of learning problems.
In addition to the proband's mother, two maternal aunts, a cousin, and the maternal grandmother were affected (see Fig. 4). One aunt (III-1) (Figs. 1C and 2C) was mildly affected with polydactyly of triphalangeal thumbs and sensorineural hearing loss. As a child, she had surgical dilation of her urethra and was in special education classes. Her first child (IV-1) (Fig. 3A–D) was a female infant born at 37 weeks gestation with multiple congenital anomalies including truncus arteriosus with overriding aortic arch and PDA; severe anterior placement of the anus; bilateral lowset, posteriorly rotated ears; bilateral polydactyly; right renal agenesis; microcephaly; hemifacial microsomia; and only 11 pairs of ribs. Chromosome analysis was 46,XX normal female. No other cytogenetic testing was done. Death occurred at 13 days of life secondary to cardiac failure.
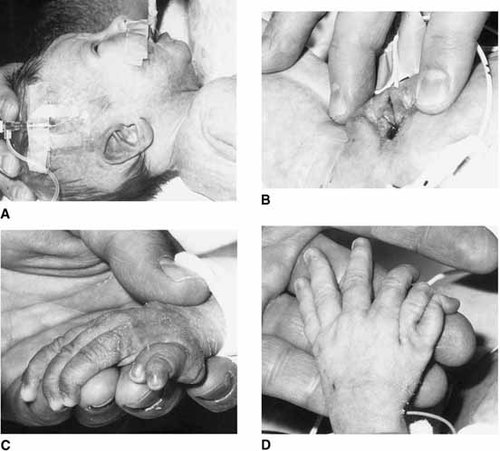
Individual IV-1 with lethal cardiac anomaly. A: Simple, lowset, posteriorly rotated ear. B: Severe anterior placement of the anus. C: Right preaxial polydactyly. D: Left preaxial polydactyly.
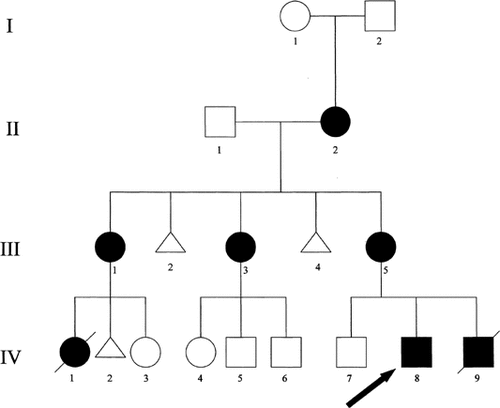
Pedigree of current family.
A second aunt (III-3) had unilateral polydactylous triphalangeal thumb, unilateral hypoplastic thumb, and bilateral sensorineural hearing loss. She had three unaffected children (IV-4,5,6). The maternal grandmother (II-2) (Figs. 1D and 2D) was also mildly affected with bilateral triphalangeal thumbs and sensorineural hearing loss.
The remaining family history failed to identify other affected individuals except the maternal grandmother's father was reported to have triphalangeal thumbs. No other family members were evaluated for subtle heart anomalies. Recently, individual III-5 presented with her third pregnancy (IV-9). Prenatal ultrasound and echocardiogram detected bilateral preaxial polydactyly, absent pulmonary valve anomaly, massively dilated main branch pulmonary arteries, cardiomyopathy, and pericardial effusion. Amniocentesis was declined. The baby was delivered at 34 weeks gestation secondary to premature labor. A cardiac evaluation revealed absent pulmonary valve anomaly with massively dilated right heart and pulmonary arteries. Other findings included anal stenosis and unilateral preaxial polydactyly. Pulmonary hypoplasia was suspected due to the massively enlarged cardiac structures and death occurred at 18 hours of life. A karyotype was not completed although a lymphoblast cuture was frozen for future analysis.
Molecular Studies
Genomic DNA from peripheral blood lymphocytes was prepared by routine procedures from eight family members, five affected (II-2, III-1, III-3, III-5, IV-8) and three unaffected (IV-4, 5, 6), and analyzed for SALL1 mutations by PCR and direct sequencing as described [Kohlhase et al., 1999]. A novel two base pair deletion 792–793delGC was found exclusively in all affected family members. This mutation was positioned within the hotspot region in SALL1 [Kohlhase et al., 1999] within exon 2 preceding the sequence encoding the first double zinc finger unit. Like the other SALL1 short deletions previously found in TBS patients [Kohlhase et al., 1998, 1999], this mutation results in a frameshift that is predicted to result in a truncated SALL1 protein lacking all double zinc finger domains presumed to be essential for gene function. However, such mutations might also result in premature degradation of the mutated transcript. Therefore, this mutation is predicted to result in haploinsufficiency for SALL1 (see Fig. 5).
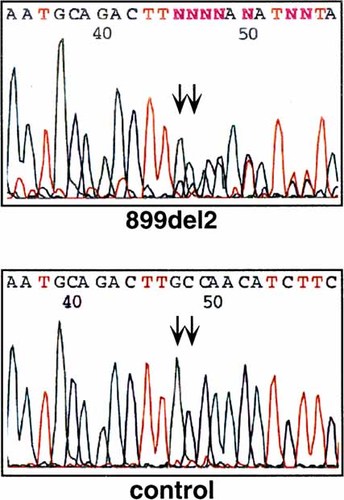
Electropherograms of the mutation detected in our family with the corresponding wild-type sequence. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Radiographic Studies
X-rays of the proband demonstrated preaxial polydactyly with a bifid first metacarpal.
DISCUSSION
We report a three-generation family with TBS and severe cardiac manifestations that highlights the wide variability of expression. An extensive review of the literature (Table I), together with a recent review by Powell and Michaelis [1999], compares the phenotypic variation between sporadic and familial cases. Due to the fact that a proband in an affected family may be more severely affected, a second comparison between the probands of the familial cases and sporadic cases is included in Table I. This table also includes a comparison of the findings in this family and in those cases in the literature with SALL1 mutations. In Table II, we compared phenotypic frequencies in all reported cases with those documented to have SALL1 mutations [Kohlhase et al., 1999]. The family reported here was included with the SALLI mutation positive cases. Phenotypic frequencies for the classic features are consistent across all cases. Sporadic cases showed a higher percentage of cardiac anomalies and nervous system manifestations. Sporadic cases and the probands of the familial cases showed similar phenotypic frequencies. In the family reported here, unique features, including congenital heart defects, reduction defects of the thumb as opposed to duplication anomalies, and a missing rib pair, are seen.
| Familial | (n = 66) | Proband | (n = 10) | Sporadic | (n = 17) | Current family | (n = 6) | SALL1 cases | (n = 16) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Anal malformation | 57/66 | 86% | 9/10 | 90% | 15/17 | 88% | 4/6 | 67% | 13/16 | 81.3% |
| Auricular malformation | 44/66 | 67% | 9/10 | 90% | 16/17 | 94% | 6/6 | 100% | 14/16 | 87.5% |
| Hearing loss | 30/66 | 45% | 7/10 | 70% | 9/17 | 53% | 5/6 | 83% | 14/16 | 87.5% |
| Hand malformation | 33/66 | 50% | 8/10 | 80% | 17/17 | 100% | 6/6 | 100% | 14/16 | 87.5% |
| Foot malformation | 18/66 | 27% | 5/10 | 50% | 4/17 | 24% | 2/6 | 33% | 14/16 | 87.5% |
| Renal abnormality | 9/66 | 14% | 3/10 | 30% | 7/17 | 41% | 2/6 | 33% | 10/16 | 62.5% |
| Hypospadius/epispadius | 2/33 | 6% | 1/8 | 13% | 1/7 | 14% | 1/1 | 100% | 3/10 | 30% |
| Genitourinary Abnormality, other (structural/reflux) | 10/66 | 15% | 1/10 | 10% | 0/17 | 0% | 1/6 | 17% | 9/16 | 56.3% |
| Total GU/abnormality | 21/66 | 32% | 5/10 | 50% | 8/17 | 47% | 2/6 | 33% | 13/16 | 81.3% |
| Atrial septal defect | 0 | 0% | 0 | 0% | 2/17 | 12% | 0 | 0% | 1/16 | 6.3% |
| Ventricular septal defect | 1/66 | 2% | 1/10 | 10% | 3/17 | 18% | 0 | 0% | 1/16 | 6.3% |
| Tetrology of Fallot | 0 | 0% | 0 | 0% | 2/17 | 12% | 0 | 0% | 0 | 0% |
| Truncus arteriosus | 0 | 0% | 0 | 0% | 1/17 | 6% | 1/6 | 17% | 0 | 0% |
| Cardiac murmur | 0 | 0% | 0 | 0% | 2/17 | 12% | 2/6 | 33% | 2/16 | 12.5% |
| Total cardiac abnormality | 1/66 | 2% | 1/10 | 10% | 10/17 | 59% | 3/6 | 50% | 4/16 | 25% |
| Dev. delay/MR | 3/66 | 5% | 2/10 | 20% | 4/17 | 24% | 2/6 | 33% | 1/16 | 6.3% |
| Cranial nerve palsy | 1/66 | 2% | 1/10 | 10% | 2/17 | 12% | 1/6 | 17% | 1/16 | 6.3% |
| Short stature/growth delay | 4/66 | 6% | 1/10 | 10% | 5/17 | 29% | 0 | 0% | — | — |
| Microcephaly | 1/66 | 2% | 0 | 0% | 3/17 | 18% | 2/6 | 33% | — | — |
| Skeletal | 1/66 | 2% | 0/10 | 0% | 3/17 | 18% | 1/6 | 17% | 1/16 | 6.3% |
| Other | 5/66 | 8% | 3/10 | 30% | 5/17 | 29% | 3/6 | 50% | 4/16 | 25% |
| All reported cases | SALL1 mutation positive cases (literature and current family) | |||
|---|---|---|---|---|
| Anal malformation | 72/83 | 87% | 17/22 | 77% |
| Auricular malformation | 60/83 | 72% | 20/22 | 91% |
| Hearing loss | 39/83 | 47% | 19/22 | 86% |
| Hand malformation | 50/83 | 60% | 20/22 | 91% |
| Foot malformation | 22/83 | 27% | 16/22 | 73% |
| Renal abnormality | 16/83 | 19% | 12/22 | 55% |
| Hypospadius | 3/40 | 8% | 4/10 | 40% |
| Genitourinary abnormality, other | 10/83 | 12% | 10/22 | 45% |
| Total GU abnormality | 29/83 | 35% | 15/22 | 68% |
| Atrial septal defect | 2/83 | 2% | 1/22 | 5% |
| Ventricular septal defect | 5/83 | 6% | 1/22 | 5% |
| Tetrology of Fallot | 2/83 | 2% | 0 | 0% |
| Truncus arteriosus | 1/83 | 1% | 1/22 | 5% |
| Cardiac murmur, unspecified | 2/83 | 2% | 4/22 | 18% |
| Total cardiac abnormality | 12/83 | 14% | 7/22 | 32% |
| Developmental Delay/mental retardation | 7/83 | 8% | 3/22 | 14% |
| Cranial nerve palsy | 3/83 | 4% | 2/22 | 9% |
| Short stature/growth delay | 9/83 | 11% | — | — |
| Microcephaly | 4/83 | 5% | — | — |
| Skeletal | 4/83 | 5% | 2/22 | 9% |
| Other | 10/83 | 12% | 7/22 | 32% |
Cardiac anomalies in this family included lethal truncus arteriosus, lethal pulmonary valve atresia, and the more common PDA. In the past, cardiac anomalies have been reported in 14% of cases (2% of familial cases, 10% probands, 59% of sporadic cases, 25% of patients with SALL1 mutations). Interestingly, all major heart defects have been conotruncal in nature with severe manifestation of truncus arteriosus and tetrology of Fallot in three sporadic patients [Barakat et al., 1988; Hersh et al., 1986; Parent et al., 1994]. Five sporadic cases have been reported with either an atrial septal defect or ventricular septal defect [Ferraz et al., 1989; Kohlhase et al., 1998; Konig et al., 1990; Kotzot et al., 1992; Monteiro de Pina Neto, 1984]. A congenital heart defect (ventricular septal defect) has been reported only in one family with an unusual presentation [Johnson et al., 1996]. This family reported by Johnson et al. [1996] illustrated the overlap of the variable features of TBS families and families with VACTERAL association and Goldenhar syndrome/Oculoauriculovertebral spectrum. It remains to be shown if the malformations in the family reported by Johnson et al. [1996] were also caused by SALL1 mutations and were therefore true manifestations of TBS. Alternatively, other genes might be mutated in this family that are possibly required within the same regulatory pathway. However, the finding of a typical SALL1 mutation in the family reported here clearly suggests that severe heart defects are indeed part of the TBS phenotypic variability, and suggests that SALL1 is required during early development of the heart. Since severe heart defects are among the rarer features of TBS, other unknown genetic or epigenetic factors may be present to explain why severe heart defects are relatively common in the family reported here.
Hypoplastic thumbs have been previously reported in three sporadic cases by Ishikiriyama et al. [1996], Monteiro de Pina Neto [1984], and Marlin et al. [1998]. This is the first report of a family with hypoplastic thumbs, including hypoplastic distal phalanx with a missing nail, and absent distal phalanx. Because of the documented SALL1 mutation, reduction, as well as duplication defects, appear to result from SALL1 haploinsufficiency. Hypoplastic and absent toes are a less frequent feature of TBS but may result from a similar mechanism of the SALL1 gene in the developing limbs.
Nervous system manifestations, including developmental delay or mental retardation and cranial nerve palsy, are seen in 12% of total cases (6% of familial cases, 30% of probands, 35% of sporadic, and 13% of patients with SALL1 mutations) (Table I). Palsy of cranial nerves VI and VII has been noted in three previously reported cases and in one individual in our family [Cameron et al., 1991; Hersh et al., 1986; Konig et al., 1990]. The mother of the proband in our family had unilateral weakness of cranial nerve VII together with Duane anomaly.
Further evaluation of the literature indicates that sporadic cases appear to be more severe than familial cases. However, given the considerable variability of TBS, it is certainly possible that there were mild manifestations in some of the family members of the “sporadic” cases that were simply missed. This is illustrated in our family by two of the individuals (II-2, III-1) that were mildly affected. III-1, who had a severely affected child, had only mild thumb changes and sensorineural hearing loss as an adult.
CONCLUSIONS
Heterozygous mutations in SALL1 result in marked variability of expression within families with TBS. Family members of all cases must be closely evaluated for minor manifestations because of the serious implications for reproductive risks and counseling. Mutation analysis will aid in identification of minimally affected family members. Our studies also indicate that cardiac manifestations in TBS must be anticipated with cardiac evaluation and echocardiograms performed on all suspected cases. In addition, hypoplastic thumbs should be considered part of the spectrum of TBS.
Acknowledgements
The authors thank the family for their time and effort toward this study. The authors also wish to thank M. Hausmann for expert technical assistance. This work was funded in part by a grant from the Wilhelm-Sander-Stiftung to J.K. Kohlhase.




