Experiences in Academic and Industry Partnerships – Forging a Path to Translational Drug Discovery
Abstract
Universities worldwide are establishing drug discovery centers to facilitate translation of exciting new human disease biology into therapeutic modalities. Drug hunting activities are typically focused on lead finding (high-throughput screening) coupled with some measure of chemical and pharmacokinetic optimization. Ideally, the research yields novel, selective drug-like molecules suitable for in vivo proof-of-concept studies and preclinical drug target validation. Preclinical activities are increasingly conducted in partnership with a pharmaceutical company seeking to access and supplement their drug development pipeline. Perpetually striving to gain a competitive edge and enhance efficiency, productivity, and profitability, the pharma industry is simultaneously experimenting with open- and crowd-source platforms and innovation incubators. Both enterprises, therefore, benefit from each other. This article is composed of a series of contributions (vignettes) from eight academic centers in the United States, the United Kingdom, Sweden, and Japan and one US-based pharmaceutical company (Table 2). The perspectives cover a range of topics including the rise of academic drug discovery and public–private partnerships; mission, objectives, and evolution of a particular center; resourcing; performance metrics; strategic and tactical lessons learned; attributes of successful projects; and open innovation initiatives. The accounts are punctuated with case studies to illustrate collective inventive capabilities.
Basic research leading to the discovery of new biology and molecular mechanisms underpinning human disease is arguably part and parcel of the academic landscape. The concept of “drug discovery” in academia, therefore, is not new. What is new is the growing emphasis and resources dedicated to translating basic research into therapeutic modalities. This is happening through a more holistic practice of drug hunting in the academic environment. In many instances, and by necessity, translational research is facilitated, in part or in whole, through active collaboration, partnership, or sponsorship with willing venture-backed biopharmaceutical companies, the pharmaceutical industry, foundations, and government-funded initiatives. The shift in attitude and growth in this arena within the span of just 10 years is remarkable. A significant number of conference proceedings, accounts, monographs, reviews, and books have appeared chronicling the precipitation and dramatic rise in what may be collectively referred to as collaborative private–public partnerships (1-87).
1 Paradigm Shift in Pharmaceutical Industry Posture
The discovery and development of new medicines to relieve pain and suffering in the world is a noble endeavor. The drug industry remains the foremost setting to develop and commercialize new therapeutic modalities. For decades, discovery, preclinical, and clinical R&D was performed inside relatively insular pharmaceutical companies. Industrial organizations having large teams of internal expertly trained scientists, relied essentially on their own ingenuity, research acumen, and financial wherewithal to drive the invention of new medicines. Invariably, the cost of R&D soared. Today, the aggregate to commercialize a new chemical entity (NCE) exceeds $2.5 billion, with a single successful drug ca. $350 million. Clinical testing, commercial launch, and marketing consume a large percentage of the expenditure. Business profitability is under pressure. The business response: industry consolidation through mergers and acquisition, layoffs of skilled workers, research dollars diverted to development and commercialization, reduction in discovery research, pressure to innovate, and more (1-6). The insular R&D model is thought not sustainable (4).
The economic reality is catalyzing action, albeit cautiously, on the part of the pharmaceutical industry worldwide (7-35). First, there is a heightened degree of scientific scouting. Recognizing that >99.9% of innovation occurs outside the walls of a drug company, scientific scouting as a mechanism to access new disease targets, preclinical and clinical agents, and technology to accelerate R&D has always been important to pharma. Previous scouting strategies at universities, in particular, were relatively passive – aligning with select high-profile research institutions and renowned academicians. In-house discovery research scientists were ostensibly tasked with identifying potential opportunities in the scientific and patent literature and conferences. Increasingly, scientific scouting strategies are organized, intentional, and proactive. Refined strategies include, for example, contact with a broad base of universities, creation of incentive programs offering “in-kind” research support (87), and panel participation and judging at university-sponsored entrepreneurial contests. Second, the industry is experimenting with novel innovation platforms: open sourcing (7-15), crowd sourcing (16-21), data sharing (22), and innovation incubators (23). Descriptions of processes (23-31), metrics (32, 33), and challenges (34-37) of open innovation platforms have been reviewed.
One example of a crowd-sourcing model is AstraZeneca's “OpenInnovation” (20). The platform is fashioned toward repurposing internal drug candidates for utility in new disease indications. The name, mechanism of action, and key references are provided for a large number of internal preclinical and clinical candidates across a range of therapeutic indications. The compounds are already optimized for drug-like attributes. Pharmacokinetic data and the preferred route of drug administration are generally indicated. Researchers are invited to submit a proposal to investigate a drug against a new disease mechanism. Researchers benefit from accessing drug-like molecules, funding, consultation, target mechanism of action proof-of-concept, and in-kind services. AstraZeneca benefits by potentially finding new life to drug candidates otherwise shelved. These activities ultimately benefit patients with unmet clinical need. Posting drug candidates on a public portal was unthinkable for a pharmaceutical company 10 years ago (88).
2 Paradigm Shift in Academic Posture
Coincident with the mutable pharmaceutical economics is the attention to discovery and translational research in academia (36-86). In the United States, the early catalysts were federal initiatives: formation of the National Institutes of Health (NIH) Chemical Genomics Center offering chemical libraries and high-throughput screening (HTS) to academics, the Molecular Libraries Screening Center Network coordinating high-throughput synthesis and screening, and the National Center for Advancing Translational Sciences (NCATS). The NIH-led incentives were designed (and successfully so) to steer the academic ship toward translational research. High-profile drug license options arising from university-based research revealed the potential high reward (revenue) of drug discovery (86). Similar government initiatives occurred in the United Kingdom (68, 69) and worldwide (4).
As a result, universities established internal drug discovery centers, subsequently organized and made visible through the creation of the Academic Drug Discovery Consortium (ADDC) in 2014 (36). There are now >150 registered centers on ADDC worldwide. Analogous organized networks include the UK Drug Discovery Centers (>50 members) and the Centro de Investigacion Medica Aplicada Unversidad de Nararra (cima) (>200 members across 20+ countries). Academic drug discovery centers may be categorized based, in part, on organizational structure and resourcing: (i) function specific, (ii) research coordination, (iii) “founder focused,” and (iv) “small-company like.” Each structure possesses a unique set of advantages and limitations (37).
Insider accounts of discovery operations at several academic drug discovery centers have been published (38-52) (Table 1), including case histories (53-56).
| Center | Capability | Highlights | Reference |
|---|---|---|---|
| Single center | |||
| Emory Chemical Biology Discovery Center | Screening, informatics, medicinal chemistry | Expertise in diverse HTS assay formats; Intra- and intermural collaborations. http://chemicalbiology.emory.edu | (38) |
| Drug Innovation Ventures at Emory (DRIVE) | Drug development | Pharma-experienced management team, virtual, not-for-profit drug development; subsidiary of Emory University. http://driveinnovations.org | (39) |
| University of New Mexico Center for Molecular Diversity | High-throughput flow cytometry, virtual screening, informatics | Identification of small-molecule probes. http://unmcmd.health.unm.edu | (40) |
| Manchester Institute Drug Discovery Unit | Biochemistry and cellular biology, medicinal chemistry, computation science | Established to translate novel research from the Manchester cancer research community; 30+ staff. https://www.cruk.manchester.ac.uk/Our-Research/Drug-Discovery | (41) |
| Institute for Therapeutics Discovery and Development, University of Minnesota | Screening, medicinal chemistry, preclinical pharmacology, GMP synthesis | Serves academic and business; collaboration and contract-based translational medicine support | (42) |
| Sanford/Burnham Medical Research Institute | Screening, chemical libraries, cheminformatics, medicinal chemistry, pharmacology | Eight functional cores support internal and external collaborations and contracts. https://www.sbpdiscovery.org | (43) |
| University of Kansas High-throughput Screening Laboratory | Screening | Traditional HTS, high-content screening (HTC) informatics, biostatistics; aligned with U Kansas medicinal chemistry labs; Serves internal and external collaborations. https://hts.ku.edu | (44) |
| Multicenter alliances | |||
| European Lead Factory | Screening, informatics | Precompetitive 30-partner collaboration between academic groups, small–medium enterprises, and pharmaceutical companies to support lead discovery in Europe. https://www.europeanleadfactory.eu | (45-47) |
| Science for Life Laboratory Drug Discovery and Development (SciLifeLab DDD) | Assay development, screening, medicinal chemistry, ADME, pharmacology, antibody development | 10 platforms comprise SciLifeLab; supports Swedish academic discovery and development; small-molecules and antibody therapeutics. https://www.scilifelab.se/platforms/ddd | (48, 49) |
| Alabama Drug Discovery Alliance | Molecular target identification, in silico and in vitro high-throughput screening, crystallography, medicinal chemistry, preclinical ADME and toxicology | Shared resources between University of Alabama at Birmingham and Southern Research Institute. Proposal/award scheme to access capability. HTS most utilized platform. https://www.uab.edu/medicine/adda | (50) |
| National Institute for Pharmaceutical Technology and Education, Inc. (NIPTE) | Full suite of preclinical and clinical R&D services | Multiuniversity partnership supporting discovery and development in academia; Headquarters in Minneapolis, MN. https://nipte.org | (51) |
| Scottish Universities Life Science Alliance (SULSA) | HTS assay development | Sources novel targets from the Scottish University community; develops high-throughput ready assays meeting submission criteria for the European Lead Factory. https://www.sulsa.ac.uk | (52) |
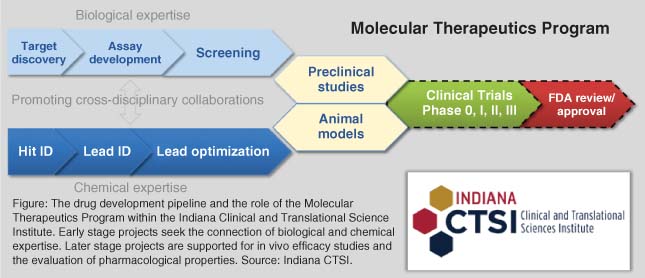
The Center for Discovery Center (CDD) at Washington University in St Louis (WUSTL) was formally established in 2016. Originally an at-cost-fee-for-service HTS core, the Center added small-molecule medicinal chemistry capability. The Center's remit is to facilitate the invention of drug-like molecules that can be used to validate new molecular targets for the treatment of human disease. There are four full-time staff: HTS core director and an associate scientist; CDD director (pharmaceutical experienced); and a scientific program manager. The CDD operates in large part as a “SWAT” team providing strategic and tactical scientific project management and preclinical expertise for principal investigators and scientists engaged in drug discovery research. Small-molecule probe design and synthesis, HTS triage, SAR-by-catalog, hit-to-lead, virtual screening, drug profiling, and pharmacokinetic studies are examples of the types of projects fielded by the CDD. As warranted, these projects are efficiently prosecuted through a network of reliable contract research organizations. The pace of academic research allows the prosecution of multiple projects in parallel. In the case of trans-multidisciplinary Wash U-industry collaborations, a dedicated scientific project leader is required. Overall, the model is cost-effective from a project management perspective. For example, PK studies are run periodically; therefore, there is little incentive to hire a full-time expert. The CDD offers a matching microgrant program. Through matched funds, principal investigators are eligible for subsidized access to screening libraries, crystallography, chemical synthesis, PK studies, etc., generating critical data to support larger extramural funding opportunities. Since its inception, the CDD and HTS core together have contributed to ca. 25 publications in Cell, Science, or Nature, >100 grant applications, and established partnerships with pharmaceutical companies, foundations, and the newly inaugurated Needleman Center for Autophagy Research and Therapeutics at Wash University. CDD research efforts, in part, have led to several high-profile licensing agreements.
There is a growing collection of literature on best practices for engineering productive academic collaborations with industry, new venture biotech, foundations, and related organizations (57-86). Optimal processes (57-69), performance statistics and success metrics (70-77), challenges (scientific, technical, intellectual property, tech transfer, funding, organizational, and cultural) (78-82), and strategies for mitigating risk (83-86) have been articulated.
This foundational article on academic–industry relationships is a first for Burger's Medicinal Chemistry and Drug Discovery. With broad readership of the professional scientist in mind, the intent of the article is to further educate and advise professionals active in academic drug discovery or those who are relatively new to this highly evolving interface. The article is composed of a series of contributions (vignettes) from eight academic centers and one pharma company (Table 2). Academic centers were selected from the United States, Europe, and Japan to provide a global perspective. Contributing authors were given creative freedom to craft a vignette in a way that fits best with their expertise and forward thinking. The authors discuss a range of topics: the rise of academic drug discovery and public–private partnerships; mission, objectives, and evolution of their center; resourcing; performance metrics; strategic and tactical lessons learned; attributes of successful projects; and open innovation initiatives. The accounts are punctuated with case studies to illustrate collective inventive capabilities.
| Center | Affiliation | Highlights |
|---|---|---|
| National Phenotypic Screening Centre http://www.lifesci.dundee.ac.uk/research/npsc | University of Dundee, UK | Founding and operations of three private–public partnerships with different aims, partners, and budgets |
| Drug Discovery Initiative (DDI) https://www.ddi.u-tokyo.ac.jp/en/ | The University of Tokyo, Japan | Formation, mission, resources, and operations of DDI. HTS and medicinal chemistry capable. |
| Center for Integrative Chemical Biology and Drug Discovery http://cicbdd.web.unc.edu | University of North Carolina in Chapel Hill, USA | Rationale for creation of the CICBDD, its successes and challenges, and the future role of academic institutions in drug discovery |
| Institute for Therapeutics Discovery and Development (ITDD) https://www.itdd.umn.edu | University of Minnesota, USA | Creation and organizational structure of the Center punctuated with project case histories |
| Chemical Biology Consortium Sweden (CBCS) http://www.cbcs.se | SciLifeLab, Sweden | Mission, organizational structure, capabilities, operational model; two case histories; lessons learned – attributes and pitfalls |
| Indiana CTSI Molecular Therapeutics Program https://indianactsi.org/researchers/services-tools/molecular-therapeutics/ | Indiana University, University of Notre Dame, Purdue University, USA | Rise of academic drug discovery centers; CTSI core facilities, operation model; intellectual property considerations, abbreviated CTSI case histories |
| Yale Center for Molecular Discovery https://ycmd.yale.edu | Yale University, CT, USA | General commentary and direct experience at Yale to find high-quality leads from academic compound libraries: solubility and aggregation behavior |
| Eli Lilly https://www.lilly.com | Indianapolis, Indiana, USA | Historical perspective on industry support of academic research. Creation and implementation of Lilly Research Award Program (LRAP). References case histories |
References
Abstract
Public–private partnerships (PPPs) are becoming prevalent enough that most R&D professionals in a pharmaceutical company will eventually be exposed to them in some shape or form. For academic biologists wanting to translate their research into tangible therapeutic applications, participating in drug discovery PPPs is an option worth considering to achieve research impact. This article attempts to explain how biology-focused PPPs between academia and industry, which are at least part funded by the pharma industry, can work and deliver useful and validated innovation for drug discovery. After exposing what drives industry and academia to engage in PPPs, we present three different exemplars of PPPs that we founded with very different aims, partners, and budgets, namely the MSD-Scottish Life Sciences Fund (MSD-SLSF), the Phenomics Discovery Initiative (PDi), and the European Lead Factory (ELF). Using five key dimensions that can be used to define collaborations, governance, administration, mutuality, norms, and organizational autonomy, we establish some of the lessons we have learnt from the creation and management of PPPs. By analyzing our PPPs in a formal way, and offering our experience from an academic perspective, we hope to educate and advise pharmaceutical professionals who wish to harness the creative force from academia and the versatility of a multistakeholder partnership to enhance early-stage drug discovery pipelines with novel biology.
1 Introduction
Public–private partnerships (PPPs) in drug discovery are becoming more common. Many of them focus on accessing novel biology from academia, because this is currently a significant bottleneck for pharmaceutical companies who have historically focused the largest part of their resources on chemistry, biochemical screening technologies, and clinical trials. The unacceptably high rates of failure in late-stage clinical trials due to lack of drug efficacy (clinical Phases II and III) is evidence for a lack of biological wisdom in the sector. Conversely, academia has amassed significant knowledge and expertise into complex biological models and pathways that are pertinent to innovation but not necessarily accessible or useful to the pharmaceutical industry. Consequently, many pharmaceutical companies are currently accessing biological innovation, expertise, knowledge, and education from academia by setting up bilateral collaborations as an integral part of their early-stage drug discovery strategy. However, valuable knowledge from academia can be difficult to translate into meaningful assets, due to a difference in experimental ethos and a misalignment of purpose, thus resulting in a translational gap.
By engaging in larger consortia, rather than in one-to-one collaborations, pharma can benefit from complementary inputs from partners that have different abilities and perspectives. These grander schemes that often include multisector stakeholders have the advantage of creating a common ethos and purpose, which can help bridge the translational gap. These coalitions often provide a broader platform from which to internalize novel assets, offer avenues to break down silo mentality, and reduce the chances of the collaboration being driven purely by academic motivators.
This article aims to present insights into biology-focused PPPs between academia and industry that are at least part funded by the pharma industry. After exposing what drives industry and academia to engage in PPPs, we present three different exemplars of PPPs that we have been engaged in, which had very different aims, partners, and budgets, namely the MSD-Scottish Life Sciences Fund (MSD-SLSF), the Phenomics Discovery Initiative (PDi), and the European Lead Factory (ELF).
The exemplars are based in Europe, although the pharmaceutical companies involved are multinationals, and all partners have, in any case, an international outlook. SMEs mentioned in this article (who are also industrial partners) are part of the PPP ecosystem but are only considered as part of consortia involving large pharmaceutical companies, not in any bilateral collaborations with academia.
By offering our experience from an academic perspective, we hope to educate and advise pharmaceutical professionals who are considering building or participating in a biology-focused PPP. Our insights include an understanding of what motivates the different stakeholders, and some lessons learnt, often the hard way, on what can enhance or degrade a collaboration. Hopefully, this practical approach will help professionals get a head start when engaging with the exciting and productive collaborative adventures that are PPPs.
2 Why Should Industry Seek New Biology from Academia?
“If I have seen further, it is by standing on the shoulders of giants” – Isaac Newton
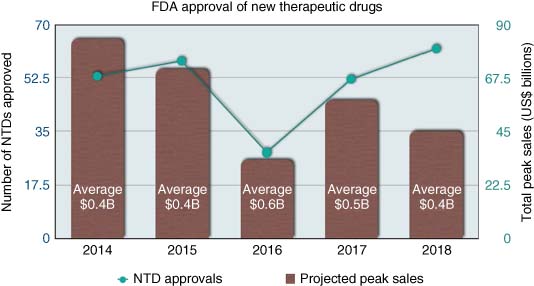
Between its instigation in 1827 and 2018, the FDA has approved around 1666 New Molecular Entities (NMEs) as therapeutics (89). The annual rate of NME approvals has varied greatly over the years. After what was thought of as the heydays of drug approval in the late 1990s (on average >36 drugs approved/year) (90), the 2000s saw a sharp reduction in approvals (on average ∼22 drugs approved/year), despite ever-increasing levels of investment from the industry. In fact, from the 1990s to the 2010s, the cost of bringing a drug to market increased from $320 million to over $2 billion (91). Also, the total global R&D spend by the industry (pharma and biotech) increased from $12 billion in the 1990s to $108 billion in 2006 and continuing to rise to $141 billion in 2015 (92). The major upheavals in the pharma industry including mergers, corporate restructuring, and pipeline streamlining were rife in the 2000s, and the disruption caused to the industry could be considered a major factor in the drop of drug approval rates around that time.
Although still hugely variable on a year-by-year basis, since 2010, the drug approval figures have slowly improved and attained, in 2018, the all-time record of 59 drugs. This encouraging observation is also supported by a trend toward improved clinical success rates, which had been in steady decline between 1997 and 2010 (93). Although clinical success rates have remained at low levels in Phase I (change of 6–7% success rate from Phase I to launch from 2010 to 2017), they are coupled with slightly increased success rates in Phase II (increase from 11% to 15% success rate from Phase II to launch from 2010 to 2017) and Phase III (increase from 49% to 62% success rate from Phase III to launch from 2010 to 2017), suggesting that pharma companies, through their business improvement strategies, may have successfully focused on improving the quality of their portfolio and are managing to fail drugs early before the cost of clinical trials becomes too exorbitant. Higher success in the clinic, coupled with more refined portfolios, has predictably increased drug approval rates.
However, realistically, success rates are still very poor, considering that fewer than 1 in 10 000 early translational programs successfully conclude in the launch of an FDA-approved drug, a process that still costs in excess of $2 billion and takes over 11 years to get to market (94). Even when a candidate enters the clinical pipeline (only 0.14% of translational programs achieve this), the probability that it will make it to an approved drug is at around 7% (just below 1 in 10, or put another way, a 93% failure rate). From a business perspective, these are unacceptably high failure rates, considering the extreme levels of investment required to be a player in this field. In fact, failures in the later stages of clinical trials have the greatest impact on the expected returns of any investment in pharma. Also, 7 out of 10 drug products that go to market do not recover the average cost of development. This means that the risk versus returns profile of the pharma industry makes it look like a very poor business model, and with this in mind, a pharma company's value can only come from a carefully considered portfolio that spreads the investment risk (95).
Until recently, the industry's business model has been predicated on launching blockbuster drugs such as Atorvastatin. Sold as Lipitor, Atorvastatin reduces de novo synthesis of cholesterol, is a competitive inhibitor of HMG-CoA reductase, and was launched by Pfizer in 1996. Atorvastatin was the world's best-selling drug, realizing $125 billion in sales over 15 years, and came off patent in the United States in 2011. Similarly, Adalimumab (an antibody which targets TNF alpha and is sold as Humira) was launched in 2003 by Abbvie to treat rheumatoid arthritis and was the best-selling drug in 2018 with sales of $19.9 billion. US exclusivity extends until 2023.
It is these phenomenally profitable blockbuster drugs that have been supporting the high-failure rates and poor profitability in the rest of pharma's R&D portfolios. Unfortunately, modern-day chronic diseases such as dementia and diabetes are more complex to address therapeutically than was reducing cholesterol synthesis with Lipitor. For complex, multifactor diseases, patient variability has a far greater impact on drug discovery outcomes and implies that achieving a blockbuster such as Lipitor is becoming less likely.
Historically, drug companies were very much built around the knowledge, skills, and logistics of creating large diverse chemical compound collections, and the medicinal chemistry expertise required to morph them into bioactive drugs.
Including biology in early-stage drug discovery is clearly necessary, but due to the adoption of reductionist high-throughput approaches in screening, it has mostly been reduced to biochemical rather than biological assays (reductionist and target-based approaches) (96, 97). This means that the more complex biological pathways that are linked to drug efficacy have been pushed out to later stages of the discovery process.
As a consequence of the spate of pharmaceutical sector mergers and acquisitions in the 2000s, several thousand industry R&D jobs were axed between 2005 and 2012 in some of the major pharmaceutical companies such as Pfizer, Roche, GSK, MSD, Johnson & Johnson, AstraZeneca, and Sanofi (98). This has resulted in reduced in-house R&D activities and an increased focus of company resources on in-licensing and supporting clinical trials. Pharmaceutical companies have in effect been revising their strategies away from trying to achieve end-to-end drug discovery and development, to focus on the parts of the process they have historically been good at, and as a result collaborating on other parts of the process they are not so good at, such as innovation. In-licensing, open innovation activities, and outsourcing the more standard R&D processes to CROs are now much more common.
Pharmaceutical companies have focused their expertise on the ability to manage and fund new therapeutic chemical entities through expensive and lengthy clinical trials to achieve regulatory approval. However, at the same time, regulatory hurdles have become more onerous, meaning that proof of clinical efficacy and added clinical value are harder to demonstrate, making approval harder.
By confounding all the changes that have occurred in the pharma industry (summarized in Table 3), it is becoming apparent that innovation, especially from biological sciences, is required to redress the pharma innovation and productivity gap and to deal with the challenges of medicine in an aging population. Over the past 15 years or so, industry has noticed this gap and is working on opportunities to plug it to be more competitive.
| The perfect storm |
|
|
- Summary of factors exposing the need for more biological innovation in pharma.
One of the opportunities is to slowly revise methods of interaction with academia by promoting more productive exchanges, collaborations, and partnerships, including PPPs. Ultimately, industry views these partnerships from the standpoint of how they are able to influence the bottom line of the company.
3 Why Would Academia Want To Collaborate With Industry?
“Progress is impossible without change, and those who cannot change their minds cannot change anything” – George Bernard Shaw
Before trying to engage with academia, it is important to understand what would motivate them to engage.
Academia is good at educating, innovating, and generating new knowledge, regardless of its utility or tractability for translation into industry applications and products. This is because success in academia is measured differently to that in industry. Quality of university education is monitored by an independent panel of experts, including academics, students, and employer representatives. Assessments are based on a set of metrics using continuation rates, student satisfaction, and employment outcomes for undergraduate students. On the other hand, university research and innovation quality are measured according to gained grant income and quantity and quality of peer-reviewed journal publications. None of these metrics are particularly conducive to creating an environment that generates solutions to industry's immediate drug discovery needs with regard to biological innovation. This is made worse, because at the outset there has been no real incentive for academics to generate results that are either specifically relevant to drug discovery's R&D safety and efficacy challenges or reproducible enough to be seamlessly translated into an industrial environment. The pharmaceutical industry often suffers from an inability to reproduce published research methods and findings. It is rather alarming that according to studies by pharmaceutical companies Bayer and Amgen, when industry labs try to reproduce academic results they are unsuccessful approximately 80% of the time (99).
Viewed by the average faculty member, a pharmaceutical corporation does not necessarily have the hallmarks of a trusted partner. There is concern from many academics that collaborating companies may try and seize any intellectual property (IP) and profits that are issued from any collaborative efforts. There is also a worry that industry collaborators will encourage a delay in publications, which are critical to academic careers. It has been argued that collaborating with industry actually reduces publication rates because industry is so keen to disclose findings in patents or the keep critical know-how secret (100). Corporate pressures could also divert scientific efforts from fundamental studies, toward applied research, which has less publication value.
However, recently, a cultural shift has occurred in Europe, resulting in universities being more engaged in impact-driven outputs, because these are now factored in as an additional measure of research quality. Impact from interactions with industry can be defined as a collaboration, knowledge-exchange activity, or technology transfer with industry that contributes toward a product or business process that measurably benefits the public or the economy. For biology-focused university departments, this has resulted in a will to translate knowledge from the bench to the bedside, as an obvious demonstrator of human and financial impact. Achieving impact, which can be described in case studies as well as publications, now comes with financial rewards for universities when they apply for core funding from the state.
Beyond the impact agenda, industry collaborations may actually benefit academic publication records. A recent study at the London School of Business has postulated that academics with industry collaborators actually published more follow-on papers than those without the relationships (101), and that publications with a corporate coauthor were more widely publicized when measured by altmetric attention score (the altmetric attention score tracks the number of discussions around a published paper, which almost doubled for life sciences papers with a corporate coauthor), increased the academics' scholarly impact when measured by h-factor, and was associated with a 26% increase in citation rates (100).
There are also other incentives for academics to collaborate with industry, such as generating new and untapped sources of income, accessing uncommercialized technologies, expanding researcher networks, generating opportunities for staff and knowledge exchanges, and using collaboration outputs as a base for commercially valuable IP generation (patent filing and licensing) and commercialization (spinout generation).
Through these relationships, faculty can keep up to date with trends in industry, students are made ready for the workplace, and university resources are often updated. The staff that is engaged can also expand horizons and develop new career skills that are relevant to commercialization and industry perspectives.
So, in summary, there are newly found reasons for university biologists to collaborate more with industry. However, it is important to note that the mechanisms, knowledge, and guidelines to do it well are not yet fully established in academic institutions. It is still very much an activity that is carried out by motivated individuals who work out how to do it themselves rather than a strategy that departments adopt seriously and comprehensively to boost their impact statements and funding goals. Tech transfer offices do help, but they generally respond to a strategy developed by the scientist and only deal with the administrative aspects of setting up the collaboration rather than providing real guidance on strategy. It is worth being aware of this situation when negotiating a collaboration with academia.
4 What Can Academia Contribute To Industry?
“The whole is other than the sum of the parts” – Kurt Koffka
The majority of innovation occurs outside of the pharmaceutical industry. The total budgets spent by academic institutions on research and innovation are higher than that spent by pharmaceutical institutions by a long shot, making them one of the main engines of innovation (Table 4). Academic freedom also promotes innovation and creativity, due to the lack of boundaries put on research goals.
| Is academia the innovation savior? |
|
One of academia's missions is to acquire and disseminate knowledge as an end in itself. Faculties tend to emphasize theory over practice, which is in direct contradiction with industry. This kind of curiosity-driven biological research has the potential to deliver novel drug targets and important enabling chemical tools.
University life scientists are defined by the specific and detailed knowledge they hold in a very narrow field of biology. They will be world-class experts on the pathway, molecule, or biological model they have spent years developing. This potentially makes each scientist unique, with a potential huge value to a pharma wanting to work in their field. The cellular assays, animal models, diagnostic tools, and know-how around the pathways that define them can be of immense value in an early-stage drug discovery program. Finding the right Key Opinion Leader (KOL) can be the defining feature of a successful early-stage drug discovery program.
University clinicians also provide industry with access to clinical samples and patients, which are useful in in vitro experiments, but also later on in clinical trials. Sourcing relevant patient material in an efficient and ethical way can be difficult, expensive, and time consuming. Pairing up with the right clinician can ease all of these bottlenecks.
Ultimately, the greatest benefit for both industry and academia is the ability to engage in academic research that benefits both the society and the economy.
5 Has Academia Historically Contributed to Industry Success in Drug Discovery?
“Many ideas grow better when transplanted into another mind than the one where they sprang up.” – Oliver Wendell Holmes
This recent contraction of in-house pharmaceutical R&D activities, combined with an amplified need for innovation, has meant that collaboration between academia and industry in the field of early-stage drug discovery should be a win–win situation. On paper, both parties are almost perfectly compatible, providing complementary skills and expertise.
Looking at the motivators and current status in academia and industry, we believe it is becoming increasingly clear that there is a real need from the pharma industry to collaborate more systematically with academia, to access biological knowledge and innovation that is useful in the early stages of the drug discovery process. However, organizations that are innovating, be they academic or industrial, only choose to do so if they feel they have to. Realistically, for IP ownership reasons, they prefer to keep as much in-house as they can.
So is there any hard evidence that academic involvement in early-stage drug discovery confers any advantages to industry with regard to increased rates of drug approval?
Pharma has in fact always benefitted from knowledge and innovation coming from academia. Bilateral – vertical one-to-one – collaborations have been quite common, and the rate of collaboration creation has been on the rise in the past 10–15 years. This is evidenced by an increased percentage of research projects supported by industry and an increased financial investment of university research by pharmaceutical companies. According to Nature Index 2017, the number of academic–industry collaborations more than doubled from 12 672 in 2012 to 25 962 in 2016, and half of those 2016 collaborations were in the life sciences (100). In 2018, industry funded about 7% of university research, which is about double that of 20 years ago (1998).
It has been proposed that increased pharmaceutical productivity can be achieved by transforming the way R&D is conducted from a fully integrated approach to one that was more highly networked, partnered, and leveraged (94). This networked approach has been predicted to improve productivity by improving the quality of the discovery pipeline in a cost-effective manner. Academia is part of this networking equation and can therefore play a role in improving productivity in the pharmaceutical industry.
Since the 2000s pharma has actually changed the nature of its overall engagement with academia, in the form of longer term and more profound collaborations such as PPPs. This shift, toward deeper and more complex relationships, has correlated with an increase in the number of FDA-approved drugs, suggesting that the collaborations have had a positive impact on drug discovery (102).
A general observation is that at every stage of the drug discovery process, projects that have benefitted from partnering (or any form: academia, biotech, etc.) deliver improved clinical success rates (93, 103), further supporting the notion that collaboration is an advantage in drug discovery.
Between 1998 and 2007, 58% of the 252 FDA drug approvals were first reported by a pharmaceutical company alone, 24% came from a university, and 18% came from biotechnology companies. In 2015, 55% of all 1453 approved drugs were first reported by academia, and finally in 2018, of the 210 NMEs approved between 2010 and 2016, all of them had an initial academic input (102). These figures clearly indicate that academic innovation is increasing its impact on drug approvals.
It is also important to note that many disruptive technologies that over the years have improved research productivity, such as PCR amplification, CRISPR/Cas9 gene editing, Western blotting, phage display libraries, and CAR-T immunotherapy, to name but a few, all originated in universities.
6 Different Types of Collaborations between Industry and Academia for Early-Stage Drug Discovery
“A rock pile ceases to be a rock pile the moment a single man contemplates it, bearing within him the image of a cathedral.” – Antoine de Saint-Exupery
There are many ways in which academia typically interacts with industry. The list in Table 5 attempts to detail different forms of engagement along with associated activities, funding, and outputs. Currently, the most common focus of industry–academia collaborations is to achieve impacts on student education (funding PhDs, for example) or develop applied research and technology transfer activities. Other more complex forms of collaboration, such as PPPs and joint research labs, are slowly gaining in popularity but are still in the minority.
| Type of collaboration | What activities are involved? | % funding from pharmaceutical | Source of remaining funding | Most likely outputsa |
|---|---|---|---|---|
| Research Fellowships | Direct funding of PhD/postdoc who work on a project of interest to the company | From 10% to 100% | University, state, or charities | Educational, research papers, and knowledge exchange |
| Collaborative Research | Long-term collaboration covering more than one research project | 100% | n/a | Sharing of confidential information, materials, and IP to meet some predefined industry need |
| Applied Collaborative Research grant | State-funded initiative to fund academia–industry partnerships | ∼50%, often in-kind but cash preferred | State | Tech transfer, novel applications, products, licensable patent, and spinout |
| Industry-led Public–Private Partnerships | Consortia 100% funded by industry | 100% | n/a | Precompetitive data and chemical and biological assets that can be used in early-stage R&D |
| Multi-sector-led Public–Private Partnerships | Consortia cofunded by pharmaceutical companies and other stakeholders | 50–100% | State, charities | Precompetitive data and chemical and biological assets that can be used in early-stage R&D |
| Consultancy | Specific consultancy task/advice/report carried out by academic expert | 100% | n/a | Designs, reports, and recommendations |
| Contract Research | Specific piece of research of immediate interest to pharmaceutical company | 100% | n/a | Results/applications that are directly relevant to pharma R&D |
| Industry Donation | Generally, activities around education or toward “good” cause that enhance corporate responsibility image | Up to 100% funded by industry | State, universities | Educational outputs and marketing: actions that demonstrate enhanced corporate responsibility |
| Exchange of best practice or novel technique | n/a | n/a | Knowledge exchange | |
| Education | ||||
| Staff exchange (often part of a larger program) | Teaching Research |
|||
| Research park | Shared office/laboratory space aimed at catalyzing partnerships between industry and academia | Variable | Variable | Academic research commercialization. Spinouts |
| Joint laboratories | Research facilities shared by academia and industry | Variable | Variable | Joint commercial ventures |
- a This does not mean that other outputs are not possible and have not been achieved in individual projects, it just denotes what outputs are most likely.
What is evident is that each form of collaboration presents a different model, with different modes of funding, different governance structures, different levels of engagement, different goals, and different outputs.
- Governance develops rules and roles to allow healthy relationships and decision-making. It is important that each partner can have a voice that is heard. Clarity, coordination, and agreement on goals are critical from a governance perspective.
- Administration builds the collaboration communication channels and executes procedures to implement decisions.
- Mutuality describes the synergistic values of the collaboration, which need to be clear for it to succeed, and realistically may be beyond the goals of individual organizations. Mutuality encompasses unique benefits members bring to the alliance or benefits members get from joining the collaboration. As the collaboration matures and morphs, mutuality may create tensions in the decision-making if alliance goals are too far from those of the individual organization. For mutuality to strive throughout the project, organizations need to provide participating staff that are fully committed to the project but have the authority to participate in decisions that affect their organization.
- Norms establish concepts of trust, where each member believes that other partners will meet their obligations.
- Organizational autonomy is the acceptance that while there may be collective interests embodied in the collaboration's goals, member organizations (and individuals) have very real self-interests.
Even collaborations that are set up meticulously well, with all five dimensions defined and agreed in advance, are likely to develop sources of tension, regardless of the nature of the collaboration. Partners may clash over IP or decisions about how much a company will pay to license a patent from a university lab. Liabilities are also a source of tension: who will be legally responsible for what, and under what circumstances will partners be sued. Each side is interested in protecting its interests from damages resulting from lawsuits. Such tensions can significantly delay a project's progress and need to be dealt with seriously as and when they emerge in the partnership.
7 Biology-Focused PPPs That Aid Education and Innovation in Early-Stage Drug Discovery
“The beauty of the universe consists not only of unity in variety, but also of variety in unity.” Umberto Eco
Drug discovery PPPs integrate subject matter experts, drug development experts, and other relevant stakeholders under a common goal to deliver novel findings that are relevant to developing new drugs. To meet their objectives, they should also be supplied with enough resources to translate these novel findings far enough down the pipeline to be seen as potential starting points for fully-fledged drug discovery programs. Underfunding these partnerships or not providing them with the correct kind of support will only result in outputs that are too early stage to be of interest to industry and therefore not ultimately translated.
The collective power and motivation of PPPs reside in the shared vision of improving human health by realizing the potential of the collaborative research. However, success requires the building of trust, open-minded debate, and incentives appropriate to each situation. Most issues in PPPs are human and come from poor communication, preparation, and expectations. It is important to have an insight into your partners' vulnerabilities to anticipate what challenges they may have. Something that is easy for one partner may be challenging for another.
The next sections of this article will aim to describe three different drug discovery PPPs that we were involved in over the past seven years, including defining their vision, establishing their governance, and administrating their delivery. Data in Table 6 summarize the partners, high-level aims, budgets, and timelines for each consortium. For each project we will also aim to describe the PPP requirements, how and why they were set up, which partners were required, how to justify the budgets and resources, and what lessons were learnt through each partnership.
| Consortium name | MSD-Scottish Life Sciences Fund | Phenomics Discovery Initiative | European Lead Factory |
|---|---|---|---|
| Acronym | MSD-SLSF | PDi | ELF |
| Timeline | 2012 to ∼2016/17 | 2016 to present | 2013 to present |
| Pharma Partners | MSD | Janssen | Bayer, UCB, Sanofi, Janssen, Merck KGa, Grünenthal, Servier, AstraZenecaa |
| Charity partners | n/a | n/a | Medicines for Malaria (MVM) |
| SME partners | 21 SMEs providing year-long industrial placements to 39 undergraduate students | n/a | BioAscent, Taros, Mercachem, Syncom, Edelris, Sygnature Discovery, Pivot Park Screening Centre, Arttic, ChemAxon, Lead Discovery Centerb |
| Academic Partners | Universities of Aberdeen, Dundee, Edinburgh, Glasgow, Strathclyde, and St Andrews. Scottish Universities Life Sciences Alliance (SULSA) | Universities of Dundee, Oxford, and Edinburgh | University of Oxford |
| University of Dundee | |||
| Lygaturec | |||
| Aim | Deliver an educational program supporting the next generation of drug discovery scientists in Scotland | Deliver a portfolio of validated phenotypic assays and hits from phenotypic screens | EU-wide platform for scientists from universities and SMEs to translate novel disease-relevant biochemical (target-based) and phenotypic assays and deliver high-quality, relevant chemical assets, with a view to discovering investable starting points for new therapies |
| Source of projects | PhDs and postdocs crowdsourced from academics in six Scottish universities | Crowdsourced globally from academia and SMEs | Crowdsourced from EU academics and SMEs |
| Cash committed by industry | £3.10M | £2.28M | n/a |
| In-kind contribution committed by industry (estimate) | n/a | £8.00M | €108.75 |
| Cash contributed from other sources | £0.65M | n/a | €98.00 |
| In-kind contribution committed by other sources | n/a | £8.00M | €0.75 |
| Leveraged funding | £2.10M | n/a | n/a |
| Total budget committed to project | £4.85M | £18.28M | €207.50 |
- a List of previous additional industry partners for ELF: Lundbeck.
- b List of previous additional SMEs: Arttic, ChemAxon, Lead Discovery Center.
- c List of previous additional academic partners: Universiteit Leiden, Leiden University Medical Centre, Max Plank Institute of Molecular Physiology, Raboud Universiteit Njimegen, University of Groningen, University Amsterdam, Universität Duisburg Essen, University of Leeds, Technical University of Denmark, University of Nottingham.
7.1 The MSD-Scottish Life Sciences Fund (MSD-SLSF)
7.1.1 Background
Launched in 2012 following the shutdown of MSD's R&D site at Newhouse in Central Scotland, the MSD-SLSF was set up as a four-year collaboration between six Scottish universities (Aberdeen, Dundee, Edinburgh, Glasgow, St Andrews, and Strathclyde), the Scottish Universities Life Sciences Alliance (SULSA – a research pooling initiative), and the pharma company MSD.
7.1.1.1 Gap Addressed by the Consortium
This PPP addressed a gap in the university education system. MSD felt that there was no formal higher education pathway in Scotland for students wishing to focus their studies on achieving a career in the drug discovery industry. While academic projects (PhDs and postdocs) did exist in this field, these were isolated and not accompanied by any specific skills-based professional training or networking opportunities that were relevant to the pharmaceutical industrial sector. MSD-SLSF aimed to address this gap by working with universities to create a comprehensive national training program at all levels of the university learning ladder to produce undergraduates, PhDs, and postdocs with the relevant skills and research capacities to enter careers in the biotech and pharmaceutical industry.
7.1.2 Scientific Focus
The focus of the collaboration was on a defined number of high-profile research activities that were relevant to MSD's strong research interests in drug discovery. For MSD, the advantage was the opportunity to engage directly with the highest quality science in Scotland through a fund that was transparently distributed by SULSA according to scientific merit across its six member universities. The hope was for MSD to have a long-lasting effect on the Scottish Life Sciences academic community and to stimulate the Scottish knowledge-based economy by potentially allowing ex-Newhouse employees the prospects of finding a new career or further training opportunities through the MSD-SLSF.
- Genetic validation of specific drug targets/pathways
- Analysis of molecular targets in vitro, such as the structure/function of GPCRs and the action of neurosteroids
- In vivo models in key therapeutic areas particularly relevant to Scotland, such as cardiovascular disease and neuroscience
- Organic synthesis methodologies for the generation of novel drug-like molecules
- Methods for biological and biophysical analysis of bioactive molecule
- Development of new chemo/bio/informatics tools for target selection and medicinal chemistry.
7.1.3 Budget
The total budget for the fund was £3.75M, with £3.1M coming from MSD, £0.3M coming from the Scottish Funding Council (SFC), and £0.35M coming from the six Scottish universities. The SFC also provided a useful interface with the Scottish Government who were able to support and advise on the skills agenda as a matter of national interest.
7.1.4 Governance
The MSD fund was administered by SULSA through a previously established reporting structure, whereby the SULSA director reports directly to SULSA University principals and the SFC.
To increase the scientific excellence of the program, SULSA crowdsourced individual projects across Scotland (for the PhD studentships and postdoctoral projects) using a panel of experts to PEER review the proposals. To maintain both academic and industry perspectives, the panel included experts from the six universities and experts from MSD.
Projects were selected according to set criteria, including scientific merit, track record of the investigators, achievement of leveraged funding, and potential for excellence and innovation. All projects were reviewed on an annual basis for progress, budget, and impact.
Each of the funded streams benefited from MSD branding, and outputs were published in peer-reviewed journals, press releases, conference presentations, networking events, and on university websites.
7.1.5 Intellectual Property
All IP generated during the project remained the property of the university in which the research was carried out. This meant that MSD were only focusing on long-term educational benefits and only seeking to collaborate with groups once the research within the program was complete.
7.1.6 Outputs
- 17 PhDs
- 17 Postdoctoral research projects
- 36 Publications (from 2013 to 2017)
- 39 Undergraduate year-long industry placements
- 14 Undergraduate iGEM teams
- 3 International Research Symposia
- An integrated training program for the MSD-SLSF PhD students and postdoctoral researchers to give them professional skills that are useful for a transition into industry, such as entrepreneurship, communication, marketing, and administration.
- Leveraged funds of around £2.1M, which were secured as part of the setup of the postdoctoral positions.
- Including leveraged funding from external resources during the period of the program; the total budget committed to the MSD-SLSF was £4.85M over four years.
Different advantages were incurred by these outputs on each partner, as shown in Table 7, demonstrating the different but compatible motivators in the partnership. For academia, MSD-SLSF provided an additional source of research and education funding that focused on much more concrete industry-focused skills development than is usual in a university. For MSD, the scheme offered a platform to showcase corporate responsibility in the skills agenda, while also accessing research findings that were relevant to active research interests in the company and developing a cohort of students with the correct profile to make a career in the pharma industry.
| MSD-SLSF Consortium activities and assets | Outputs for academia | Outputs for industry |
|---|---|---|
| Postdoctoral positions | Funding for ambitious transitional projects that are vetted by a large pharma. External source of funding for research | An insight into relevant new research findings in relevant fields |
| Potential source of highly qualified young researchers | ||
| Matched funding/leveraged funding for postdoc research projects | Ability to carry out larger research projects. Funding is sometimes easier to obtain due to translational nature and the fact that it is backed by need from industry | Additional due diligence on the proposed projects |
| PhD studentships | Funding for ambitious transitional projects that are vetted by a large pharma. External source of funding for education | An insight into relevant new research findings in relevant fields |
| Potential source of highly qualified young researchers | ||
| Professional career development program for PhDs and Postdocs | Develops students with professional skills which makes them more employable. As parts of the program were defined by industry partners, they had high relevance to the work place | Potential source of highly qualified young researchers with a far more relevant and up-to-date set of skills |
| iGEM teams | Develops undergraduate skills, making them more employable | Source of highly skilled undergraduate students, with strong team-focused skills |
| Year-long Internships | Develops concrete undergraduate skills, making them more employable | Source of highly skilled undergraduate students, with industry experience |
| Research Symposia | Networking platform | Networking platform |
| Publications | 36 publications, with possible longer term impact statements | 36 publications relevant to industry research needs |
7.1.7 Lessons Learnt from MSD-SLSF
7.1.7.1 Governance
Using SULSA as the main governance structure and managing entity was very successful because the committees were already used to working together and were made up of people who were able to detach themselves from the interest of their individual institutions to focus on national educational interests.
7.1.7.2 Administration
Delays in the initiation of certain aspects of the project meant that these were not realized, and there were as a result some underspends in the budget. These budgets were reallocated to different tasks that had shorter timelines and were easier to set up and manage, possibly to the detriment of the integrity of the project. This was in part due to poor communication between project partners, and a lack of leadership to redress the balance of the program early on, resulting in taking the path of least resistance. Anticipating issues early enough and communicating them to all partners so that constructive mitigations could be implemented would have prevented this unfortunate situation.
Running a program over six universities has some serious administrative challenges for communications, finances, and approvals. It is important to have an internal champion in each university, with significant local admin knowledge, to be able to navigate each of the university systems. Failing to have this resource will lead to delays, frustration, and ultimately be detrimental to the project.
7.1.8 Mutuality
One cannot underestimate how important it is to carefully select people to sit on PPP committees. It is important that they understand the vision for the PPP, are fully engaged and committed to its goals, and also have good knowledge of how it interfaces with their organization so they can intervene and make decisions on its behalf. It is more helpful if they are seniors and are not relying on the outputs of the PPP to make their career. This sounds counterintuitive, as one would expect personal gain to be a great motivator and thus an advantage to the PPP, but in our experience this causes the project to be skewed toward outputs that benefit the individual's organization, which risks destabilizing the partnership.
7.1.9 Norms
One of the hardest tasks in the MSD-SLSF was delivering the program to the specific deadlines required by industry. Industry seemed suspicious of academia's ability to deliver, prompting some overly harsh deadlines as a fail-safe. This required many of the programs to be started in parallel, which caused the mobilization of significant resources. Through this experience, it was learnt to better manage the expectations of the industry partners at the outset of any collaboration, so that future programs could be deployed according to more sensible deadlines. It would have been useful to build trust earlier on in the program.
7.1.10 Organizational Autonomy
With well-chosen committee and panel members, there were few issues with conflicting interests.
7.2 Phenomics Discovery Initiative (PDi)
7.2.1 Background
PDi was a PPP between industrial pharmaceutical companies and the National Phenotypic Screening Centre (NPSC) that launched operations in 2016. NPSC is an academic phenomics center with laboratories in the Universities of Dundee, Edinburgh, and Oxford.
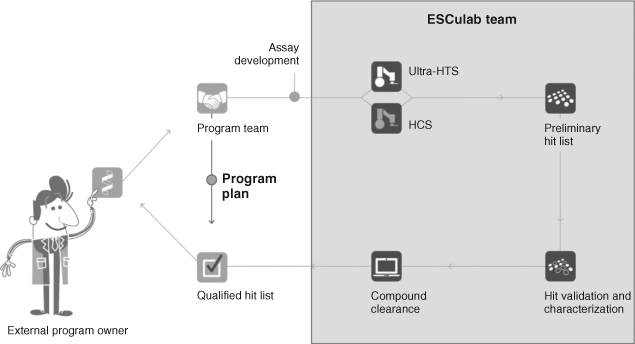
PDi was a PPP addressing industry's biological research priorities for phenotypic drug discovery. Each industry partner paid an annual fee (£650K) to NPSC to access novel disease-relevant phenotypic assays that were recruited globally from academia, developed to industry standards for high-content screening and then screened against two compound libraries (annotated and diverse). Assays were crowdsourced globally from academics (assay owners) who put their assays forward for selection by a panel of experts.
Using the data from the screens that were selected to be in the portfolio, PDi aimed to deliver its partners with validated assays that were automatically licensed to the main consortium partners who could exploit them privately and validated hits and hit structures that are owned by the academic but formed part of the shared consortium data that could be used to inform downstream strategies.
7.2.1.1 Gap Addressed by the Consortium
The PDi addressed several gaps that are pertinent to the current lack of productivity in the pharmaceutical industry. Through smart crowdsourcing the consortium had a sophisticated mechanism for efficiently accessing near-patient physiologically relevant biology, which is one of the bottlenecks in the industry. PDi also allowed this biology to be developed in industry standard assays, addressing the reproducibility crisis in translational efforts. Focusing on phenotypic rather than target-based approaches means that the hits that were obtained from screening were more likely to be successful in the later stages of the drug discovery process due to the chances of efficacy being higher.
7.2.2 Scientific Focus
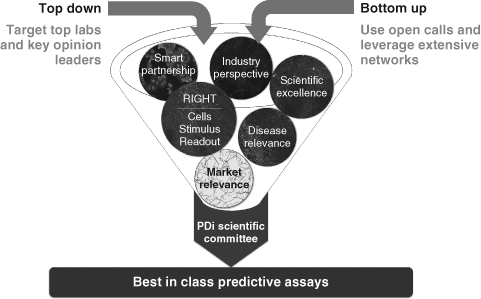
PDi sought to identify, develop, screen, and validate innovative phenotypic assays that were relevant to human disease. PDi phenotypic assays were crowdsourced globally from academia and small businesses in the form of project proposals made by academics, clinicians, and SME employees. These were submitted to the NPSC online recruitment platform (Figure 1). Best in class-predictive assays were sought out using top-down and bottom-up approaches, accompanied by a ruthless selection mechanism, which took into account the quality of the model (right cell, right stimulus, and right readout), scientific excellence, disease relevance, market relevance, and need from industry (Figure 1).
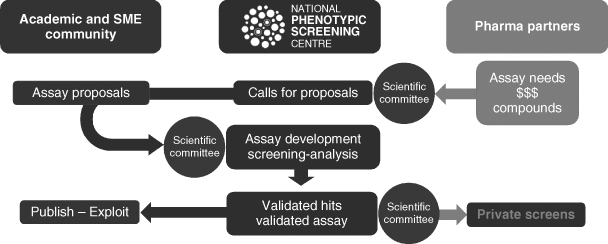
All applications were made online and submitted to a tailored recruitment platform, allowing them to be accessed and tracked globally. Projects that were selected were fully funded through assay development and phenotypic screening at NPSC against a ∼1000-compound annotated small-molecule collection and an ∼80 000-compound industry small-molecule collection (Janssen's Jumpstarter library) as shown in Figure 2.
The portfolio of projects was selected by a scientific committee made up of experts in the field, who at each yearly project recruitment round iteratively select projects to best meet industry needs. The portfolio contained a range of assays from different disease areas: Oncology (6), Neurology (2), Viral respiratory (2), Immunology and Inflammation (3), and Metabolic (2).
7.2.3 Budget
- Access to a ∼1000 cpd annotated compound set from Janssen
- Access to Janssen's 80 000 Jumpstarter diversity set (both libraries combining to an estimated value of around £8M)
- Leverage of SFC's £8M Capital Expenditure investment, which founded the NPSC phenotypic screening facilities in 2015 (Dundee and Oxford laboratories).
7.2.4 Governance
- At a board level – The PDi Board aimed to meet industry needs by setting the consortium strategy and approving and funding a phenotypic screening portfolio put forward by the PDi Scientific Committee. The Board was made up of industry and academia experts.
- At a scientific level – a scientific committee, made up of representatives from industry and academia, reviewed incoming crowdsourced projects and recommends a portfolio of screening projects to the board. Projects were selected on a set of defined criteria such as scientific excellence, disease relevance, tractability, potential for translation, and quality of the research team. They also appointed suitable project teams to manage the research projects and scrutinize results and outputs on a day-to-day basis.
- On a project-by-project basis – a project team made up of a team leader from academia and one from industry who established and managed research plans that were specific to the project.
NPSC's executive team managed the screening operations at the three university sites carrying out the assay development and screening operations.
7.2.5 Outputs and Portfolio
Multiparametric, near-patient phenotypic assays were generally complex and challenging to develop into high-throughput format for screening. Many of the cellular models contain human-derived cell types that are hard to source, expensive, and difficult to culture in vitro. Furthermore, the assays often involve building multicellular models or organoids, which require patience and skill to miniaturize for screening formats. These pathophysiology-relevant assays that were painstakingly developed at NPSC were one of the main assets of the PDi. Combined with the novel research aspect that comes from academia, their disease relevance made them attractive to industry. The assays represent novel biology that is hard to come by and is often not reproducible enough if taken straight form the academic lab into industry.
PDi's portfolio, shown in Figure 3, was made up of 15 programs, 4 of which have been completed by the NPSC and are moving into downstream screening cascades. Other assays are at various stages of assay development and screening.
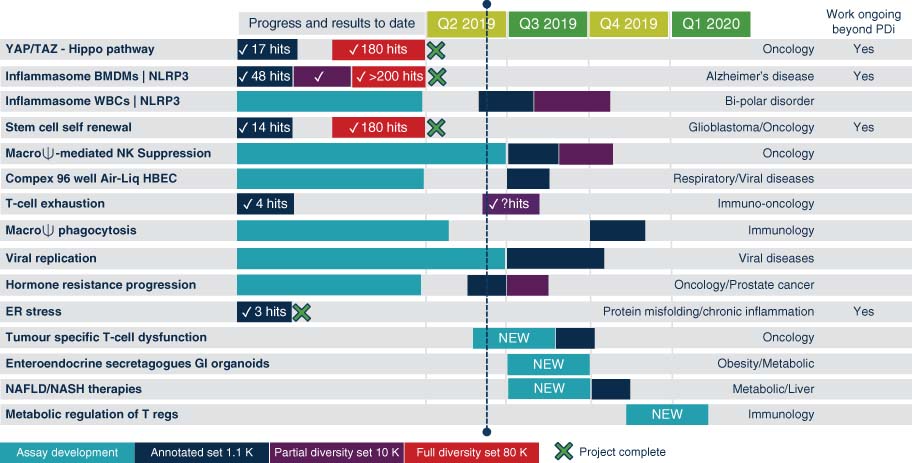
For academia, PDi provides a translational platform for knowledge and education, because all the innovative outputs are validated to industry standards, making them more valuable to industry. For industry, reproducible assays that explore new biology can easily be turned into proprietary material (Table 8).
| PDi consortium activities and assets | Outputs for academia | Outputs for industry |
|---|---|---|
| Validated assays with comprehensive SOP | Validated translational asset: An assay that is built to industry standards, which can be used as a primary assay in screening cascades | Complex near-patient assay that can be screened on private collections to generate private IP |
| Validated annotated hit list with 10pt dose–response curves | Insight into the biology at play in the near-patient model. Hits that inform on possible deconvolution strategies | Insight into the biology at play in the near-patient model. Hits that inform on possible deconvolution strategies. |
| Validated hits from diversity set with 10pt dose–response curves | Validated hits that can be used as staring points for a drug discovery project | Ideas for further drug discovery strategies |
| Access to raw and processed data | Research material | Research material that can be cross-referenced against other programs |
| Staff exchanges | Training of staff on high-throughput robotics systems | n/a |
7.2.6 Intellectual Property
The IP model for this program is of particular interest, as it is simple and promotes the creation of ongoing relationships and collaborations to take projects forward into development.
The industry-standard data that is generated by PDi (validated assay, SOP, and structures of up to 80 phenotypic hits) is owned by the academic/SME applicants who are free to publish these results and use the hits as starting points for novel drug discovery programs. This is possible because the compound collection used for screening is made up of commercially available compounds.
Assay development and screening results data are also shared precompetitively between all consortium members. Industry participants and the NPSC are free to use the validated assays in-house on proprietary compound sets in the so-called private screens, and this is where the industry partners generate IP and benefit from the consortium. This means there is a clear distinction between the consortium-owned results, which are automatically licensed for use to all partners, and the potential for privately generated IP which is private to the party that generates it.
7.2.7 Lessons Learnt from PDi
7.2.7.1 Governance
PDi taught us that having an internal industry champion who truly owns the program is really important. Having an industry coordinator who is simply assigned to be in charge of the program is not sufficient.
7.2.7.2 Administration
Dealing with corporate restructuring in pharma companies is detrimental to PPPs, as the change of staff can destabilize the consortium, especially when trying to grow it with the entry of new partners. Accelerating the decision cycles and making them more discrete and iterative can mitigate the effects of this problem by setting shorter term objectives that can be met within the committee lifetime of staff members or can easily be revised without too much upheaval on the joining of a new member.
7.2.7.3 Mutuality
It is critical to anticipate and mitigate the issues partners may have to preserve the synergistic values of the collaboration. For example, when crowdsourcing programs from the public, having very simple and beneficial T&Cs makes academic uptake easier. With PDi, the clear-cut split between the public assets and the potential for private screens by industry partners meant that academics were happy to engage with the program because they could easily understand the T&Cs. Perception of hurdles is often more important than the hurdles themselves.
Helping academics with assay development is really important in making transitional projects a success. Most academics neither have the skills nor the equipment to make assays robust or reproducible enough for high-throughput operations. By seeking the best scientific ideas and then putting resources into making them fit for industrial grade screening is a very productive way to translate science, rather than trying to select projects that are already robust enough for industrial applications.
In PDi, it was important to keep individual industrial participants sharp with their needs, interests, and expectations from the consortium, as this is directly linked to achieving the success anticipated by the company as a whole. This was difficult to do because for each individual contributor, the PPP probably only represented a small fraction of their workload, which may not be critical to their career. This means that it is important to make sure they understand the synergistic value of the consortium and what they have to do to make it work, regardless of whether it is any benefit to them. Hopefully, it is possible to find good reasons for them to be involved which are indeed beneficial to them.
7.2.7.4 Norms
As with most collaborations, communication is key to achieving success. Regular small updates at every management level of the project are more productive than adopting a top-down managerial approach. This habit will establish trust early on and build an environment where partners are keen to engage and deal with issues as they appear and before they become a treat to the project. However, with project partners dotted around the globe in different time zones, this can be difficult to achieve in a smooth and fruitful manner.
7.2.7.5 Organizational Autonomy
From a biology perspective, the pharmaceutical industry is mostly organized in therapy areas (TAs). Each TA has a director (or VP) who is a very important decision-maker and budget holder. PDi was initially set up and mostly funded through the discovery arm of the pharma partners (screening technologies and chemistry) who are not therapy focused. This meant that defining the disease focus and getting buy-in was difficult. If collaborating on biological assets, involving the Heads of TAs is critical to success, as it is their organizational interests that drive many critical aspects of the project for decision-making and financial support. As the project evolved from being funded through discovery to the TAs, this lack of engagement became a real issue.
7.3 The European Lead Factory (ELF)
7.3.1 Background
The ELF was launched in 2013 and entered its second round of funding in May 2019. It is funded by the EU commission's Framework 7 funding scheme via the Innovative Medicines Initiative (IMI).
The ELF consortium is currently made up of eight EFPIA pharmaceutical companies, nine SMEs, and four academic partners. It is an EU-wide platform for scientists from universities and SMEs to translate novel disease-relevant biochemical (target-based) and phenotypic assays and deliver high-quality, relevant chemical assets, with a view to discovering investable starting points for new therapies. Researchers from academia or biotech would not be able to achieve this rapid progression without ELF's industry standard facilities, financial support, staff expertise, and access to small molecule libraries. The contributing scientists are called program owners.
- 200 000 highly novel small molecules that were crowdsourced and developed in the early stages of ELF from academic and SME partners
- Around 350 000 in-house compounds from the eight EFPIA partners
- Different selections from the library are available for phenotypic screens, including a small collection of annotated compounds and chemical probes.
7.3.1.1 Gap Addressed by the Consortium
ELF bridges an important gap between basic research and drug development. It facilitates the translation of fundamental biological insights and chemical novelty into innovative drug starting points. The setup allows innovators from EU academics and SMEs to have access to state-of-the-art industry-grade facilities, drug discovery expertise, and a top-quality, curated library with over 550 000 unique compounds suitable for screening potential drug targets. These are amenities they would never normally have access to. The JECL compound library in particular is one of a kind, as it includes proprietary compounds from eight different EFPIA companies as well as compounds with truly creative chemistries that were sourced from academia. Screening this unique library against novel targets and novel biological models from academia and industry has a great potential for addressing the lack of productivity in drug discovery due to a lack of innovation. The size and scope of the consortium also bridges an investment gap by providing funders with an attractive proposition for exploitation of the consortium findings. Ultimately, this will result in novel medicines bridging a therapy gap for patients.
7.3.2 Scientific Focus
The eight EFPIA partners that are engaged in ELF will screen around 130 programs using the unique ELF library over five years on programs that are proposed and selected through a program selection committee, made up of members of industry, SMEs, and academia. The program selection committee ensures that the program portfolio is novel, balanced, has therapeutic and commercial potential, is tractable, and has no duplication. As in the first iteration of the program, ELF will crowdsource novel screening ideas such as high-value biological targets and disease-linked phenotypic assays from the European research community (public partners from academia and SMEs). The aim is to source around 50 public screening programs in this way, which are selected like the EFPIA programs according to a strict set of criteria set by a program selection committee made up of members of industry, SME, and academic partners. MMV will also propose five screens, relevant to their mission, to be prosecuted by the public partners, and additional screens (around 20) will be prosecuted and externally funded by other charities and private organizations. Program recruitment efforts are supported by therapeutic area champions and aimed at a range of different disease areas, with a special focus on neglected tropical diseases (Figure 4).
7.3.3 Budget
- In the first cycle (2013–2018), the consortium was funded with €196M, including an €80M cash contribution from the IMI, €91M provided as in-kind contributions from the participating EFPIA companies, and €25M in a variety of contributions from non-EFPIA participants.
- In the second cycle (2019–2024), the consortium is funded with €36.5M, around €18M is a cash contribution from the EU commission, €0.75M is contributed by the Medicines for Malaria Venture (MMV – a charity focused on finding novel antimalarials), and the rest (around €17.75M) is contributed as in-kind contributions by the eight EFPIA companies.
7.3.4 Governance
- A coordinator from the public partners and project leader from the EFPIAs are the key intermediaries between the IMI and the other consortium members and actors
- A project management board supervises the overall progress and strategy of the project
- A project executive – responsible for the operational governance of the consortium activities and overall coordination of the partnership
- Three committees dealing with legal matters and IP, ethics, and program review
- Three theme-led teams dealing with portfolio management, project operations, and ELF sustainability
- A working group that manages the crowdsourced programs
- A general assembly involving representatives from all companies and institutions, to present and review results and strategies on an annual basis.
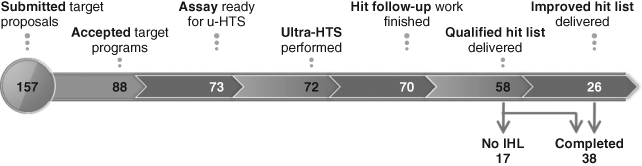
Most governance structures and bodies have a mix of partners from industrial, SME, and public partners, allowing a balanced approach to manage strategy and operations.
7.3.5 Intellectual Property
The following description of the IP conditions for ELF is at a very high level and is just a rough distillation of a far more complex agreement. IP in ELF mostly resides in the structures of the qualified hit lists (QHLs) that are delivered after screening and triaging the JECL library against a public or EFPIA assay, and also to a lesser extent in the screening results, and assays themselves. Qualified hits have gone through an extensive triaging process, giving them significant value for downstream drug discovery activities. Program assets (JECL compounds) and results generated under the project (assay results, screening results, QHL results, and other results) initially belong to the party that generated them. However, the driving principle behind the rights and obligations of the consortium is that program owners should be granted access to, and be in control of, any results from which they can generate value from their program. This means that result ownership, depending on its importance for generating program value, can be transferred to and/or shared between the party generating the data and the program owner. To facilitate this, compound owners (EFPIA or public) grant program owners with royalty-free research use to relevant program results and any relevant background IP required to execute the project. If an asset is directly exploited by the program owner, there are predefined milestones to compensate the compound owner for direct exploitation and also an obligation to purchase newly synthesized compounds from one of the chemistry SMEs that are engaged in ELF. If program owners decide to partner the program, the ELF EFPIA partners have first right of refusal. If an EFPIA directly exploits a public target or assay, the downstream compensation scheme is negotiable with the program owner (Figure 6).
7.3.6 Current Outputs from ELF
ELF is an ambitious long-term project, which in time is aiming to move from the earliest stages of drug discovery (screening and delivery of hits) into later preclinical stages such as lead and candidate generation. The project has so far accepted 88 screening programs, for which 58 QHLs have been delivered.
The ELF will accumulate many valuable assets as a result of the longevity and scope of the consortium activities including novel chemistry, novel biology, world-class facilities and translational platform, shared knowledge, and IP. These include assets that were delivered in the first round of funding and those that will be delivered in the second. Put together, these outputs provide significant benefits to the consortium partners and correspond to a level of screening activity that might exist in a reasonably sized pharma company. Data in Table 9 shows the activities since ELF's inception in 2013; it has had a number of significant outputs as a result of activities downstream of the screening, which are listed in Table 9.
| Type of activity/asset | What has ELF delivered so far? | New goals and benefits to partners |
|---|---|---|
| Access to world-class drug discovery infrastructure and expertise | 88 accepted target programs, of which 58 have been screened and delivered QHL |
Delivery of the facilities, logistics, and expertise for up to 185 new drug discovery programs
Screening efforts will include phenotypic screening as well as target-based screening |
| A translational platform delivering QHLs | 58 QHLs that are ready for translation | Translation of up to 50 novel biological targets and phenotypes from the European Research community (academia and SMEs) |
| Novel chemistry | ∼200 000 novel chemical compounds sourced from public partners | EFPIA partners and public program owners will have access to a unique compound library made up of a mix of ∼550 000 proprietary industry and academic compounds. The large-scale screening activity will generate a significant portfolio of new innovative and biorelevant hits that have the potential to be progressed up the value chain |
| New biology | Development of >250 bespoke assays | ELF will source 185 novel targets from industry and EU scientists. Novel insights are expected to emerge from the implementation of phenotypic and high-content screening. These insights can be exploited for drug discovery activities or to further life sciences knowledge |
| The delivery of 58 public QHLs | ||
| Resolution of >40 crystal structures of target–compound complexes | ||
| New businesses | The generation of two spinout companies: ScandiCure AB and Keapstone Therapeutics, who have both secured funding for their ventures through different sources | ELF is expected to facilitate the creation of more start-ups and business opportunities |
| New collaborations | Translation of two programs into either preclinical or in vivo trials | The ELF partners will actively build new partnerships that will benefit the public and will strengthen the EU life sciences community |
| New intellectual property | Ongoing generation from round 1 funding | ELF will continue to support the generation of patents around chemical matter and cellular models |
| Filing of one patent to date | ||
| Leveraged funding | Ongoing | To expand and extend its activities and services, ELF partners will continuously and actively seek additional financing from a range of different sources |
| Economic and health impact | n/a as too early | Taking academic and biotech research programs to the next stage will create more value, more jobs, and result in new therapies, an improvement in global health, and a direct effect on the economy |
| Shared knowledge | >60 peer-reviewed journal papers | ELF will actively pursue publications and dissemination of novel results in journals, at conferences, and by digital means that enhance the available knowledge and provide tool compounds to the scientific community |
| Education and training |
>90 academic postdoctoral fellows trained in industry methods and approaches ELF data has contributed to three PhD theses |
ELF will continue to support education and training opportunities |
| New IT platforms | Two custom-built data management platforms | ELF will continue to implement bespoke IT platforms to address issues of partner confidentiality, rights, and obligations |
7.3.7 Lessons Learnt from the ELF
7.3.7.1 Governance
ELF's governance structures generally work well. The size and scope of the consortium mean that the setup is a little large and complex, but this seems inevitable.
7.3.7.2 Administration
When the ELF grant was applied for to the IMI, it was in two halves in the first round of selection: chemistry and biology. In the second round, the two halves of the consortium had to come together into a single project. This caused some issues with the administration, because both parties had processes and structures that were already in place to manage the project, including management entities. These management entities were sometimes at odds with each other. This caused some confusion at the start of the project. With hindsight it may have been more efficient to have a single step in which to form the consortium, which would have included a single management entity.
7.3.7.3 Mutuality
The ELF's vision was to recruit novel biology from the academic and industry communities, and screen it against a unique compound library, in the hope that this kind of shake-up would produce some true innovation. This approach means taking risks, which does not sit well with the pharmaceutical industry. However, with a clear communication of the PPP's visions, and the fact that it was part funded by public funds, we have a position where around 1/3 of the screening programs are indeed really novel and do not fit into more common categories of targets that are screened by the pharmaceutical partners, such as enzymes, receptors, and ion channels. It was only by forcing through the obvious synergies and repackaging them into values that could be understood by each of the partners to account for their different perspectives and objectives that brave decisions could be achieved. It was therefore important to adapt the message to each stakeholder to get the best outcome for the PPP as a whole.
The vision for ELF is to be a platform that is available to scientists from all EU countries. However, some member states, with greater research capacities, are benefitting more from the platform.
7.3.7.4 Norms
With so many partners involved in ELF, establishing trust was a lengthy process. The project had two natural splits: pharmaceutical industry versus public and chemistry versus biology. At the start of the program, there was an “us-and-them” attitude between the four camps, which resulted in trust issues and disagreements on liabilities. It took a massive collaborative effort between a large group of talented lawyers from all the different ELF entities to establish a suitable legal framework that would allow trust to flourish. It is surprising what a good legal team can do! Their detachment from the scientific substrate of the operations allows them to be much clearer on the legal and administrative processes that need to be put in place to have an operational PPP. The complex legal framework actually catalyzed some of the initial innovation in the program by creating one of the IT management platforms that allows confidentiality, rights, and obligations to be respected and monitored in the consortium. This is a tool that everyone relies on and trusts to facilitate the correct flow of data within the partnership.
7.3.7.5 Organizational Autonomy
Some aspects of the partnership were initially too focused on the way pharmaceutical companies regard biological research. This comes from the fact that biochemical reductionist approaches dominate in this sector, and reproducibility is of paramount importance to succeeding in high-throughput screening. This led to very high barriers to entry for project owners when submitting programs for review, as the assay requirements in terms of reproducibility, signal to noise, and coefficients of variation were too high. Most academics are neither trained nor equipped to deliver this kind of data, meaning that they were unlikely to submit a project to ELF. This led to a gap in assay development, where either the ELF screening centers or regional screening hubs had to put more resources into this activity. We believe this is an example where the interests and motivations of one type of partner (pharmaceutical partners) took precedence on that of the consortium as a whole. Fortunately, this issue was addressed early on in the project, and academics are now supported by local screening centers and with local funding agencies to get their assays developed enough to meet ELF recruitment requirements.
8 Discussion and Conclusion
Through the experience of three PPPs, we can extract some common guidelines and points of discussion that are useful to bear in mind when setting up this type of collaboration.
Good governance comes from choosing the right team. Having team members who are seniors is also an advantage, as they will most likely be natural decision-makers and strategists. Giving every partner a voice that is truly heard requires effort from all parties and has to break through different cultural, sector, and personal barriers. As in most ambitious projects, recruiting the best people is always a winning ticket.
In terms of administration, it is critical to have an internal champion in each participating organization who truly owns the program and also has a very good understanding of the admin procedures and chain of command in their organization. Most operational issues in collaborations are due to human failures, so ensuring good communication and resolving conflicts as they arise will enhance the quality of the partnership. Nothing is more important than clarity, coordination, and agreement on the PPPs goals.
The mutuality of the collaboration and its benefits needs to be rational and understood by all parties. There also needs to be an agreement that partner benefits may evolve during the course of the partnership, and any changes should be discussed and agreed by all in a timely manner. The synergistic values of the partnership are often what motivate the human and emotional aspects of partner involvement, so are a strong component for engagement and action. If the individual benefits of each partner are matched with a larger positive societal benefit, then the stars are truly aligned for a successful PPP.
Gaining trust comes with time and is in general poor at the start of the partnership. Establishing norms by being open minded about different cultural and professional approaches to a problem is important at the start of a PPP. With patience and understanding, partners can learn to appreciate the strengths and weaknesses of their colleagues and eventually avoid “us-versus-them” politics.
Each partner suffers from the issues of organizational autonomy, but these can be mitigated if the partners are well chosen to be compatible, meaning that their involvement in the project leads to outputs that they can easily defend with their line manager or board. They should also ideally be allowed to make decisions on behalf of their organization, yielding smooth and efficient engagements.
References
Abstract
Drug discovery initiative (DDI) at the University of Tokyo has maintained a public research platform for chemical screening since 2007. Researchers in Japan can receive small-molecule samples from the DDI's compound library to discover active compounds for medicines, agrochemicals, and research reagents. This service is open to academic and industrial researchers on the condition that the recipient accepts the terms of use, including confidentially disclosing the intended use and the assay results to the DDI. The recipient will only be liable to container and shipping costs. If no elements of collaborative research with the DDI are involved, the intellectual property of the results will belong to the user. In addition to samples, the DDI provides technical advice and offers semi-hands-on technical courses yearly to popularize technologies. Moreover, the DDI's Lead Exploration Unit consists of professional researchers in medicinal chemistry, pharmacokinetics, and metabolism. This organization is strongly supporting hit-to-lead synthesis for academic drug discovery research in Japan.
1 The State of the Transfer of Japanese University Research Findings to Industry
Amidst discussions of pharmaceutical industry challenges such as expiring patents for compounds on the market and depleting novel drug targets, major pharmaceutical companies continue to appeal for research collaboration and are actively working to partner with external organizations, or “open innovation,” including academia.
The Japanese government equally continues to promote its project aimed at strengthening the links between the latest findings in life science and pharmaceutical industries. The Ministry of Education, Culture, Sports, Science, and Technology (MEXT); Ministry of Health, Labour and Welfare; and Ministry of Economy, Trade and Industry have begun initiating unique programs to this cause. However, as a result of overlapping policies, the Japanese Agency for Medical Research and Development (AMED) was established in 2015 to intensify the implementation of policies for medical-related research and development and to partner on increasing their efficiency.
There are many notable successes documenting academia-initiated drug discovery. Pharmaceutical development cannot be achieved solely depending on academia; hence, universities have been cooperating with pharmaceutical companies. However, university governance has recently been called into debate. In the past, a typical Japanese university's administration did not strategically direct collaborations with the pharmaceutical industry. In terms of research, universities have long been an aggregate of the so-called private shops, which are managed by each professor. Alternatively, universities only act as shopping malls, where each shopkeeper (professor) talks to familiar companies, calls the relevant people, and markets his/her groups' discoveries based on personal judgment. Subsequently, a good number of the drug discovery leads introduced to these companies tend to be courteously declined, or distant level affiliations are built with the professor, which eventually fade away.
Although there have been successful cases where a handful of university pioneers collaborated with industry and flawlessly developed their research into a pharmaceutical product, the vast majority of university researchers are only amateurs in the field of drug discovery research. Basically, it is a general trend for companies to reject academia-based researchers mostly because the research fails to align with the company's needs and sometimes due to concerns of insufficient or irreproducible data.
After incorporation in 2004, Japanese national universities modified their approach to intellectual property rights, establishing the Technology Licensing Organization (TLO) as a point of contact for academic–industrial collaboration. Despite the recent effort by universities to accumulate knowledge, the necessary support to exploit this innovation are either insufficient or completely absent (in some universities). Consequently, the relationships between individual faculty members and companies remain relevant.
2 Barriers Faced by Basic Researchers Getting into Drug Discovery Research
Excluding coincidental discovery, basic researchers need to conduct compound screening in order to discover small molecules that control biological functions. Having a high degree of originality is of utmost importance in academic research. Hence, academic projects themselves are not so competitive. However, “drug discovery” researchers while drafting their research plan must review the previous research through the lens of existing drugs and disease treatments, including alternate treatment methods not involving the target molecule of interest. If such research exists, the researcher is then tasked with clarifying the superiority of the drug they aim to develop. Basic researchers need to have a sufficient understanding of the different facets of drug discovery outside science, including clinical and business aspects. The required compound screening skills cannot be acquired from university lectures or practical laboratory courses, making basic researchers inexperienced in these skills. Multisample screening is thought of as an easy, repetitive task consisting of investigating whether a small number of compounds have effects. However, the skills and knowledge vary, so guidance from experienced individuals is ideal. Forcefully conducting multisample screening with inadequate preparation may result in large variations, poor reproducibility, and data which cannot be analyzed/interpreted, leading to a waste of time and resources.
Daily compound screening is conducted within pharmaceutical companies, and it is not uncommon for a company to screen hundreds of thousands of samples in a day. High-throughput screening (HTS) technology, in which multiple samples are tested at a high speed, was hailed as a dream technology for discovering drug “hits.” However, with the rate of “hit” discoveries failing to meet up with the resources invested in HTS, many companies are drifting toward areas with higher development efficiencies such as antibody drugs. Despite this drift, the cheap prices of small molecules that control biological target molecules compared to biopharmaceuticals, as well as their applicability to highly versatile oral drugs, make them difficult to replace. Thus, screening cannot simply be stopped, as it is an irreplaceable method for the discovery of small molecules with pharmacological activity.
3 Chemical Screening Support of DDI
The University of Tokyo founded the Chemical Biology Research Initiative (CBRI) in 2006; it was the organization responsible for building a large-scale compound library and compound screening facilities in order to utilize the findings of a large-scale structural biology project started by MEXT in 2002. Switching government grants, the CBRI was then renamed twice: first, the Open Innovation Center for Drug Discovery (OCDD) in 2011 and then the Drug Discovery Initiative (DDI) in 2015 (Figure 7). The organization continues to develop its support activities for researchers based on the research base. Currently, DDI is a member of the Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS) program of AMED (105).
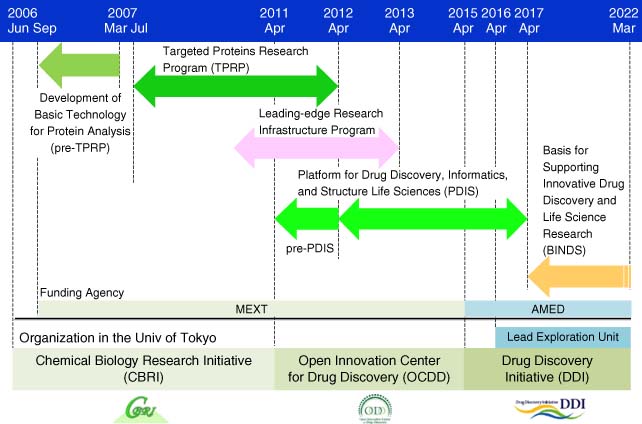
The DDI provides the same drug discovery research support to academic and industrial researchers. The drug discovery targets may be medicines, agrochemicals, or research reagents. The main condition is that confidential information such as the intended use and assay data be disclosed to the DDI. Chemical samples are equally provided free of charge, although charges covering the costs of sample plates (container fee) and delivery (delivery fee) are paid by the user. The DDI only uses the reported assay data to improve the quality of the compound library by discarding compounds with nonspecific functions, for instance. Recently, assay ready plates, which allow for direct reagent and culture media addition and assay measurements without creating intermediate dilutions of chemical samples, make up a big portion of the total usage. The Echo® liquid handlers, which can dispense solution of chemical samples at the nanoliter level, are used for preparing the assay ready plates (Figure 8).
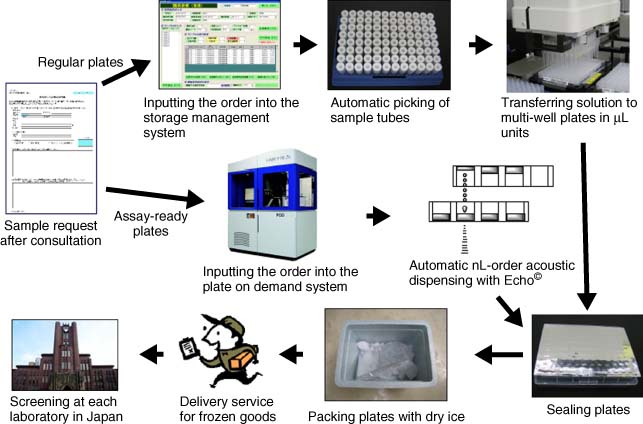
The DDI's compound library currently maintains and manages approximately 280 000 samples (Figure 9). In 2007, when the plan to build the compound library in Japan was made, only major pharmaceutical companies had HTS compound libraries with over 100 000 samples, and no large-scale library was available to academic researchers in Japan. Such libraries already existed in countries such as the United States and South Korea, with the United States standing out, as it is not uncommon for individual universities to have them. We observed compound libraries at such institutions and at pharmaceutical companies as reference.
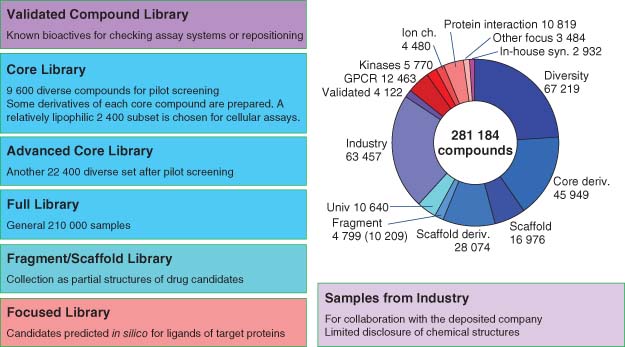
Several people have the opinion that “drug discovery cannot be accomplished within academia” or question if anything can be accomplished with such a scale of foundation. Even with 280 000 samples, the DDI's compound library is insignificant compared to the libraries of the major pharmaceutical companies with over 1 million samples. However, considering the time when no academia-targeted drug discovery research foundation existed, we believe that the difference between 280 000 and 1 million is not high. Rather, an increased number of samples in turn increase the management and assay costs, which would make the library less accessible to academia.
The compound library consists of several commercial compounds made by foreign suppliers, selected for their diversity in chemical structure, predicted affinity with biomolecules, and “druglikeness.” In addition, the library contains approximately 10 000 noncommercial compounds from university laboratories with unique chemical structures and approximately 60 000 compounds deposited by pharmaceutical companies. Compounds from pharmaceutical companies typically require a link between the providing company and the research, so the conditions for use of these compounds are maintained in accordance with the intentions of the providing company. If academic researchers agree to those conditions, they may use these samples, but the sample amount and number of chemical structures disclosed are considerably limited. Hence, these samples are only used in research assay systems that have previously been cleared from any issues associated with using generally provided samples.
All samples are prepared in solutions of dimethyl sulfoxide (DMSO) at either 10 or 2 mM, which is miscible with water in all proportions. The quantity needed is dispensed into microplates, and the plates are quickly supplied. Outside pharmaceutical company samples, solid samples are stored for repreparation of solution samples. Generally, solid samples are not provided for bioassays. For research such as animal studies, the supplier information will be disclosed to the researchers to enable self-procurement of the required samples. Academic researchers are often forced to keep their screening to a small scale due to limited funding and manpower. If there is any information such as the three-dimensional structural information of target proteins or information on known active compounds, a computer may narrow down the candidate compounds, but when searching for changes in phenotypes before and after the administration of a compound with no clear target molecule, researchers are forced to use a standard random search. To that end, a Core Library of 9600 samples selected for pilot screening or a validated compound library containing existing medications/samples reported to have pharmacological activity is commonly provided to researchers. Furthermore, for cell-based assays testing, the Whole Core Library is normally not feasible. Thus, we have constructed an intracellular target core library which consists of a subset of 2400 Core-Library samples predicted to relate to membrane permeability. We are also devising alternative diverse subset collections such as the Advanced Core Library (contains 22 400 samples), to enable efficient screen where conducting assays on the full library is impractical.
We also provide support for building assay systems that meet the needs of users. It is necessary to build individual systems suited to various targets for assay methods. Trial and error and a certain amount of experience are required for this. It often takes at least a year to build a suitable assay system, and commonly takes longer than the assay itself. Commercial luminescence kits for measuring kinase activity are expensive, so it is unfeasible to conduct large-scale kinases screening within academia. However, a new fluorescence assay method developed by the DDI has made it extremely low cost to run these analyses (106). A laboratory (Figure 10) is open to users who wish to use it. The laboratory has staff with extensive experience in exploratory research at pharmaceutical companies who are called in to guide academia-linked researchers with no experience in building large-scale compound libraries or conducting HTS. Advice alone is useful, but they equally provide practical guidance which allows beginners and students to ease into their research. Semi-hands-on compound screening technical courses are also offered a few times a year, which hundreds of people attended.
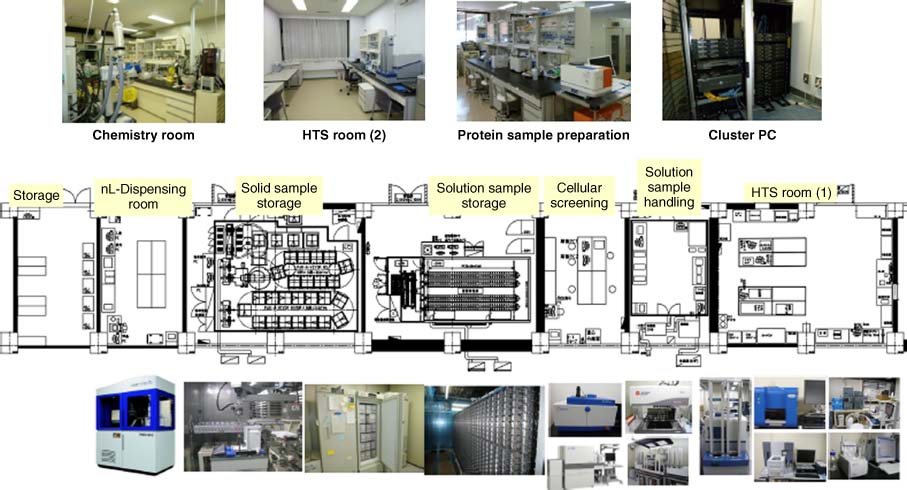
The DDI is a system which provides chemical samples to all researchers. Another popular system is screening centers, where samples are not provided to common researchers, but assays are contracted to screening professionals. This system is common, owing to its high efficiency and yield of good-quality data. However, successful drug discovery research requires perseverance, hard work, and enthusiasm; hence, having researchers conducting their respective goal-oriented research facilitates research progression. Moreover, above anything, this is connected to popularizing know-how in screening and drug discovery research in academia, and training drug discovery research personnel and instructors, which we believe can contribute to a bottom-up approach to drug discovery research in Japan.
Promising drug targets are said to be buried in academia, and pharmaceutical company representatives are eagerly searching for such existing targets; multiple companies publicly appeal for proposal-based targets. Furthermore, AMED conducts target search surveys, and mostly existing targets may have been played out. However, because drug discovery research continues to evolve, it is therefore crucial to continually release new drug discovery ideas, improve the ability to validate them, and cultivate an environment where target development is easier.
Even if screening is contracted to the DDI, from a resource perspective, research themes must be highly scrutinized and prioritized; thus, there are concerns that this may narrow the door to opportunities in drug discovery research. We believe that amidst the fierce global competition in drug discovery research, our limited resources and a small library with commercially available compounds cannot compete with pharmaceutical companies even when conducting similar research on a small scale. Thus, we need to cherish the unrestricted approach of academia with undefined drug discovery targets. Meanwhile, speed is extremely important in drug discovery research. Researchers should consider applying to the iD3 Booster project of AMED, in which research funding is given to promising research themes relating to drug discovery (107), in order to accelerate research through collaborations with companies seeking such opportunities. Given that “transferring to pharmaceutical companies” is one of the goals of academic drug discovery, academia should work to partner innovation with companies that have a strong strategic interest in the target indication.
Based on these points, we believe that a system which provides necessary support to individuals in need, specifically recipients who propose novel drug discovery ideas, while moving forward with screening research, is ideal.
4 Hit-to-Lead Synthesis and ADMET/Physical Property Evaluation Support of DDI
In cases where compound screening results in “hits,” those compounds do not necessarily have satisfactory characteristics, owing to poor activity, nonideal targets, nonselective subtypes, or poor oral absorbability/pharmacokinetics. In other cases, the compound may be metabolized in the body and converted into a different compound or easily excreted. In such cases, improvements need to be planned using hints from the structures of the “hits” from screening, followed by chemical modification and substitutions. It is not recommended to transition to animal studies without evaluating the pharmacokinetic properties of a compound; hence, if disregarded, its results (favorable or not), chemical structure stability, and effective concentration will remain unclear, creating controversies and decreasing the persuasive power of the data.
The DDI's lead exploration unit, which has a team of researchers presently working at pharmaceutical companies, was set up in 2016 with the aim of consulting with biologists or medical researchers not specialized in “hit-to-lead” generation or pharmacokinetics and taking charge of the kinetic parameter measurements. Requests for this support can be made through the BINDS program.
5 Regarding Intellectual Property Rights
When no elements of the DDI's collaborative research are involved in research conducted with DDI-provided general compound samples, the intellectual property of the results belongs to the user. Synthesis support entails collaborative research with academia; hence, the intellectual property rights will be handled accordingly. Findings obtained from the use of public compound samples should be published. However, the nature of the research field may require that the information be retained until the publication of a patent application or a paper with the findings. Users working for companies make up approximately 10% of all users, but company-affiliated users generally seem to have no challenges with following this rule.
The possession of findings that are considered inventions in drug discovery research leads to patent application issues. It usually takes 10 years or more from the discovery in basic research to the actual sale of the drug, including the clinical trials. If a patent expires, other companies could sell the generic versions of the drug, making the patent term of utmost importance in the drug discovery business. It is therefore obvious that patent applications must be submitted as rapidly as possible, given the competitive environment. However, given that the term of research and development after the date of application is subtracted from the effective term of the patent, filing early applications with immature data packages may not always be the best approach to secure exclusivity. Moreover, application details are published a year and a half after the application is filed; hence, competing companies will be notified of the novel technology. The application timing for the technology in question is based on a “strategy” in anticipation of commercial development. Conversely, in academia, publishing papers on research findings and attending conferences are of utmost importance, as opposed to commercializing a pharmaceutical product. This is especially true in cases where graduate students contributed to an invention and have to fulfill their requirements to publish their research thesis to obtain the respective degree. It is in the nature of academic researchers to publish their research findings as soon as possible, to avoid getting scooped if other researchers end up publishing first. The department handling intellectual property at their university requests that the patent application is considered before the research findings are published. Hence, there is no so-called strategy in patent applications based on research findings from universities, leading to substandard patent applications (patent omissions are acceptable), failing to meet company demands, causing a barrier to cooperation between the two parties. To ensure university rights, filling out opportunities for collaborative research with companies after applications is virtually unavoidable, although the patent is initially meant for company activities. If the contents of the patent application cannot be applied in the pharmaceutical industry, then there is no point to it. In this field, it is ideal for inventors and candidate companies for future invention development to be matched in many cases, so that collaborative applications can be based on a business strategy that allows for the progress of collaborative research. It is ideal to understand that the most important things to each party are different and find amicable compromises such as allowing the publication of academic papers only on the “hits” that are not being developed, on a case-to-case basis.
6 Examples of Past Findings
The DDI has provided a total of approximately 25 million samples for over 600 research themes as of March 2019. The target diseases of about half of the research themes were cancers or infectious diseases. At the drug discovery stage, projects are often in their early development, with more than half being in the idea stage.
Major findings obtained using the DDI's compound library have been involved in the discovery of AdipoRon (prospective hypoglycemic agent) (108), the invention of RK-20449 (prospective leukemia medication) (109), the repositioning of a biliary tract carcinoma drug for use in organoids (110), and the discovery of RK-287107 (tankyrase inhibitor and prospective colorectal cancer drug) (111). Additionally, an inhibitor of autotaxin, which is a chronic renal disease target, discovered in collaboration with Dr. Junken Aoki of Tohoku University and Dr. Osamu Nureki of the University of Tokyo, was successfully used as a lead compound by Shionogi & Co., Ltd.
The number of yearly requests for support remains fairly unchanged. In the future, the support of the DDI holds promise in contributing to the creation of new “hits.”
Acknowledgments
The DDI's activities and findings listed in this article are made possible by the constant hard work of the members at the organizations involved. Special thanks go to the current DDI director, Dr. Hidenori Ichijo, and the former director, Dr. Tetsuo Nagano, as well as Dr. Takayoshi Okabe, who provided instructions on drug discovery research technologies.
References
Abstract
This article describes the initial impetus for creation of an academic drug discovery center, its successes and challenges, and why we believe that academic institutions should embrace a fully open model of drug discovery.
1 Introduction
When transitioning from a 20-year career in the pharmaceutical industry more than a dozen years ago, one of us (SVF) had the opportunity to create an academic drug discovery center at the University of North Carolina at Chapel Hill – the Center for Integrative Chemical Biology and Drug Discovery (CICBDD, http://cicbdd.web.unc.edu). During this transition, collaboration between the CICBDD and the Structural Genomics Consortium (SGC, https://www.thesgc.org, led by AME) contributed greatly to our discovery of many small-molecule chemical probes (112-114). Over the years, this collaboration and our practical experiences in the academic drug discovery ecosystem have informed our thinking about the future of efforts in this area. This article aims to describe the initial impetus for creation of the CICBDD, its successes and challenges, and why we believe that the future role of academic institutions in drug discovery should embrace a fully open model of drug discovery (115).
It should be noted that our discussion will focus on small-molecule drugs not because other approaches, such as antibodies, cell, and gene therapies are not important, or subject to some of the same challenges, but simply because this is the area of our expertise.
2 Problem Statement
“Discontent is the first necessity of progress.” – Thomas Edison
Spurred by access to human genome sequence information, tremendous technological advances, and massive increases in funding by the public and philanthropic sectors, scientific progress in the biomedical sciences has accelerated enormously over the past two to three decades. The cellular signal transduction processes and biochemical pathways that enable life are increasingly being defined at the molecular level, and the aberrations that result in disease can be addressed within this rational framework. The sequencing and manipulation of model organism genomes has further explicated the identity and roles of specific proteins in pathophysiologic processes, increasing our ability to preclinically assess pharmacologic targets. We are clearly approaching the “end of the beginning” in our efforts to comprehend the molecular basis of disease.
The pharmaceutical industry has responded to these exciting advances in basic science and technology by increasing their own investment in R&D, with the aim of translating these advances into a revolution in the availability of potent, selective, and safe drugs. However, the rates of approval of completely new medicines have not kept pace with these massive increases in investment. Consequently, pharma is rethinking how to organize its research, particularly in the light of additional business challenges, such as pricing pressures and litigation (116). This reimagined pharma has increased emphasis on external research and also relied on frequent mergers, reorganizations, and reductions in scientific staff across the industry to maintain profits. Indeed, given the rate of organizational change in the industry, and the long timelines associated with drug discovery, it is increasingly difficult for a research project or strategy to bear fruit before it is abandoned (117).
The travails of the drug discovery sector, coupled with pressures on health care systems, are now causing some to rethink the fundaments of the business model. It is increasingly being voiced that the current economic incentives that society has put in place to support the discovery of new medicines, which are based on protection of intellectual property (IP) and prolonged periods of data secrecy, are inherently inefficient; they result in many organizations simultaneously exploring the same molecular target hypotheses, in secret, with failure as the most likely outcome. To support this process, pricing of new medicines must be set at levels needed to recover the costs of these failures, to drive profit expectations, and to turn every new drug, even ones that treat only a few hundred patients (https://thehill.com/policy/healthcare/445451-fda-approves-worlds-most-expensive-drug-at-%242.1M), into blockbusters (annual sales >$1 billion). In our view, this scenario presents an opportunity for public sector drug discovery efforts to contribute, especially to pilot alternative business models.
3 Initial CICBDD Organizational Model
“The beginning is the most important part of the work.” – Plato, The Republic
- Collaborate directly with basic scientists and academic physicians both at UNC and beyond to create drug discovery efforts on targets not known to be under pursuit in the pharmaceutical industry. The anticipated products of these collaborations were small-molecule probes to test initial biological hypotheses in preclinical models, which may ultimately become leads to optimize to clinical candidates.
- Establish an area of unique scientific excellence and meet all the requisite academic metrics of success: publications, grant funding, student training, and visibility through seminars and presentations.
Part of the rationale for pursuing these dual objectives was that they provided the center flexibility in controlling its portfolio, staff requirements, training culture, stakeholder support, and funding options: increasing the resiliency of the center while also providing differing advantages and limitations. Drug discovery projects were responsive collaborations in which the center assessed targets proposed by disease-centered PIs and then engaged to cocreate projects where there was a perceived opportunity to make a unique contribution. To establish an area of scientific excellence, the CICBDD focused on the chemical biology of chromatin regulation and creation of open-access chemical probes, frequently in collaboration with the SGC. For these projects, the center selected its own scientific problems and proactively aligned with the best collaborators to enable success. It should be noted that the objectives of our drug discovery efforts and basic science were often complementary; both led to publications, funding, and training opportunities.
Although our drug discovery and scientific objectives were complementary and often synergistic, they differed significantly in how IP was handled. In order for drug discovery projects to advance into human studies, we anticipated needing to raise significant (∼$5–10 million) funds to enable lead optimization (LO) and preclinical development (toxicology (tox) and chemical manufacturing and controls work (CMC)). Should LO, tox, and CMC all be achieved, we would require even more funding (∼$5–10 million) to pursue initial Phase 1 studies to establish human pharmacokinetics (PK) and tolerability and, only occasionally, evidence of efficacy. The cost for each subsequent stage of clinical development is in turn even higher – perhaps $20–50 million to achieve evidence of efficacy in Phase II, the typical stage for a first readout of a compound's potential as a medicine. This need for capital, especially when focused on high-risk endeavors and when adopting the traditional business model, means that we felt we needed to protect the IP around any new chemical entities in order to attract private investors and offer them a potential for return, should the project lead to a useful therapeutic. We appreciated though that this approach was not without limitations. For example, patent costs can also be a considerable burden as projects progress and national phase filings are required. And we also realized that if any of our discoveries led to a new medicine, the price would be set by downstream investors to maximize profits, not to ensure access for the taxpayers who funded our initial discovery work.
Our chemical probes program adopted a different IP strategy. Since chemical probes are most impactful when shared, we decided they require no IP protection. This strategy eliminates concerns about publications or presentations creating prior art, facilitates transfer of material among scientists, and has been shown to accelerate subsequent scientific discoveries (112).
To be successful in both our missions, the CICBDD recruited staff with industry expertise in protein expression and purification, assay development, computational drug design, and medicinal chemistry. This breadth of expertise means the center has all the resources and knowledge required to optimize small molecules for potency and selectivity in vitro and in cells. We rely on collaborators and outsourced activities for data in animal models and PK/tox. Because the core staff and research faculty of the center provide effort and input to drug discovery and chemical probe projects equally, they increase their opportunities for scientific growth and have greater connection to the overall mission of the center. In addition to research faculty and staff, the CICBDD is home to a number of tenured/tenure-track faculties with research programs in medicinal chemistry, natural products, and chromatin regulation. These faculties enrich the scientific life of the center and benefit from ready availability of expertise and shared equipment that support the center's drug discovery efforts.
A major design principle behind the CICBDD's two-part mission is the capacity it provides to optimize the training experience for students. Our drug discovery projects are well staffed, involve several years of focused effort, and necessarily have to tackle multiobjective optimization of a compound series for potency, selectivity, PK, pharmacology, and tolerability. These projects also face rigorous “go, no-go” decision points as they progress. This team-based science is an ideal training ground for postdoctoral fellows who are aiming for careers as medicinal chemists or other careers that involve multidisciplinary activities. Our typical postdocs in these roles arrive with a Ph.D. in organic synthesis and are able to learn drug design for structure–activity relationship development, biochemistry of their target, PK assay parameter interpretation, and some elements of drug development. In contrast, development of a chemical probe for a novel protein–protein interaction (PPI) involved in chromatin regulation, for example, is an excellent thesis material for graduate students. Students can participate in selecting their probe target with an emphasis more on the biological knowledge to be gained from the use of a probe than on established or potential disease relevance. New technologies are frequently in need of development (119), and because no IP is created on probes, students are able to collaborate with anyone in the world, sharing compounds freely (no MTAs) throughout the discovery process. We learned that avoiding IP also eliminates the potential for conflicts of interest in trying to balance a student's need to publish with timing of patent filing and alleviates this important concern about drug discovery in academics. However, our postdoctoral fellows engaged in traditional drug discovery projects publish or speak externally about their work only coincident with filing or publication of a related patent. Team meetings and a journal club in the center enable students, postdocs, faculty, and staff to learn about the issues specific to drug discovery programs as well as chemical biology-focused probe development strategies, reinforcing our educational mission. Journal club is also open to collaborators, where they frequently give disease-focused seminars, and because this is an internal UNC meeting, we can openly discuss our drug discovery projects and avoid creating a veil of secrecy that conflicts with academic culture.
Funding for the CICBDD, which has an annual operating cost of ∼$3 million, derives from competitive grants and contracts to center principal investigators (PIs) or collaborators (∼70% of total funding) and from institutional support from the University Cancer Research Fund (UCRF ∼30% of total funding, administered by the Lineberger Comprehensive Cancer Center). These funding sources have been supplemented by a gift from a donor and a small amount of indirect cost returns over the years. An active decision was made when creating the center not to carry out any research on a fee-for-service basis. The thinking behind this was twofold: fee-for-service implies a routine and predictable activity with minimal intellectual input, which is certainly not the case for drug discovery, and there might be few customers – very few academic labs have funds to cover the real costs of drug discovery through fees. To engage academic labs, the center decided to operate on a collaborative basis, utilizing UCRF funding to cover some of the initial costs of projects to create preliminary data for joint grant applications.
While scientific and technical skills are obviously important to the CICBDD's success, the training in leadership, organizational design, and strategic planning from the time spent in industry (SVF) were the basis for the purposeful design of the center described above. Explicit training in these areas has recently become more accepted in academic medical centers and is a welcomed addition to faculty skills. “An investment in knowledge pays the best interest.” – Benjamin Franklin.
4 What Works Well
All academic centers struggle with developing meaningful quantitative metrics for drug discovery efforts. The ultimate, and perhaps best, measure of impact would be the number of safe, effective medicines approved for significant unmet medical needs, delivered at lower cost than would have been spent in industry. Unfortunately, the long timelines associated with drug discovery make the use of this metric of success impractical. And thus, academic centers must work to imperfect proxy metrics, such as the derivation of clinical candidates, the novelty of the drug target, and the knowledge gained by drug discovery efforts. It should be noted that these long timelines create the same, and perhaps even greater, challenges in industrial drug discovery. As long as the half-life of senior leadership is much less than the cycle time for the organizational change they impose on pharma to meaningfully impact end-to-end productivity in discovery and development of approved medicines, the “tail” of mergers and acquisitions to feed profits will always wag the “dog” of the early discovery engine (117).
With these caveats about metrics, the CICBDD has identified two clinical candidates for previously unprecedented drug targets. One is now progressing through Phase 1 under the auspices of Meryx Pharmaceuticals (http://www.meryxpharma.com) and targets the Mer tyrosine kinase (MerTK) (120), and the other is under consideration for out-licensing. The center also contributed to the creation of IP that helped launch four additional start-up companies from UNC that are actively pursuing drug discovery programs.
Drug discovery projects for unprecedented drug targets usually involve both “validation risks” (likelihood that modulation of the target will have a favorable outcome in patients) and “technical risks” (likelihood that a tolerable molecule can be discovered that modulates the target in patients) (121). Our projects bear risks in each of these categories, and tellingly our most successful project, MerTK, was of lower technical risk due to the accumulated expertise in drug discovery versus the kinase target class and the ability to use structure-guided approaches (121). Over the course of these drug discovery efforts, more than 20 postdoctoral fellows received training in medicinal chemistry, and more than 30 publications resulted. These projects were funded through the CICBDD's participation in the NCI's Chemical Biology Consortium (https://next.cancer.gov/discoveryResources/cbc.htm) and using grants from the National Institutes of Health (NIH).
Our chemical biology program is based on targeting methyl-lysine-binding domains (Kme readers) and methyltransferases. The scientific challenges in probe discovery for Kme readers provided excellent fodder for students and fellows because the probes target a PPI modulated by the eponymous posttranslational modification (PTM). Methyltransferase enzymes were considered inherently more technically feasible than PPIs, but the knowledge base for this class was minimal at the initiation of our program, and we contributed significantly to the progress in the field (122). The methyltransferase project was initiated by Prof. Jian Jin, now at Mount Sinai, as a close collaboration with the SGC, and this and Kme readers program resulted in six high-quality chemical probes for these target classes (114, 121). These probes have been purchased more than 3000 times from commercial vendors and requested via the SGC probe library more than 6000 times. Five students will have soon completed their Ph.D. training based on targeting Kme readers, and more than 10 postdocs also received training in chemical biology as part of this program. Forty papers have been published describing this research, and our chemical probes have been shared freely with many labs around the world, both through the SGC and directly from the CICBDD. Perhaps, the most gratifying aspect of our chemical probe program has been our ability to share probes freely and then see them used as tools in other labs to increase scientific understanding of the proteins they target – an IP-free approach is truly liberating.
Indeed, the creation of chemical probes is ideally suited to academic models of funding, training, and collaboration. Once an initial knowledge base and strategy is in place for a target class, such as Kme readers (121), the efforts of one to two students/postdocs can progress a target to the chemical probe stage during their training. The absence of IP constraints enables sharing of probes with multiple biological collaborators who can test many hypotheses in differing model systems and uncover unanticipated connections between a target and biological consequences of its modulation – often with implications for treatment of disease (https://news.oicr.on.ca/2019/01/new-potential-treatment-for-leukemia-discovered-by-oicr-scientists-draws-major-industry-investment%EF%BB%BF/). While NIH funding is always competitive, the more exploratory nature of probe discovery better fits the expectations of study sections for innovation and creation of foundational knowledge; accordingly, we have been successful in maintaining funding for our Kme reader probe program for more than a decade, and the majority of our NIH funding has come from our chemical probe research. While open-science chemical probe efforts may not have the immediate allure associated with drug discovery, they are in fact one of the best opportunities for chemists, structural biologists, and biochemists to create de-risked drug discovery opportunities. Dozens of proprietary drug discovery programs have been launched from the data generated from open chemical probes created by our center and the SGC.
5 Challenges
“The only people who claim that money is not important are people who have enough money so that they are relieved of the ugly burden of thinking about it.” – Joyce Carol Oates, American author
An ongoing challenge in our drug discovery efforts, and in most other academic drug discovery efforts, is the limited number of target proposals that are novel, have some promise of validity, or are even remotely technically feasible. Of course, risk is always high prior to some investment of effort, and it also takes time; technical de-risking through assay development, hit discovery, and hit-to-lead chemistry is minimally a 2-year endeavor, and biological de-risking of target validity, best achieved using a chemical probe/lead in preclinical models, only adds to these timelines. In other words, good projects do not come knocking on your door; they are almost always created through expenditure of resources, by cultivating internal expertise in a system, and through partnerships with disease experts. One must also distinguish drug discovery, which requires these significant investments in time and intellectual resources, from hit discovery, which is a more generic, platform-based endeavor, and can be achieved with any of a number of approaches, such as high-throughput screening. Our thesis is that to be impactful, academic drug discovery centers should specialize in a target class (121) or a unique technology and thus acquire and apply distinct insights.
Another limitation of academic drug discovery settings, indeed of all drug discovery settings, is that of funding. Grant funding can be used to diminish technical and validation risks, to discover a high-quality chemical probe, and to generate an interesting therapeutic hypothesis, but grant funding is less likely to support a 2–3-year LO effort whose next major scientific payoff – testing the hypothesis in humans – is many years and many millions of dollars down the road. It is understandable that public funders are reluctant to finance such efforts (and are often unqualified to review them), but starting a new company or partnering at this early stage is often equally difficult. For these reasons, initially competitive academic projects can move at such a slow pace that they are no longer in a leading position when a candidate is identified. Alternative funding mechanisms are clearly needed if the traditional, IP-driven model of academic drug discovery is to flourish, and this issue is being partly addressed by firms or funders that aim to fill this gap for LO. These include pharmaceutical companies (https://www.tritdi.org/), contract research organizations (https://www.evotec.com/en/innovate/bridges), and pools of private capital (such as Deerfield's investment into UNC (https://uncnews.unc.edu/2018/10/22/unc-chapel-hill-and-deerfield-management-announce-the-creation-of-pinnacle-hill-to-accelerate-the-discovery-of-new-medicines/). These funding mechanisms may be the solution to enable support for efforts across the spectrum of activities required in drug discovery, activities that cannot be supported by traditional grants.
6 New Approaches
“Ymgasglu, i rannu, er daioni” (Gather, to share, for good) – Robert Gwilym Thomas, Welsh poet.
Whether early-stage drug discovery is carried out in industry or in academia, the core scientific problem is the same – our understanding of disease is too poor to adequately predict the efficacy of a new treatment targeting an unprecedented mechanism, and many exciting therapeutic hypotheses will prove wrong. And whether early-stage drug discovery is carried out in industry or in academia, if the traditional drug discovery business model is pursued, the process will be kept confidential until after patents are filed, the licensor will negotiate for the most lucrative terms, and any resulting drug will be priced as high as the market can bear. In practical terms, there is little to distinguish an academic drug discovery operation from a biotech company – except that academics are using students and fellows as their labor source.
However, we believe that academic drug discovery efforts have one distinct advantage: the public good mandate of these institutions and their associated faculty's commitment to service provide university-based drug discovery efforts the freedom to explore completely new business models – models that might aim for impact, affordability, and accessibility as opposed to maximizing returns on investment. We have decided to explore one such model, in which the data and reagents are shared openly and frequently as the project goes along, and marketing rights of any developed product will be protected by regulatory data exclusivity and not by patents (115). By eschewing patenting, the model both promotes partnerships and reduces the many conflicts of interest that emerge when one tries to maximize financial gain within a publicly funded institution.
The genesis of our open science business arose from two observations. First, we noted that there were massive amounts of funding available in the public sphere. The sum total of global nonprivate investment in biomedical research totals well over $150B per annum. This is an immense pool of funds compared with the ∼$10B that venture capital spends annually in biotech. Second, we were convinced that most academic scientists and institutions would be willing, even delighted, to contribute and align their intellectual capacity and laboratory funding to the discovery of a new medicine under the following conditions: (i) the science must be impactful and have the potential to lead to high-quality publications; (ii) there must not be any restriction on publication of the scientific outputs; (iii) the science must be shared in real time, as the project progresses; (iv) all materials (i.e. chemical probes and pre- and clinical candidates small molecule or antibodies) must be shared with the community as the project progresses, and under terms that restrict actions that may encumber any resulting research (such as patenting); (v) the pricing of the new medicine must be as low as is possible; and (vi) any profits from drug sales must be given away to charity – no participating institution or scientist should receive any compensation (equity or any form of income) from the sales of an approved drug.
This approach might sound overly optimistic of the willingness of institutions and individuals to forego financial rewards, and even naïve, but it has already been implemented in a company called M4K Pharma, and the two-year experience in operating the company has only increased our confidence as to its potential. Our commitment to openness and affordable pricing has attracted dozens of collaborators and resources from both the public and private sectors. We have also observed that scientists in pharmaceutical companies are more than willing to donate their time to consult for the project – a phenomenon that has also been observed by malaria and neglected disease drug discovery researchers. We have seen that every single academic institution with whom we have wished to collaborate, including those in Canada, the United States, Spain, and the United Kingdom, have been willing to forego IP rights in the M4K Pharma projects. Clearly, this model provides a vehicle for many academics to fulfill their desire to make a difference and participate in the development of a new medicine, yet not forsake their commitment to open knowledge generation for the public good.
7 Conclusions
“Live the questions now. Perhaps you will then gradually, without noticing it, live along some distant day into the answer.” – Rainer Maria Rilke, Poet
A dozen years into our collaboration and the life of the CICBDD, we have learned a great deal about the challenges and opportunities in academic drug discovery. While it has proven possible to balance the traditional business model for drug discovery with the training and knowledge-generation mission of an academic center, there are inherent conflicts and the time seems ripe for a new approach. Our open-science chemical probe effort has created new small-molecule tools for unprecedented targets. Importantly, these widely shared tools have proven highly effective to explore new biology and to identify new therapeutic hypotheses, as well as to seed new drug discovery programs – notably without the incentive, the cost, or the encumbrances of patents nor with any hint of sacrifice of our public good mission. Indeed, with the impact achieved by inventing and sharing open science chemical probes, it is increasingly difficult to justify allocating public funding to proprietary drug discovery.
It may be time for academic drug discovery centers to reconsider their mission. In many ways, these centers are structured ineffectively. They are given the aims of a biotechnology company but not the resources. They are embedded within public good institutions, yet are forced to adopt both the proprietary concerns of industry and the financial models of an early-stage corporate investor. Given that there may be a viable path to achieve public good through open science drug discovery, and a mechanism to license assets under terms that promote affordability and access, should not academic drug discovery centers abandon the traditional model for one that seeks to maximize public good and embrace the academic ideals of knowledge creation and sharing? Of course, there is an element of risk. Given that the discovery of completely new medicines often takes decades, there is no evidence now that the open model will be effective. But what are the downsides? At worst, we will generate openly available knowledge and research tools about unprecedented targets and mechanisms, including data about the therapeutic relevance of the target or mechanism in patients, and these will spur others to discover anew or to abandon unproductive paths. At best, our new drugs will achieve clinical proof-of-concept in one or more diseases, and we will then develop the drugs ourselves, or with an industry partner aligned with our values, and provide society with a safe, and affordable, new medicine.
Acknowledgments
We gratefully acknowledge the contributions of scientists in the CICBDD and SGC. Chemical probe work in the CICBDD was supported by the National Institute of General Medical Sciences, U.S. National Institutes of Health (NIH) (Grant R01GM100919) to S.V.F. and the National Cancer Institute NIH (Grant R01CA218392) to S.V.F. The MerTK project was supported by the University Cancer Research Fund, a gift from Dr. Fred Eshelman, and Federal Funds from the National Cancer Institute, National Institute of Health, under Contract HHSN261200800001E. The SGC is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, CHDI, the Bill and Melinda Gates Foundation, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, Merck KgA, Merck Sharp & Dohme, Novartis Pharma AG, Pfizer, São Paulo Research Foundation-FAPESP, Takeda, and the Wellcome Trust. M4K Pharma has received funding from the Ontario Institute for Cancer Research and the Brain Tumour Charity and in-kind support from the SGC, Charles River Laboratories, and Reaction Biology Corp.
Conflicts
S.V.F holds stock in Meryx Inc., which is developing MerTK inhibitors discovered in the CICBDD. Aled Edwards is a chief executive of the SGC and chair of the Board of M4K Pharma.
References
Abstract
The Institute for Therapeutics Discovery and Development (ITDD) was founded in 2007 as part of the College of Pharmacy of the University of Minnesota (UMN). Its mission is to carry out interdisciplinary research, help educate the next generation of scientists, and to enhance the biomedical research infrastructure at UMN. We do this by creating opportunities for drug discovery and early pre-clinical drug development through collaborations between various schools, colleges and research centers, as well as industrial and academic partners throughout the State of Minnesota and nationwide. The institute is divided into five scientific cores: High-Throughput Screening and Assay Development, Lead and Probe Discovery, Medicinal Chemistry, Therapeutics Process Development, and Preclinical Pharmacology. Collaborations can involve a single core or multiple cores. Projects in the ITDD span almost all therapeutic areas including cancer, infectious diseases, contraception, glaucoma, epilepsy, and Alzheimer's disease, among others.
1 Establishment of the ITDD
The University of Minnesota (UMN) has a long history of biomedical innovation, including drug discovery and development. Perhaps most notably, in the 1980s, a family of carbocyclic nucleosides known as carbovirs were designed by Professor of Medicinal Chemistry Robert Vince. In 1992, UMN exclusively licensed a series of patents for these antiviral agents to Burroughs Wellcome (later Glaxo Wellcome, later Glaxo Smith Kline or GSK). In 1998, Glaxo started marketing the new anti-HIV drug abacavir (Ziagen). After a legal dispute over whether abacavir was covered by UMN's patents was settled, royalties from the sales of this life-saving drug eventually brought in over $600 million to the University. This showed that academic drug discovery is not only scientifically rewarding but can also be financially rewarding. In the early 2000s, partially inspired by the success of Ziagen, the UMN made a strategic pivot toward developing the infrastructure to support translational sciences. The general scientific funding environment was favorable as well, thanks to the creation of the new NIH Roadmap program supporting academic translational science, including drug discovery and development. The University decided to create a comprehensive drug discovery and development center as part of the Medicinal Chemistry Department within the College of Pharmacy. This initiative was spearheaded by Dr. Marilyn Speedie, the dean of the College at the time, and Dr. Frank Cerra, who was the head of the Academic Health Center. Professor Gunda Georg was recruited from the University of Kansas to lead the new center named the Institute for Therapeutics Discovery and Development, or ITDD.
The ITDD opened its doors on 8 January 2007 in a newly renovated facility with an initial staff of two dozen scientists and 20 000 sf of premium scientific space. In the following months, the institute director, Dr. Georg, and the associate director, Dr. Vadim Gurvich, who oversaw the facility renovation effort, established four scientific cores and recruited industrially trained scientists as the core leaders. The structure was intended to mirror the typical industrial drug discovery approach. It included a highly automated High-Throughput Screening and Assay Development Core, a Lead and Probe Discovery Core, a Medicinal Chemistry Core, and a Therapeutics Process Development (TPD) Core with a GMP facility. Several years later, a Preclinical Pharmacology Core was added. With its collective expertise among the five cores, the Institute's structure allows the development of a drug candidate starting from a biological target and moving it all the way through preclinical development. The ITDD was also designed to leverage UMN's outstanding basic biomedical research infrastructure and excellent clinical capabilities. Through its highly collaborative network, the ITDD established access to various complementary capabilities, such as X-ray crystallography, computational chemistry, pharmaceutical formulations, bioanalytical chemistry, toxicology, translational medicine, cancer biology, and many other specialties. Within the first few years, the Institute became involved in dozens of internal and external collaborations, including several funded by the NIH. The Institute's flexible business model was designed to accommodate the diverse funding needs of its collaborators and available opportunities from funders. The Institute's strategic goal is to discover, design, and develop drugs that can reach patients. With this in mind, intellectual property protection is at the heart of its activities. Projects in the ITDD are not focused on a single therapeutic area, but rather span a wide range of areas that include cancer, infectious diseases, contraception, glaucoma, epilepsy, and Alzheimer's disease, among others.
2 Organization of the ITDD
The ITDD is housed within the Department of Medicinal Chemistry at the UMN. One of the primary benefits to this organizational structure is that graduate students in the Department have exposure to modern methods of drug discovery that they might not experience elsewhere in their training. Additionally, undergraduate and graduate students from other departments often work in the ITDD laboratories, thus gaining valuable experience. Each of the core directors in the ITDD has an unpaid research faculty appointment in the Department of Medicinal Chemistry. In general, the directors are not part of the graduate faculty of the Department but teach in the graduate and professional programs in the College of Pharmacy, participate in departmental and collegiate committees, and informally mentor students.
The ITDD is organized into core groups, much like those that exist in biotechnology companies. There are five cores in the ITDD: (i) High-Throughput Screening and Assay Development, (ii) Lead and Probe Discovery (LaPD), (iii) Medicinal Chemistry, (iv) TPD Core, and (v) Preclinical Pharmacology. The cores allow the ITDD to work on a variety of projects ranging from assay development and optimization to scale-up and GMP manufacturing for Phase I and II clinical trials. The cores are staffed by permanent scientific personnel and also by graduate students and postdocs. Projects that enter the ITDD can involve either a single or multiple cores.
2.1 High-Throughput Screening and Assay Development
This core develops 384-well plate assays and conducts high-throughput screening, fragment screening, and high-content screening and performs hit characterization and SAR-generating assays. There are three essential components of this group: (i) a structurally diverse high-quality library of compounds for screening purposes (currently ∼330 000 compounds in both diverse and focused subsets), (ii) liquid handling and plate readers necessary to develop and perform almost any assay, and (iii) the assay development expertise to bring the two other components together. Having all three of these components is critical to the discovery of new chemical matter as starting points and subsequent SAR studies for lead optimization. The assay capabilities of the HTS Core range from fluorescent and luminescent biochemical and cellular assays for HTS to biophysical assays such as differential scanning fluorimetry (DSF), surface plasmon resonance (SPR), and isothermal calorimetry (ITC) for fragment screening and hit characterization. The HTS Core conducts screening and SAR assays for a broad range of therapeutic areas and target classes, including kinases (e.g. CDK2), other enzymes (e.g. guanylate cyclase), protein–protein interactions (e.g. FANCM), ion channels (e.g. CatSper), nuclear hormone receptors (e.g. RAR), transporters (e.g. ANT), and epigenetic readers (e.g. BRDT) as well as phenotypic screens (e.g. synapse formation).
2.2 Lead and Probe Discovery
The main function of the LaPD Core is to assist in the triage of the results of assays in the HTS Core. The LaPD also provides parallel medicinal chemistry, protein production and purification, small-molecule purification and analytical services, and cheminformatics and computational support for projects. One unique specialty of the LaPD is its ability to determine the causes of assay interference using a variety of counterscreens including aggregation, reactive-thiol, and ALARM NMR assays. Dr. Michael Walters, the director of the LaPD, helped bring the concept of PAINS (pan-assay interference compounds) to a broader scientific audience with his copublication of an article with Dr. Jonathan Baell, the coiner of the term (123). Dr. Baell had described the concept of PAINS in 2010. This joint manuscript brought the PAINS to the attention of medicinal chemists and high-throughput screening scientists around the world and sparked a controversy in the field. Dr. Walters' recent article on “The Essential Medicinal Chemistry of Curcumin” that focuses on the assay interference potential, IMP-like (invalid metabolic panacea) nature, and bioactivity of the titular compound is the most viewed article in the history of the Journal of Medicinal Chemistry (124).
2.3 Medicinal Chemistry
The Medicinal Chemistry Core is the largest of the five cores because academic and start-up groups typically need synthetic services more often than the services the other cores provide. The Medicinal Chemistry Core is primarily involved in the optimization of active compounds into leads. It also has ligand–protein modeling capabilities using standard software suites. One of the core's specialties is the design and development of prodrugs, and several of the Institute's most advanced projects involve the application of prodrugs to enable the use of previously discovered drugs or natural products in new indications (see Section 3).
2.4 Therapeutics Process Development
The TPD Core scales up compounds for use in animal pharmacology studies and ultimately clinical studies. It has GMP (good manufacturing practices) capabilities rarely found in an academic setting. The TPD Core can perform analytical and stability studies required for FDA investigational new drug submissions and produce clinical supplies to support early phases of clinical trials.
2.5 Pharmacology
Early studies on pharmacokinetics (PK) and pharmacodynamics (PD) are enabled by the Pharmacology group of the ITDD. This work is aided by the extensive expertise and services offered by the Research Animal Resources group sponsored by the UMN.
Beyond the ITDD, many other groups support translational drug discovery at UMN. These span the spectrum from other cores that support basic science, such as the Mouse Behavior Core in the Department of Neuroscience, to groups that direct funding to translational projects, such as the Committee on Pharmaceutical Development which is part of the UMN Clinical and Translation Sciences Institute (CTSI). Additional key resource groups are the Center for Translational Medicine (CTM) that performs preclinical animal studies and the Technology Commercialization (TechComm) office that connects inventors to industrial partners and manages the UMN's intellectual property.
Currently, about one-quarter of the ITDD's infrastructure and personnel costs are supported financially by the College of Pharmacy, but the bulk of its funding comes from federal, state, and private grants and contracts. Philanthropic funding has also become increasingly important over the past decade.
3 Project Examples
3.1 Minnelide: A Prodrug of the Natural Product Triptolide with Strong In Vivo Anti-Cancer Activity
Triptolide is a natural product isolated from the plant Tripterygium wilfordii used in traditional Chinese medicine to treat rheumatoid arthritis and inflammation. It has also been reported to have potent anticancer activity in vitro against a number of cancers. Our collaborator, Dr. Ashok Saluja, then with the Department of Surgery at UMN, was particularly interested in Triptolide's strong activity against pancreatic cancer. However, Triptolide's poor aqueous solubility, narrow therapeutic window, and poor patentability as a natural product made clinical development of triptolide extremely challenging.
We were able to overcome these difficulties by designing and developing the prodrug minnelide (Figure 11), which incorporates a phosphonooxymethyl group that greatly increases its water solubility (125). This promoiety allows for rapid and reliable cleavage in vivo, releasing Triptolide and formaldehyde after activation with alkaline phosphatase. Minnelide showed very good efficacy at low doses against multiple animal models of cancer, including human colon adenocarcinoma (Figure 12). Further studies showed similar results in models of pancreatic cancer (126).
Source: From Patil, S., Lis, L. G., Schumacher, R. J., Norris, B. J., Morgan, M. L., Cuellar, R. A. D., Blazar, B. R., Suryanarayanan, R., Gurvich, V. J., & Georg, G. I., Phosphonooxymethyl Prodrug of Triptolide: Synthesis, Physicochemical Characterization, and Efficacy in Human Colon Adenocarcinoma and Ovarian Cancer Xenografts. Journal of Medicinal Chemistry, 58(23), 9334–9344. © 2015. American Chemical Society.
The discovery and development of minnelide at the ITDD was supported primarily through internal UMN grants, including funds from the Masonic Cancer Center, the College of Pharmacy, and the Academic Health Center. Collaborations between the ITDD and other UMN translational groups, such as the CTM, enabled the internal development of minnelide through the IND-preparation stage. At that point, minnelide was licensed to Minneamrita Therapeutics. It is currently in Phase I and II clinical trials.
3.2 CKLP1: A Water-Soluble Prodrug with In Vivo Ocular Hypotensive Activity
Elevated intraocular pressure (IOP) is a major risk factor for glaucoma, and most treatments designed to slow the progression of glaucoma focus on reducing IOP. Our collaborator, Dr. Michael Fautsch of the Mayo Clinic, identified adenosine triphosphate-sensitive potassium (KATP) channels as key cellular effectors of IOP. He found that administration of KATP channel openers such as diazoxide or cromakalim led to significant decreases in IOP in a normotensive mouse model. These drugs also lowered IOP in cultured human anterior segments. Utilizing pharmacologic KATP channel openers with selective subunit specificity, and a combination of immunohistochemistry and treatment of Kir6.2(−/−) mice, Dr. Fautsch determined that the IOP-lowering effect of KATP channel openers was due to their action on KATP channels that comprised SUR2B and Kir6.2 subunits.
However, cromakalim and diazoxide have limited aqueous solubility, and in his initial in vivo studies Dr. Fautsch used DMSO and Cremophor EL to solubilize the KATP channel openers and permeabilize the cornea so that the drugs could be administered topically. Because the toxicity of DMSO makes its clinical use in eye drops undesirable, we prepared a series of water-soluble derivatives of KATP channel openers and worked with Dr. Fautsch to evaluate whether they could be administered topically to lower IOP (127).
The compound that we identified with the best mix of efficacy, solubility, stability, and synthetic scalability was CKLP1 (Figure 13), a prodrug in which a phosphate group is directly attached to the alcohol moiety of cromakalim. CKLP1 was highly water soluble (>10 mg/mL), allowing it to be administered in a simple phosphate-buffered saline (PBS) vehicle. Once daily, CKLP1 showed good IOP-lowering efficacy in normotensive mice (Figure 14) as well as in rabbits, monkeys, and dogs. It also worked to lower IOP in an additive manner with existing glaucoma therapeutics (128).
Source: From Roy Chowdhury, U., Viker, K. B., Stoltz, K. L., Holman, B. H., Fautsch, M. P., & Dosa, P. I., Analogs of the ATP-Sensitive Potassium (KATP) Channel Opener Cromakalim with in Vivo Ocular Hypotensive Activity. Journal of Medicinal Chemistry, 59(13), 6221–6231. © 2016. American Chemical Society.
Funding for this project was initially obtained via grants from the Mayo Clinic and the UMN Office of Discovery and Translation, followed by a larger grant from the Minnesota Partnership for Biotechnology and Medical Genomics. The Partnership is a program funded by the State of Minnesota designed to encourage collaborations between UMN and the Mayo Clinic. Once a potential lead compound was identified, we secured an additional grant from the Partnership to fund development work on the compound. CKLP1 was licensed in early 2019 to a start-up company that plans to begin human clinical trials with the compound in 2021.
3.3 Development of Gut-Restricted Bile Acid Analogs Designed to Inhibit Clostridium difficile Spore Germination
Clostridium difficile is a spore-forming bacterium that can cause a potentially lethal infection of the colon. Clostridium difficile infection (CDI) normally occurs after a course of antibiotics disrupts a patient's intestinal flora, allowing spores of C. difficile room to germinate and propagate in the colon. The typical treatment for CDI is an additional round of antibiotics. However, this causes further disruption to the patient's indigenous microbiota, and approximately 20–40% of patients relapse.
Rather than finding new ways to eradicate vegetative cells, our approach to solving the problem of recurrent CDI is to focus on preventing C. difficile spores from germinating in the colon and reestablishing the infection after the cessation of antibiotics. Spores of C. difficile use cues from their environment to determine if they are in the right location to germinate. In particular, the bile acid taurocholic acid (TCA) promotes spore germination, while derivatives in the chenodeoxycholic acid (CDCA) and ursodeoxycholic acid (UDCA) families typically inhibit germination. We have synthesized a large set of CDCA and UDCA derivatives and evaluated their ability to prevent TCA-promoted spore germination in vitro. We have identified several synthetic compounds that inhibit germination significantly more potently than naturally occurring compounds (129). We then focused on incorporating features into these molecules that reduce their uptake from the digestive tract into enterohepatic circulation by working to block both the active and passive transport mechanisms (Figure 15). We are currently evaluating our compounds in animal models of CDI to determine their efficacy.
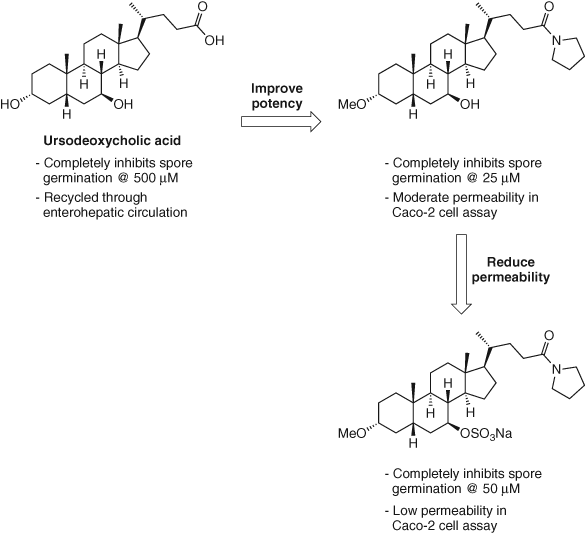
To advance this project we have brought together a project team that includes Dr. Alex Khoruts, a gastroenterologist who regularly treats CDI patients and has access to clinically relevant strains of C. difficile, and Dr. Michael Sadowsky, an experienced microbiologist who has the facilities necessary to work with the infectious disease. The program was initially funded by a grant from the UMN Academic Health Center (AHC) that was specifically designed to encourage collaborations between investigators in different colleges of the AHC. Our continuing research in this area is funded through a pair of grants from the US Department of Defense.
3.4 A Prodrug/Activating Enzyme Delivery System to Treat Epileptic Seizure Emergencies
A key advantage of academic drug discovery is that the university environment can sometimes bring together project teams that would not naturally occur in an industrial setting. This can lead to solutions to problems that would not be arrived at by a single researcher working alone or even a team working in a corporate environment. One example of this is our project to develop diazepam derivatives that can be delivered nasally during epileptic seizures. The project team members include Drs. Gunda Georg (a medicinal chemist), James Cloyd (an expert in epilepsy and orphan diseases), Ronald Siegel (Pharmaceutics), and Edward Paterson (Veterinary Medicine).
Benzodiazepines derivatives such as diazepam are used clinically for the treatment of seizure emergencies. In many cases these drugs are administered intravenously, which can lead to delays in treatment, especially when seizure emergencies occur outside of a hospital setting. Rectal administration can also be effective but is hindered by poor patient compliance. Nasal delivery is an attractive alternative to these other routes of administration, but the limited volume of the nasal cavity requires dosing with highly concentrated solutions. Diazepam is not water soluble enough to be administered nasally in aqueous solution, and the use of organic cosolvents causes nasal irritation and pain. One solution to this problem is to administer a water-soluble prodrug of diazepam, such as avizafone (Figure 16). When administered by injection, avizafone is cleaved enzymatically to intermediate 1, which is then chemically converted to diazepam. Unfortunately, the nasal cavity has insufficient amounts of enzymes available capable of rapidly activating avizafone. To overcome this problem, the project team developed a dual-delivery system that administered avizafone along with an enzyme to cleave the promoiety. The resulting supersaturated solution of diazepam was highly permeable (130), and excellent PK properties were observed upon nasal application in rats, providing in vivo proof of principle. This project was funded through a mix of UMN internal grants and philanthropic and foundation funds. Efforts to commercialize the discovery are underway.
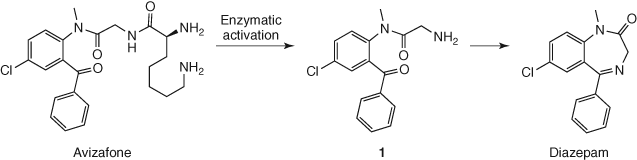
3.5 Discovery of Nonhormonal Male and Female Contraceptive Agents
A major focus of the ITDD is the discovery of nonhormonal contraceptive agents to regulate male fertility by pharmacological means. Sixty years after the female birth control pill came to market, a similar option still does not exist for males, who are restricted to condom use and vasectomy. To provide couples and men with alternative methods for birth control, the ITDD has been engaged in targeting multiple validated contraceptive targets in collaboration with reproductive biologists. One such genetically validated target is the testis-specific plasma membrane alpha 4 isoform of Na,K-ATPase. In collaboration with Professor Gustavo Blanco from the University of Kansas Medical Center, we have developed a picomolar inhibitor of the alpha 4 Na,K-ATPase that is a completely selective and orally bioavailable analog of the natural product ouabain (Figure 17). The analog reduces both total and progressive sperm motility in rats to levels that are predictive of male infertility according to WHO standards (131).
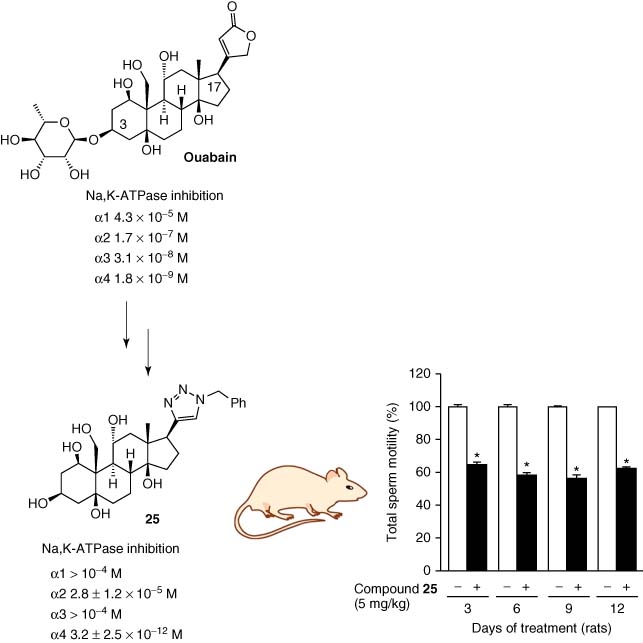
Source: From Syeda, S. S., Sánchez, G., Hong, K. H., Hawkinson, J. E., Georg, G. I., & Blanco, G. (2018). Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPase α4 Isoform Inhibitors for Male Contraception. Journal of Medicinal Chemistry, 61(5), 1800–1820. © 2018. American Chemical Society.
The ITDD is currently (2017–2021) the recipient of a U54 Center grant from the NICHD Contraception Research Branch to discover and develop male contraceptive agents. We are developing selective antagonists for the retinoic acid receptor alpha in collaboration with Dr. Debra Wolgemuth from Columbia University; inhibitors of the testis-specific bromodomain BRDT with Dr. Jun Qi from the Dana-Faber Cancer Institute; and inhibitors of testis-specific serine/threonine protein kinases (TSSKs) with Dr. Pablo Visconti from the University of Massachusetts, Amherst. Protein crystallography is supported by Dr. Ernst Schönbrunn from the H. Lee Moffitt Cancer & Research Institute. The U54 Center also involves a behavioral research component led by Dr. Jennifer Barber from the University of Michigan.
4 Lessons Learned
Over the past 12 years, many lessons have been learned regarding the best practices for the operation of a drug discovery and development institute at a public university in the Upper Midwest.
4.1 Good Collaborators Are Key
Perhaps unsurprisingly, collaborators who are able to effectively contribute to the project can make the difference between a successful project and disappointment. We have also found that collaborators who understand and appreciate the complexities of drug discovery and development are the best partners. However, most researchers in an academic environment will not initially have a practical understanding of drug discovery and development. Another potential stumbling block is that principal investigators often find it impossible to give up control of the medicinal chemistry aspects of a project. This can get in the way of effective collaborations.
4.2 Drugs are not the Only Successful Output
It may go without saying that making drugs is a difficult process and is fairly elusive in the academic setting. In many cases, the development of a biochemical probe that may help better understand the mechanism or disease relevance of a biological process can also be regarded as a success.
4.3 Communication is Very Important
The need for effective communication between laboratories and specialties in industry is dealt with head-on using standard project policies and procedures. These policies and procedures do not exist in academia. We recommend, therefore, that collaborative projects start with a written charter outlining the expectations for communications and data exchange between collaborators working on the project.
4.4 Operating as a Team is Only Possible with Full Team Funding
Nothing stalls a project better than to have key collaborators who are unfunded or underfunded. Usually, this means long delays in experiments and data communication that may slow down a project for years. Do not rely on unfunded collaborators on the critical path to desired outcomes.
4.5 An Entrepreneurial Mindset is Important
Projects and collaborators will not typically show up at your office door, nor will funds magically appear in your mailbox. Extensive networking at all levels (department, college, university, state, and nationally) will be necessary to bring forward the best projects. After the best projects are secured, the entrepreneurial mindset will help (though not guarantee) funding to bring the projects toward well-established goals. Finally, significant determination is often needed to bring even the best of drug candidates to the attention of potential investors and licensees.
4.6 Flexibility is a Virtue
The best-laid plans of mice and research scientists ofttimes go astray. Because of the typically slow nature of academic drug discovery, persistence and flexibility are true virtues that will make or break a project. Assays might not always be up and running, mice may not show up when planned, and experiments may take six months or more to execute. Flexibility means that it may be necessary to chart many paths to success and not to be too rigid in following the screening tree exactly every time and in every situation.
4.7 Patience is Required
The flexibility described above is founded on a base of patience. The progress of academic drug discovery, with its fits and starts and stops, is often especially exasperating to institute directors who are accustomed to industrial standards of productivity. This is just the nature of doing what is, at its best, a highly organized, interdisciplinary endeavor in an arena that often is not. Sometimes effectively managing one's patience quota requires that even good projects be dropped.
We have found that all the necessary resources for successful projects are available at the UMN, but getting everyone working toward the same goals is challenging: The UMN, as a large university, has researchers and laboratories that have the expertise to perform all aspects of drug discovery and development. Getting all of these resources together at the same time and rowing in the same direction can be difficult. The solution? Choose your partners wisely and consider outsourcing (as funding permits) those tasks that can be performed more efficiently externally.
5 Features of Successful Projects
- All partners on the project have a clear understanding of the target and its biological liabilities.
- The project has clearly defined plan and success points along the translational pathway. These may be publications, patents, probes, etc.
- The project features a culture of active and respectful communication between all collaborative partners.
- The best projects involve partners who understand the drug discovery process or are willing to learn the basics.
- Partners have an appropriate appreciation of the timelines and rate of success of drug discovery projects.
- The project partners trust each other as scientists and deserve that trust.
- All key partners have sufficient funding, and the project has a funding strategy that considers a wide variety of sources.
References
Abstract
Chemical Biology Consortium Sweden (CBCS) is a national infrastructure for chemical biology in Sweden. CBCS aims to assist research groups to identify and develop small molecules that can interact with biological processes for their further use as chemical research tools. High quality basic research in this field of chemical biology is a critical foundation for Life Science innovations, with emphasis on medicine and health – drug discovery, but also more unexplored potential in plant sciences and environmental research. Starting in 2010, CBCS provides services in assay development, access to and screening of small molecule compound libraries, and follow up chemistry support. CBCS support in these areas enable high quality research, made available through open access publications, and cross-fertilization between universities and industry in the life sciences arena.
1 Mission
Chemical biology is an evolving, multidisciplinary field at the interface of chemistry and biology. Detailed definitions of chemical biology research are still being discussed (132) but in general involve manipulation and interrogation of biology using chemical entities. One subcategory of chemical biology concerns the generation and validation of new small-molecule-based tool compounds. These serve as excellent tools to modulate biological systems and possess unique characteristics that nicely complement some of the shortcomings with modern precise genome editing. Needless to say, high-quality basic research in this field is a critical foundation for life science innovations, with emphasis on medicine and health, and with more unexplored potential in plant sciences and environmental research. However, access to well-characterized molecules with appropriate biological selectivity is limited to a small portion of the proteome, such that significant investments are motivated to broaden the repertoire of accessible biologies.
Chemical Biology Consortium Sweden (CBCS, http://www.cbcs.se, (133)) was inaugurated in 2010 as a distributed national infrastructure for chemical biology in Sweden. The mission of CBCS is to perform collaborative research with research groups in Sweden, aiming to discover, optimize, and use new chemical tools for exploration of complex biology. CBCS aims to enable high-quality basic research in this field, generate open access publications, and foster cross-fertilization between universities and industry in the life sciences arena. Although CBCS' mission is primarily focusing on enabling basic life science research, the operations have after 10 years led to an excess of 130 co-publications and 11 patent applications and has served as the basis of the formation of six start-up companies.
2 Description
2.1 How CBCS was Formed and Organization Today
CBCS was formed in 2010 after an initiative by employees of the pharmaceutical company Biovitrum (former Pharmacia and Upjohn). This led to the establishment of a chemical biology research node at Karolinska Institutet, including a consolidation of already existing chemical biology infrastructures in Sweden at Umeå University and Uppsala University (134). In 2013, CBCS became part of SciLifeLab, a national center for molecular biosciences (135), and is now part of a combined platform for chemical and genetic perturbations of biological systems. Today, CBCS comprises a multidisciplinary team of 16 staff scientists in areas spanning from biochemistry and cell biology, organic/medicinal/enabling/computational chemistry to compound logistics and management (see Figure 18 for a schematic illustration of CBCS operations).
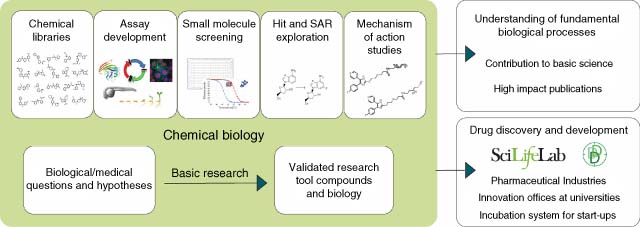
As a complement to CBCS, SciLifeLab established a dedicated platform for drug discovery and development (DDD) in 2014 (136) with the primary goal to support academic research projects with improved translational potential through offering of industry standard services, both in small-molecule projects and also using other drug modalities. The combined chemical biology and drug discovery platform provides a fantastic opportunity for Swedish academics to initiate a chemical biology-related research project, which later can extend into the drug discovery value chain and mature into a lead therapeutic for further development. In fact, several projects that were initiated and validated at CBCS have later continued their drug discovery journey within the SciLifeLab DDD platform (137).
3 CBCS Services
Most CBCS projects are characterized by a combined basic research and human health application focus, and a large portion of the users have a strong strive to understand disease biology and a wish to advance their projects toward drug discovery. CBCS provides services in assay development – both biochemical and cell-based assays, access to and screening of the SciLifeLab compound collection and medicinal and computational chemistry for validation, structure–activity relationship (SAR) exploration and optimization of hit compounds, and chemistry for mechanism of action elucidation efforts (chemical proteomics) (Figure 18). The goal is to generate validated compounds and targets – assess “ligandability” of pathways and targets – and create a foundation toward initiation of drug discovery projects.
3.1 Biological Assay Development
Assays developed for screening purposes can be either biochemical (isolated protein targets) or cell based (targeted to specific mechanisms or phenotypic). The quality, reproducibility, and feasibility of the bioassays are of utmost importance for a successful screening project. CBCS staff have extensive experience in assay development from CBCS years of operation and from industrial experiences. The combination of this capacity with the individual users' expertise in the scientific research field allows for relevant, innovative, and informative bioassays to be tailored for each scientific enquiry. In the current operating model, users refines their crude assay concept under guidance of CBCS staff in our laboratories, thus enabling parallel service and user education in principles of assay design and development.
3.2 Compound Screening
The screening process involves parallel bioactivity evaluation of a large array of compounds by applying the developed bioassay(s). In contrast to large, integrated screening platforms, CBCS uses multiple parallel screening workstations serving dedicated tasks. This approach results in a greater flexibility to address several protocols at once, a lower cost to the user, and a reduced risk of technical failures. This is particularly important because many of the screens carried out at the CBCS premises rely on complex assays that cannot be adapted to the typical screening setups used within the pharmaceutical industry. In addition, CBCS screening workstations can work either individually or in concert to handle larger screening initiatives (up to 50 000 compounds per day); the throughput is thus not the primary limiting factor in an academic setting.
3.3 Hit Validation
Solid validation of bioactive compounds from compound screens is crucial and commonly insufficiently emphasized. Although small molecules are excellent tools for modulation of biological systems, there are also many examples of “pan assay interference substances” – referred to as PAINS (138) or in other way promiscuous and unwanted compounds. These may act through reactive mechanisms and undesirable interference with the assay readout. CBCS has experience in identifying unwanted compounds and an established repertoire of follow-up assays to ensure a sound pharmacology.
After a screen, CBCS selects hit compounds for a three-points dose–response as a hit validation step and performs suitable counterscreens (i.e. with/without assay reagent and protein concentration variations) to remove false positives. Of high importance in this process is also the history of results from the many screens performed within CBCS alongside routines for assessment of the quality of hit compounds. This allows for direction of efforts toward compounds with suitable biological activity. The remaining hits (and potential selected analogs from CBCS larger compound collection) are then tested in full concentration–response studies with fresh compound solutions to verify the pharmacological response and to identify initial SARs.
3.4 Enabling Chemistry
As mentioned, CBCS aims to develop high-quality research tool compounds and to ensure that correct conclusions are drawn based on established data. To achieve this, the validated hit series of compounds emerging from a screening campaign generally require further optimization and verification of SARs. However, while the collaborating research groups are generally experts on their biological systems, they frequently lack this ability. CBCS' expertise in organic and medicinal chemistry has therefore proven to be very important to advance the projects toward validated research tools and starting points for drug discovery efforts. In addition, CBCS' chemistry experience is also important in the selection of hit compounds from the screen.
3.5 Computational Chemistry and Modeling
The selection and development of targeted compound collections, the prioritization of suitable screening sets, in silico profiling of hits and the follow-up chemical exploitation of hits, and the accompanying cheminformatics are dependent on computational chemistry support. In addition, the diversity of research projects and ideas that CBCS encounters requires multiple strategies besides conventional small-molecule screening projects, many of which are based on computational techniques. CBCS has therefore acquired expertise in this area including structure- and ligand-based design, statistical molecular design, pharmacophore modeling, docking, and virtual screening.
3.6 Compound Libraries
The success rate of screening campaigns is highly dependent on the quality of the applied compound collection. CBCS houses more than 200 000 small molecules which are chemically and structurally diverse and routinely quality controlled by analytical methods. The collection is based on an initial donation from the former pharmaceutical company Biovitrum and has during the years of operations been expanded with sets from different commercial vendors and other donations from biotech companies. The strategy has been to build a modular collection that enables selection of subsets tailored to the needs of each academic screening project as dictated by assay throughput and cost per data point for the user. Besides the structurally diverse primary screening set of 35 000 compounds, the collection contains modules such as targeted compounds (kinases, G-protein-coupled receptors, nuclear receptors, agrochemicals, and antimicrobials) and annotated research tool compounds including approved drugs, known as chemogenomics compounds. CBCS runs a “Compound Center” (https://compoundcenter.scilifelab.se/, (139)) with automated storage systems, where compounds are housed under controlled conditions. The Center maintains and makes available robotic handling as well as acoustic dispensing equipment. This secures reliable distribution and delivery of nanoliter amounts of compounds and screening sets to CBCS, the drug discovery development platform, and their respective users.
4 CBCS Operational Model
4.1 Operational Model
The most successful projects are based on a tight collaboration with the academic research group. Research groups interested in comprehensive CBCS services comprising assay development, small-molecule screening, and synthetic chemistry activities are defined as a “large collaborative project,” and principal investigators (PIs) have to send in an application to a CBCS Project Review Committee (PRC). In addition, CBCS assists in small collaborative projects that require limited resources, e.g. use of instrumentation/compound libraries or intellectual input. CBCS projects are categorized according to the type of project: isolated protein, targeted cell based, phenotypic cell based, plant biology, technology development, diagnostic tool, and others (i.e. ADME profiling and chemistry analysis); see project distribution in Figure 19.
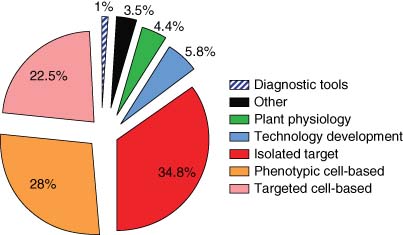
- Overall biological rational and potential scientific impact
- Plans and availability of assays and models for validation of research tool compounds
- Technical feasibility for CBCS and the user
- CBCS resource requirements
- The importance of CBCS efforts
- Publication strategy
4.2 Project Review Committee
CBCS uses an unbiased PRC for the evaluation and ranking of project proposals submitted to CBCS based on the defined criteria (see box above). The committee has been operational since 2011 and ranks project proposals twice a year, which forms the basis for CBCS resource allocations. The PRC consists of experts in the chemical biology fields, all of which are independent of the CBCS infrastructure and include industry representation. With this procedure, CBCS secures that the available resources are used in a transparent and unbiased way.
4.3 Large Collaborative Projects
The fact that large collaborative projects are given resources based on the PRC evaluation leads to that only technically “feasible” projects with a high potential scientific impact enter CBCS. Currently, research groups can apply for assay development, small-molecule screening, or synthetic chemistry activities such as chemistry follow-up or synthesis of affinity/imaging probes. Despite the fact that a project may include all three of these parts, it has proven important to separate them and to set milestones with regard to avoiding an accumulation of lengthy research projects. The division between assay development and screening is intended to highlight the importance of a high-quality assay that is compatible with screening. It further helps to avoid expectations of immediate screening results that end up in years of assay development before the screen can be even considered. CBCS encourages active participation in the assay development and screening of members from the research groups, which also impact an important educational aspect.
A project may also apply for support from a chemistry perspective. This often involves chemistry optimization to improve potency and other preclinical properties. The increase in applications for cell-based phenotypic screens has increased the interest in target identification efforts, which often require chemistry modifications, e.g. for producing affinity labeling probes or imaging probes.
Before a large project is initiated, a detailed project plan with stop–go criteria is set up and agreed upon between CBCS and the PI. This is of importance to avoid commitments in projects that run into obstacles out of CBCS control and to have mutual expectations. The project plan may run over a maximum of two years.
4.4 Small Collaborative Project
CBCS is frequently contacted by research groups that need rather limited support from the infrastructure. These projects do not need a PRC application but are supported based on resource availability and “first come, first served” and may use a maximum of two weeks resources. The projects mostly include PIs that can perform the screening campaign in their own lab. Here, the PI only needs access to a screening collection that can be supplied by the Compound Center in assay ready plates. As a continuation of this, another example of small projects may be limited chemistry support and consultation on the identified hits. A commonly underestimated task in projects that are performed outside of CBCS is hit evaluation. This often makes appropriate support difficult, e.g. to avoid that pan assay interfering compounds are pursued.
4.5 Cost Model
CBCS user fee model is based on the philosophy that access to the infrastructure is not for free. Instead, it is partly subsidized to ensure access for a broad user base, i.e. services are not restricted to well-financed research groups. As a general principle, the user covers all materials and consumables needed for the specific project, including a compound access fee for compound library plating.
4.6 Technology Development Projects
On top of the user-initiated projects, CBCS performs technology-based method development to refine and enhance its service portfolio. This work can in some cases be project driven and performed within projects approved by the PRC, claiming approximately 20% of the individual CBCS researchers' time. As CBCS labs are located in the vicinity of leading experts within chemical biology at the host universities and at SciLifeLab, CBCS provides a unique opportunity to perform method development in collaboration with both scientists and other infrastructures.
4.7 Infrastructure Accessibility and Publication Output
A main objective for CBCS is to be available in a transparent way to all users in the Swedish research community. Accordingly, CBCS has engaged in collaborative projects resulting in a project portfolio of >400 projects in various stages from ∼236 individual users between 2010 and 2018 with users from all major universities with a national spread, see Figure 20, but focus on the location of CBCS nodes. User interest in CBCS services has grown steadily during this period with approximately one new project discussion meeting, often with a new PI, per week. In these meetings, including CBCS experts with different expertise, all potential users are provided with useful input to their projects. Thereof, 25% results in full PRC applications, whereas other projects get CBCS limited support through a small project. CBCS publications normally have a lag time of a couple of years after project completion and have currently reached a steady state of about 20 publications annually. Besides the publications and educational impact, CBCS operations have generated more than 11 patent applications and the start-up of six companies.
5 CBCS Projects – Examples
We have chosen to exemplify two projects representing the different CBCS services: (A) This is a collaborative project that started with a phenotypic screening campaign in primary cells and altogether required significant assay development, screening, and chemistry support for elucidation of the mechanism of action of low nanomolar compounds (Figure 21). This project has resulted in a first publication (140) and awaits disclosure of a more complete study. The project has also generated patent applications, and the project is now being pursued by a biotech company. (B) This represents a major technology development around the cellular thermal shift assay (CETSA), which started as a user-driven project and lead onto internal technology development within CBCS (Figure 22). The overall project has so far generated six CBCS publications and have significantly impacted the business of a Swedish biotech company Pelago Bioscience. Further developments are still ongoing in this active project.
Source: Based on Molina DM, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science (80- ) [Internet]. 2013 [cited 2019 Jul 5];341:84–7.
(b) High-throughput adaption of CETSA using AlphaScreen® and first large-scale compound screening using CETSA.Source: Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundb\xE4ck T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–22; Almqvist H, Axelsson H, Jafari R, Dan C, Mateus A, Haraldsson M, et al. CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil. Nat Commun [Internet]. 2016 [cited 2019 Jul 5];7:11040.
(c) Comparison of CETSA data with functional cellular responses.Source: Seashore-Ludlow, B., Axelsson, H., Almqvist, H., Dahlgren, B., Jonsson, M., and Lundbäck, T. (2018). Quantitative Interpretation of Intracellular Drug Binding and Kinetics Using the Cellular Thermal Shift Assay. Biochemistry 57: 6715–6725. doi: 10.1021/acs.biochem.8b01057.
(d) Intracellular drug binding and localization using high-content imaging.Source: Images are adapted/reprinted from the respective journal articles. Source: Axelsson H, Almqvist H, Otrocka M, Vallin M, Lundqvist S, Hansson P, et al. In Situ Target Engagement Studies in Adherent Cells. ACS Chem Biol. 2018;13:942–50; Axelsson H, Almqvist H, Seashore-Ludlow B. Using High Content Imaging to Quantify Target Engagement in Adherent Cells. J Vis Exp [Internet]. 2018 [cited 2019 Jul 10];e58670.
A. Exploring the druggability of Diamond-Blackfan anemia: Johan Flygare at Lund University has an interest in a rare hereditary disease called Diamond-Blackfan anemia (DBA). The mechanism of this bone marrow failure syndrome, caused by a mutation in ribosomal proteins, is poorly understood but involves reduced proliferation of erythroid progenitor cells. Johan Flygare contacted CBCS in 2012 and asked for guidance in a screen for small molecule that could rescue the viability of erythroid precursors and use such compounds and mechanisms to explore DBA biology.
Together with CBCS, a phenotypic assay was developed using fetal liver erythroid progenitors from a doxycycline-inducible DBA mouse model (see Figure 21). The addition of doxycycline to the culture medium induces the growth defect phenotype and reduces proliferation to <10% of normal such that rescue of proliferation could be used as a simple readout for screening. The primary screening of the CBCS collection, which at that time amounted to 10 500 cmpds, was followed by hit validation activities and generated several hit series of which one was particularly promising showing a crude SAR. Based on these data, the PI could apply for a follow-up project aiming at elucidating the mechanism of action of this hit series, and the project was transferred from a biology-oriented to a chemistry-focused program.
Substantial “detective work” was carried out by the chemists including in-depth literature and database mining, synthesis of hit series analogues and known reference compounds, and multitarget assay panel testing. Making a long story short, this resulted in a proposed target responsible for the rescuing effect of proliferation of the mutant mice erythroid progenitors. The rescue effect for this and other chemotypes for the same target was later validated using patient-derived cells. Based on these findings the researcher applied to the SciLifeLab DDD platform, which supported the filing of a patent and initiation of a collaboration with a biotech company that is presently pursuing this project toward the clinic.
B. Method development around the cellular thermal shift assay (CETSA): The ability to directly verify the interaction of small molecules with their putative protein targets in native cellular environments is a critical step in elucidating and validating pharmacological concepts. The CETSA was developed as a novel biophysical approach to investigate ligand binding in intact cells (141). CETSA is based on the principle of increased thermal stability of proteins upon (concentration-dependent) ligand binding and its detainments in the soluble fraction of cell lysis fractions after heat challenge. In contrast to most other target engagement techniques, no modification of the ligand or cloning work to include reporter groups on the target protein is required. Instead, the methodology is based on ligand-induced resistance of target proteins from thermal denaturation when the cells are heated, i.e. while ligand-stabilized protein remains in solution, unbound protein denatures and aggregates.
Already in the original CETSA paper, CBCS' intellectual input was acknowledged (Figure 22a) (141). This formed the basis of a collaboration in which CBCS, together with Professor Pär Nordlund's group and Pelago Biosciences, has demonstrated that CETSA is amenable to automation and screening, thus making it possible to apply large chemical libraries for screening purposes (Figure 22b) (142, 143). CBCS has now successfully developed several homogeneous AlphaScreen®-based assays (142-145) to monitor target engagement and also performed a first 384-well format screening campaign in live nonengineered cells expressing thymidylate synthase (TS) (143). The screen successfully identified all drugs within the test library that are known to modulate TS as well as novel compounds capable of binding and inhibiting this enzyme (Figure 22b) (143).
This work nicely showed the opportunity to identify cell-permeable compounds that show direct target engagement already at early-screening stages of a project. In a follow-up internal technology development project, CBCS scientists developed a method for quantitative interpretation of intracellular drug binding and kinetics using CETSA (144). This work describes the experimental path that is required for making proper comparisons of CETSA data with functional cellular responses at 37 °C and is believed to have broad implications in the appropriate use of CETSA for target and compound validation. A follow-up technology development project developed a CETSA protocol for detecting intracellular drug binding to p38α (MAPK14) in live A431 cells in situ, using high-content imaging (Figure 22d) (146, 147). The assay concept was further validated using a number of known p38α inhibitors, and the potential of this technology for chemical probe and drug discovery purposes was demonstrated performing a small pilot screen. Importantly, this protocol creates a workflow that is amenable to adherent cells in their native state and yields spatially resolved target engagement information measurable at the single-cell level. Finally, CBCS has translated this expertise into additional projects, including, for example, the first example of the use of CETSA as the main SAR-driving cellular assay in a high-impact publication on NUDT5 (148).
CETSA is today broadly used within academia and the pharmaceutical industry, which have also adopted the CBCS microplate format (e.g. AstraZeneca and GSK) (149). In addition, Perkin Elmer recently launched a number of Alpha SureFire® CETSA® Target Engagement kits for use in combination with Pelago Bioscience's CETSA® technology assessing target engagement in live cells (https://www.perkinelmer.com/, (150)).
CBCS has established itself as a world-leading authority for developing modifications of this technology for high-throughput screening and target-identifications purposes. Investigators collaborating with CBCS have access to our full arsenal of expertise and technology, allowing for powerful, conclusive scientific deduction of molecular mechanisms of action.
6 Lessons Learned and Future Directions
CBCS have during these 10 years successfully built an efficient and sustainable national platform for chemical biology open to all researchers in Sweden with qualified projects. Several lessons were learned on the way which has impacted the future directions of which some are mentioned below.
6.1 National Spread
Based on the statistics during the years of operation (Figure 20), it is clear that vicinity to the infrastructure is a merit. Having said that, CBCS do regular outreaches and contact suitable points of interactions such as innovation offices and centers at all universities throughout Sweden, but it cannot replace the informal network possibilities beefing on site. Awareness of the existence and easy access to meet and mingle and also to visit the lab is of great value. CBCS is presently trying to establish small nodes at the universities outside the host universities and have a pending application for that purpose.
6.2 Attributes of Successful Projects
CBCS can clearly identify attributes for successful projects with the potential to reach conclusive and sustainable scientific results. For example, such projects commonly have internal know-how (in many cases chemistry-based competences) and resources to validate the screening results in more advanced assays. CBCS specifically emphasizes this aspect in the PRC application form with the intention to make the PI aware about this early in the project.
6.3 Pitfalls – Pan-Assay Interference Compounds (PAINS)
The literature is full of incorrect conclusions drawn from low-quality chemical probes that either suffer from potency, stability, or selectivity issues or are unknowingly pursuing artifacts through promiscuous bioactivity or assay interference behavior. In addition, the correct use of chemical probes, e.g. dosage regimen and formulation, in advanced biological assays such as animal models can be a challenge for those that lack the right experience. CBCS experience and expertise is highly needed to enable that quality conclusions are drawn based on data. As an example, a project suggestion from an experienced academic researcher requested the identification of mechanism of action of a lead candidate series that had even been the basis for a small biotech company. After CBCS evaluation of the biological activity in the primary assay (luciferase reporter assay) the compound was concluded to be a luciferase inhibitor, and all presumed activities in the various complex downstream assays showed to be a result of a too strong belief in the correctness of the data. However, even when CBCS is involved from the beginning of the project, doubts of the soundness of the compound mode of action can be experienced which is why CBCS has established a good practice for an extended repertoire of confirmation assays to corroborate the expected mechanism of action.
6.4 Mechanism of Action Elucidations and Target Identification
In contrast to our experience of working in industry where the majority of the projects were isolated target based, we soon experienced that a large portion of the requests from academia were for phenotypic cell-based screens, e.g. utilizing high-content imaging screens in primary cells (Figure 19). This has led to an increased need for identifying the mechanism of action, which is also a necessary requirement for the drug discovery platform to take over the project. We believe that the understanding of the mechanism of action is critical for high impact research and successful commercialization. Importantly, no single methodology for target identification is applicable across the proteome, and thus the application of several methods is often necessary to corroborate findings. In collaboration with other platforms and researchers at SciLifeLab, CBCS is focusing on three key areas to support the user needs:
(1) Target identification through chemoproteomics and mass spectrometry (MS) CETSA: The current CBCS offering is largely built on chemoproteomics (151), where bioactive compounds are synthetically modified to become baits for fishing in cell lysates. Both classical “in lysate” affinity-based chemoproteomics (also known as “pull-down”), where cell lysates are incubated with resin bound small molecule or “baits” to enrich noncovalent binding partners, and in situ affinity-based chemoproteomics where live cells are incubated and labeled with photoreactive derivatives of the small molecules are used. This is complemented with MS-CETSA (152), where compound impact on protein thermostability is used to identify which proteins are bound to a compound. Building on internal CETSA expertise, CBCS has built a relationship with the SciLifeLab Facility for Chemical Proteomics run by Roman Zubarev. Recent work from Zubarevs group discloses an improved protocol for data analysis with dramatically increases throughput and sensitivity (153).
(2) Target identification through high-throughput genetic approaches: Some projects involve the identification of compounds that target pathogenic microorganisms, and in this case their mechanism of action can be identified through the generation of resistant mutants and subsequent whole-genome sequencing. In addition, a facility for high-throughput genome engineering, which is coorganized with CBCS in a SciLifeLab platform, is specializing in pooled genetic loss- and gain-of-function screening using CRISPR/Cas9 technology. This is being explored in combination with chemical proteomics for mechanism of action elucidation by CBCS users.
(3) Compound-induced biological signatures: Broad measurements of compound-induced cellular responses, and comparisons with those observed for known pharmacology's, can be used to understand which pathways are affected by treatment. One such methodology “cell painting,” developed by scientists at the Broad Institute (154), is presently being implemented by CBCS for mechanism of action studies. In addition, together with a SciLifeLab research group, CBCS has been granted funding for developing protocols for compound-induced transcriptional fingerprints with the potential of genome-wide coverage, low cost, and high throughput, thus having a potential advantage to the “L1000” transcriptional profiling platform (155). Finally, new approaches for measurements of compound-induced changes to the proteome (156), developed in Roman Zubarev's laboratory, are available through CBCS.
7 Concluding Remarks
Small molecules are excellent tools to unravel complex biology and “enlightening the (un)targeted proteome.” The understanding of gene and protein function in disease biology is of utmost importance for drug discovery and explains the majority of small-molecule application failures in the clinic. In order to maximize this development, there are lots of opportunities ahead of which some are excellently exploited by the SGC (https://www.thesgc.org/, (157)) and their collaborators (158). New and already explored high-quality chemical probes need to be made available for the entire research community (159). Expert scientists in drug discovery and chemical biology national infrastructures widely present in many countries have a great role to enable this development by expert guidance in selecting and using these tools. The development will further be enabled by cross-disciplinary collaborations and new method development together with related scientific research areas such as single and spatial omics and imaging, genome editing, advanced integrative data analysis, and artificial intelligence. CBCS is really excited and has great expectations for the future within the field of chemical biology.
Acknowledgments
The authors would like to express their sincere gratitude to Annika Jenmalm-Jensen, Anja Reithmeier, and Thomas Lundbäck for contributing to this article.
References
Abstract
The contributions of academic research to drug development process has been a subject of vigorous debate. Regardless, there is agreement that it is an outstanding source of a broad range of fundamental scientific discoveries and these have served as the foundation of innovative therapies. Most research universities have established drug discovery centers in an effort to promote translational biomedical research and a number of administrative support strategies have been implemented with varying success. This article outlines the author's experience in the creation of a number of programs seeking to support drug discovery at the University of Notre Dame as well as his understanding of these strategies at other institutions. The essay covers the potential benefits, issues, and opportunities with seed funding, research core facilities, technology innovation centers, cross-institutional partnerships, and partnerships with the pharmaceutical industry. A few case studies are provided as successful examples as well as advice on maximizing return on administrative investment.
1 Introduction
1.1 New Opportunities in Rare Diseases
There are more than 7000 known rare diseases, and the further incorporation of genomic and other bioinformatic information into clinical trial development will likely lead to increased numbers of diseases classified as rare. Unfortunately, the resulting splintering of disease populations, and their corresponding commercial market, has directly resulted in a significant rise in drug prices. Although the total number of people affected by these approvals may appear limited, many of these people had no prior treatment available heralding these drugs as significant scientific discoveries but potentially less impactful on overall public health. However, this perspective underestimates the true potential of rare disease research and the likelihood of identifying connections to multiple other, but currently poorly understood, rare diseases. Moreover, research in rare diseases frequently supplements our currently understanding of biological processes associated with more common diseases and development of novel treatments for more common ailments.
More than 35 years ago, the Orphan Drug Act created incentives for pharmaceutical companies to develop drugs for rare diseases. The incentives were meant to mitigate the concerns that drugs that target small patient populations were not considered financially viable and provide more companies financial savings that they could put back into research and development (161). The incentives include seven years of market exclusivity, tax breaks for research and development expenses, and prescription drug user fee waivers. More recently, Congress added the priority review program (2007) as a means to further stimulate the commercial development of drugs for neglected infectious diseases for developing countries (162). Following FDA approval of a treatment for a neglected or rare pediatric disease, the developer receives a voucher for priority review for a different drug. Two drugs receive priority review for each voucher: the drug winning a voucher for a neglected or pediatric rare disease, and the drug using a voucher for a subsequent marketing application. The second part of the voucher can also be sold to another company, who can gain months of additional time on the market with their new drug for any indication. Although the recent value of the voucher has dropped due to the decreased emphasis on developing potential new blockbuster drugs, a recent sale by Spark Therapeutics garnered just $110M. The combination of the rising drug prices and the number of annual FDA-approved rare disease drugs has led the FDA to reconsider whether these incentives continue to be necessary.
2 Academic Drug Discovery Centers
If the revenue from new medicines cannot support their own research and development programs, the business model is no longer sustainable. While the role of academic research in the drug development process has been a subject of some, and often including high-profile, debates, there is agreement that it is an outstanding source of a broad range of fundamental scientific discoveries. Moreover, it is clear that these discoveries can be the foundation that ultimately leads to innovative therapies with proper shepherding through the development pipeline. According to the latest industry research, about 90% of drugs that reach the clinical stage development never make it to FDA approval and commercialization with most of these candidates has shown to have limited efficacy (163). This suggests that even the recent industry trend toward “quick-kill” decision-making strategies, which aim to reduce late-stage attrition, suffers from cognitive biases and inadequate predictors of success (164). Contemporaneously, pharmaceutical companies have also drastically cut early-stage research budgets and reduced research staff as a measure to de-risk their business models and focus on short-term gains. Thus, academic and not-for-profit research sectors have been given the opportunity and are taking an increasing proportion of responsibility in early-stage drug discovery (165). In response to these opportunities, there has been an increase in the number of academic drug discovery (ADD) centers, where often these programs are a part of a university's basic research support structure.
Internal support for translational research has a number of benefits beyond their potential to impact on societal health including but not limited to strengthening education and training programs, promoting faculty and institutional reputation, and enabling potential revenue through licensing and commercialization. Drug discovery centers at some universities are focused on single research groups and small collaboration between complementary researcher programs within specific focus areas, such as the Vanderbilt Center for Neuroscience Drug Discovery (VCNDD). The VCNDD is an outstanding example of a collaborative model for ADD based on the complementary expertise in neuroscience biology and industry experience in drug discovery and development. VCNDD facilities, which have been staffed by scientists with significant pharmaceutical industry experience, replicate the infrastructure typically found in the industrial sector. As a testament to their translational capabilities, this Center recently received approval from the FDA to advance one of their compounds into clinical trials for Alzheimer's disease.
At the University of Notre Dame, we established the Warren Family Research Center for Drug Discovery and Development in 2014 through philanthropic support. As is typical of many academic research programs, the Warren Center was built upon a strong foundation of faculty colleagues with a history of drug discovery research interests. In this case, the Department of Chemistry & Biochemistry at the University of Notre Dame had long been known, in part, for excellence in antibiotic research including bacterial cell wall biochemistry and the discovery of related and novel small-molecule inhibitors. Over the years, this has been well complemented by a diverse group of faculties with expertise overlapping synthetic organic chemistry, medicinal chemistry, natural product research, and diagnostic tool development. Prior to the establishment of the Warren Center, faculty researchers had two avenues for translating their early-stage biomedical discoveries: (i) Develop expertise within their individual labs or (ii) identify expertise outside the university and develop personal relationships, collaborations, and partnerships which secured complementary capabilities and experience.
2.1 Research Core Facilities
Notre Dame's path to a fully functioning ADD center began with internal support from the dean's office in the College of Science and the Office of the Vice President of Research. Initial funding established of a new university core facility that would be staffed to provide chemical synthesis and medicinal chemistry support services to faculty-generated projects. It was envisioned that the Chemical Synthesis and Drug Discovery Facility (CSDD) would be particularly valuable to biologically focused laboratories that currently lacked chemical synthesis expertise and allowed them to procure expert staffing for short-term needs. Moreover, groups with overlapping capabilities may also find value in working with such a facility by shifting projects which lacked particular innovation such as hit and lead resynthesis, compound scale-up, and the generation of small analogue libraries or affinity-labeled probe compounds. The proposal also gained favorable support from administrative leaders by partially dedicating staff effort to the physical and chemoinformatic organization of a university compound collection. Through the procurement, purification, and storage of the products of the past, current, and future chemical synthesis projects, the Notre Dame Compound Collection is a growing resource of unique chemical entities and a platform for promoting collaboration between biological and chemical researchers across the university and with external partners such as regional universities and pharmaceutical companies.
Over the years, pharmaceutical companies have attempted to improve research and development efficiency through a number of business strategies. Studies conducted by the Tufts Center for the Study of Drug Development have not found these efforts to be particularly transformative and the subsequent results to have marginal impact (166-168). However, in response to the recent decrease in the rate of return on R&D expenditures, companies such as Eli Lilly have created open innovation programs to create connections to external researchers, research institutions, and academics. Pharmaceutical companies have long expressed interest in developing collaborations with individual academic labs. This form of distributed innovation would mitigate some of the costs and risk of early-stage discovery programs, but truly mutually beneficial relationships have only recently begun to take shape. From a minimal perspective, university researchers can request access to proprietary compounds from a company's library for exploration in new research areas in return for varying degrees of IP rights. Our lab has utilized these programs on a few occasions. Almost 25 years ago, Merck provided our lab with a sample of epothilone B, a compound isolated by their natural products group. With access to the material, we utilized NMR techniques to assign the compound's relative stereochemical configuration and determine its conformational preference in solution. More recently, we obtained an appreciable sample of one of Bristol-Myers Squibb's proprietary compounds to explore a novel drug conjugate for cancer applications. Access to both compounds required a fairly straightforward and relatively short research proposal submitted electronically. More recently, pharmaceutical companies have offered greater access to their expertise and capabilities. Lilly's Phenotypic Drug Discovery program (PD2) enabled researchers to submit compounds for evaluation in proprietary biological assays, thus expanding upon the chemical diversity of their own, in-house chemical library (169). Exclusive rights to the results remained with the submitter, and the initial digital submission process is structure blind, making this both an attractive opportunity and promoting broader participation. Researchers at the University of Notre Dame have participated in PD2 submissions since its inception and, in fact, received the program's first license deal. Compounds previously created in the laboratory of University of Notre Dame by Professor Marvin Miller, as antituberculosis agents, were found to have antitumor activity by a potentially novel mechanism. In 2010, Pfizer established a more focused relationship with specific academic institutions. The Centers for Therapeutic Innovation (CTI) is a collaborative model for the development of partnerships with academic medical centers and disease-focused foundations (170). In contrast to previous Pfizer's previous academic alliances, their CTIs are located near their eight academic partners to facilitate greater scientific interaction. Projects, which are funded by the pharma giant, are pushed forward by teams of researchers that include both academic and Pfizer scientists.
The Warren Family Research Center for Drug Discovery and Development continued to electronically submit compounds for PD2 screening as they were added to the compound collection. Unfortunately, Lilly's OIDD program was put on hiatus for review in 2019 despite having a standard assessment framework for evaluating the effectiveness of the program when it started (171, 172). However, it is certainly likely that the resources required to manage a growing collection of external relationships, particularly with programs lacking significant drug discovery experience, outweighed the potential benefit. The National Center for Advancing Translational Science (NCATS) Assay Development and Screening Technology program and their CANVASS natural product evaluation program are additional opportunities for drug discovery researchers to add complementary biological data and thus increase the value of an institution's chemical compound collection. Through the development of the Canvass library of natural products, NCATS has recognized the continued importance of natural products in the drug discovery process. Through this program, participant-submitted samples were subjected to quantitative high-throughput screening (qHTS) across a diverse set of cell-based and biochemical assays. The library was characterized in terms of physicochemical properties, structural diversity, and similarity to compounds in publicly available libraries. The resultant assay profiles were used to develop structural class and activity profiles. For some time, my own research group has been working on the polyketide herboxidiene (GEX1A) and found the bacterial-derived natural product to be a potential lead for the rare lysosomal storage Niemann–Pick Type C characterized by aberrant cholesterol storage (173). Based on our understanding of the mode of action, we also identified certain rare mutations of acute myeloid leukemia (AML) as an alternative indication. Submission of a sample of GEX1A to CANVASS provided a trove of interesting data from more than 60 individual assays (174). From a similar perspective, the University of Queensland in Australia has created the Community for Open Antimicrobial Drug Discovery (CO-ADD) as a not-for-profit framework for discovery and development of new therapies against multidrug-resistant (MDR) bacteria. CO-ADD is willing to perform primary antibacterial screening for academic research groups as a free service and, like the Lilly's OIDD program, makes no claims on the results or IP. Submitted compounds, as little as 1 mg, are screened at no cost against a key panel of susceptible and drug-resistant bacterial and fungal pathogens including key ESKAPE pathogens, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus (MRSA), and the fungi Cryptococcus neoformans and Candida albicans. In return, participants must allow the results to be made accessible through an open-access database for use by the community following a confidentiality period. CO-ADD also provides the opportunity to engage their experts in collaborative ventures that investigate the mode of action, target validation, and genome sequencing projects. This is an excellent paradigm, which could be mimicked for other indications/fields, for connecting complementary researchers with expertise across chemical and biological interface.
While active, Lilly's OIDD program also provided chemical synthesis services through their Indianapolis-based Automated Synthesis Laboratory (ASL) (175). The robotically operated reactors are linked to automated workup, purification, and characterization modules run by off-site, non-Lilly collaborators. The ASL allows researchers to access its synthesis capabilities in a remote fashion through internet access, 24/7. The ASL's versatility has been successfully demonstrated on a broad range of reaction types. ASL-related projects were designed for early-stage discovery work and the preparation of roughly 100 mg of products arising from sequences up to eight individual steps. Lilly provided full support for the projects including the initial development of each transformation and material costs in return for half of the final materials. Researchers at Notre Dame participated remotely in several collaborative projects utilizing the ASL including the preparation of a roughly, 100-member library of beta-lactams for use as protease-inhibiting probe compounds that are now included in the university's chemical compound collection.
CSDD facility staff are assigned with the tasks of executing material transfer agreements, familiarity with electronic submission processes, and the processing of physical material transfers associated with these types of external collaborative opportunities. This aspect or their charge is a particularly valuable service since it lowers the barrier for broad faculty and student participation and further justifies university-subsidized support of these facilities. The Warren Center also supports two additional core facilities to provide services in computational chemistry and biological screening. The Computer-Aided Molecular Design Facility (CAMD) provides a range of computational support, from protein modeling to docking studies. CAMD recently contributed significantly to an international collaboration that determined the molecular mechanism of artemisinin resistance in Plasmodium falciparum through the building and MD simulations of a homology model of PfPI3K (176). The Biological Screening and Development (BSD) Core provides access to equipment and expertise for the biological and translational assessment of chemical compounds including the development of high-throughput assays. Additionally, this core also provides evaluation of in vitro pharmacological properties of potential lead compounds which are services typically not available in individual labs.
As discussed earlier, these facilities enable the procurement of complementary expertise for short-term projects, relieving the need to hire staff through more typical annual appointments. Staffing of core facilities is frequently done through postdoctoral researchers with the idea of maximizing FTEs on a limited and hopefully sustainable budget. However, the value of including core facility leadership and/or staff with significant of even better, real world, pharmaceutical industry experience cannot be stressed enough. Core facilities must reach out to faculty across the campus and promote the value of shifting aspects of their projects to the core as both financially and scientifically beneficial. Well-staffed core facilities with expertise not typically found in the academy can significantly benefit the research infrastructure of a university and justify university subsidies that keep their usage fees reasonable and ensure their broad usage.
2.2 Incentivizing Research through Seed Grant Programs
Academic administrations have often utilized small- to mid-sized seed grant programs to support early-stage research programs or projects that do not yet meet the criteria necessary to secure external funding. Frequently, these opportunities are created to incentivize researchers to prod their programs into burgeoning areas, establish new interdisciplinary relationships, or extend the goals of their current efforts beyond fundamental discoveries. Grants for early-stage investigators may be valuable for the initiation of new projects and help them create connections in their new environment. And for more established researchers, seed funding can help redirect their program, enabling new directions or reinvigorate a well-established but potentially stagnant research program. Seed programs are often evaluated by typical academic metrics including resulting publications, grant submissions, and awards as well as invention disclosures and/or patents. Program assessments rarely consider the likelihood that similar outcomes would have been achieved without seed funding. Seed programs that seek to stimulate collaboration, including across disciplines, often create a conundrum for application reviewers since newly established partnerships can be somewhat forced and initiated to meet program requirements. This is in contrast to long-standing collaborations that have a record of productivity and synergy but may not be in need of smaller grant resources.
One important issue is that seed funding is often provided as discretionary dollars to the project's principal investigator(s) (PI), with significant oversight, making it difficult to ensure that the funds are utilized to best achieve the overarching goals of the program. For good reasons, program administrators typically do not want to micromanage the utilization of the funds and thus limit accountability communications to routine progress reports and previously discussed metrics. In an effort to circumvent some of these issues, the Warren Center has utilized seed grants to drug discovery projects across the Notre Dame campus, while simultaneously promoting the utilization of their research core facilities. Their annual requests for applications (RFA) provides up to $25 000 for pilot projects that are associated with drug discovery in need of small-molecule synthesis (libraries or molecular probes), hit validation, lead optimization, mid-sized scale-up, assay development, computational screening, protein purification, and ADMET screening. However, in contrast to discretionary PI support, the funds are provided directly to one or more of the Center's three research cores: Chemical Synthesis and Drug Discovery, Computer-Aided Molecular Design, or Biological Screening and Development core.
2.3 Intellectual Property Protection and Enterprise Development
Beyond the potential impact on human health and subsequent benefit to the academic reputation of the institution, further justification for the internal support for biomedical research has included the promise of potential financial gain. Several universities have benefited significantly from royalty payments associated with commercialization of their pharmaceutical technologies including Florida State University, the University of Minnesota, Emory University, Princeton University, UCLA, and Northwestern University. The Bayh-Dole Act, signed into law in 1980, gave academic institutions IP rights on the products of federally funded research. A recent evaluation of Bayh-Dole has shown that the legislation is working as intended helping American universities contribute to the American life science ecosystems and maintain economic leadership in the life sciences (177). Thus, universities are incentivized to patent research discoveries and inventions and license the IP to private-sector entities which then become responsible for the sometimes lengthy, difficult, and risky development effort. Unfortunately, as previously discussed, the age of the blockbuster drug has passed, and newer pharmaceutical business models are seeking to spread the risk of the development process shifting licensing decisions to later in the development process.
Traditional academic technology transfer offices are responsible for the protection of university IP through a process that first promotes faculty submissions of invention disclosures. The invention disclosure describes a potentially new idea, a current understanding of its commercial potential, and a brief evaluation of the current state of the societal problem the idea seeks to address. This information is supplemented, to varying degrees, by a market analysis and patent search, in an effort to determine whether or not there is adequate justification for proceeding with patent protection. From the author's experience, the process is highly reliant on the experience of the tech transfer staff, the promotional ability of the inventor, and the willingness for decision-makers to seek specific outside expertise. When it comes to drug discovery, patent type, timing, and jurisdiction are all critical. Protecting states of matter, their observed biological activity, and their therapeutic potential prior to their readiness for clinical development can eliminate interest from potential licensees. It is important to remember that the drug discovery development process is long and expensive. Shortening the lifetime of the patent, 20 years from filing, by protecting IP too early can negatively effect its commercial potential. Thus, a process that enables the delay of first public disclosure is preferable through the use of confidentiality agreements and carefully controlled dissemination of technical information.
Despite the opportunity to obtain revenue from IP licensing, in any given year, it is well documented that most universities fail to recover the costs of running their tech transfer offices. In fact, only a small number of universities consistently secure lucrative licensing deals that produce significant revenue. While blockbuster discovery can alter a university's fortunes, the vast majority of licensing deals yield little or no money, and for most universities the royalty returns are low. The problem is not only related to the high-risk nature of scientific research. Only a fraction of fundamental discoveries ultimately relate to and provide a solution to real-world problems. Much has been made of the so-called Valley of Death relating to the gap in external support for academic research that is found between early-stage, federal funding for basic research and proof-of-concept, where routine but critical supporting data is needed to attract pharmaceutical companies and biomedical investors (178). To the PI, this chasm not only exists in the paucity of external funding opportunities but also in access to internal university support structures; i.e., seed programs and research cores support fundamental discoveries and intellectual property offices that are most excited by projects with clear commercialization potential.
Notre Dame, like many other R1 universities, has begun to expand their technology transfer services beyond classic IP protection and into the realm of enterprise development. The lack of early-stage capital and support structures for innovation development is a major challenge in advancing promising university technology from the lab to commercialization. Research institutions are implementing gap funding and accelerator programs to provide support for proof- of-concept prototype development and start-up ventures. These programs have evolved into sophisticated evaluation, development, investing, and commercialization support structures that can provide the necessary space, services, and expertise for idea development, commercialization, business formation, prototyping, and addressing the need for entrepreneurial education for both students and faculty.
Indianapolis-based Parmelee consulting is also providing services to start-ups associated with the University of Notre Dame's IDEA Center. Standing for Innovation, De-Risking and Enterprise Acceleration, the IDEA Center is the exclusive home for commercialization and entrepreneurial activities at the University of Notre Dame. In addition, to overseeing entrepreneurship-related educational programs at the undergraduate, graduate, and professional levels, the IDEA Center is a commercialization and enterprise development program that seeks to advance new technologies and innovations from the Notre Dame community to the market. In its first two years, the IDEA Center founded more than 40 start-ups, which exceeds the number of ND-related commercial entities in its history. As with typical university technology transfer offices, the IDEA Center provides IP-related services from invention disclosure through patent protection and licensing agreement negotiation. In addition, projects are now managed through several stages of lean-canvass and lean-start-up process, which often includes financial support for prototype development and supplemental data collection through a de-risking fund. In the drug discovery space, this support can enable the hiring of external consultants and obtaining important pharmacological and toxicology data to complement fundamental biological and efficacy data already in hand. For example, our current effort to develop a natural product lead compound for AML that is being supported by the IDEA Center's commercial engine has proceeded from their risk assessment stage, called the two-stroke, to the four-cylinder de-risking phase. Funding has been provided to recruit the oncology-focused, contract research organization (CRO) TD2, Inc. (Translational Drug Development; Scottsdale, AZ) to provide additional preclinical data necessary to support an investigational new drug (IND) filing to the FDA for permission to support human clinical trials. Technologies that have been sufficiently de-risked are eligible to pitch for funding from the IDEA Center's Venture Fund as a complement to external funding sources such as STTR and SBIR grants. The IDEA Center has recently supported a drug discovery-based venture generated out of the Notre Dame's Department of Chemistry & Biochemistry. Extending on basic and translational research conducted with diabetic animals and human diabetic wound samples to elucidate the dysregulated mechanisms of wound healing, Professors Chang and Mobashery have developed a topical drug that addresses the underlying condition in pathology of the recalcitrant healing of diabetic wounds. The only currently approved drug has modest efficacy and comes with an increased risk of cancer and mortality. The new lead compound is currently undergoing IND evaluation through a $5 million Therapeutic Development Award from the Department of the Army with the goal of starting Phase I clinical trials in 2021 through the start-up, SalvePeds.
A recent $50 million dollar gift allowed the creation of a similar support structure at Harvard University. The Blavatnik Biomedical Accelerator provides strategic, monetary, and advisory support for the translating applied life-science research discoveries building upon their Office of Technology Development. The Accelerator has provided over $20 million in direct research support of more than 100 projects from across Harvard targeting most major disease areas. Supported projects have included the development of therapeutic strategies, new diagnostic tools and biomarkers, and other biomedical technology and instrument development. This effort has already demonstrated tangible results as nearly half of all completed projects have developed licenses with pharmaceutical and biotech industry or other major industry-sponsored research agreements as well as new venture start-ups. One particularly strong example demonstrates the value of providing support for the advancement of potential lead compounds to a relatively late stage of preclinical development. A faculty researcher in Harvard University's Department of Chemistry & Chemical Biology discovered a potentially novel therapeutic strategy for AML through the inhibition of kinases associated with transcription regulation. The technology was licensed to Merck in 2016 for an upfront fee of $20M along with future milestone payments. In return, Merck will shepherd the further development of these new cancer lead compounds including clinical development and commercialization.
3 Cross-Institutional Models for Promoting Academic Translational Research
3.1 The Tri-Institutional Therapeutics Discovery Institute
Traditional university–industry collaborations within biomedical research could be characterized as bilateral arrangements providing financial support through contract research, consulting, and research agreements in return for an option to license potential IP associated with drug candidates and technologies. Core differences between their approaches have frequently been viewed as obstacles to closer joint research efforts. Academia's focus on curiosity-to-publication-driven research contrasts with industry's need for limited disclosure and faster timelines to go-no-go decisions. The more recent open innovation models allow university researchers to access pharmaceutical industry technologies with hopes of identifying additional licensing opportunities. Neither of these models is particularly collaborative. However, recent examples suggest that these relationships have begun to evolve into more horizontal, multistakeholder public–private partnerships (PPPs) that allow each side of the collaboration to contribute their complementary expertise and proprietary technology to biomedical challenges of mutual interest (179). The Tri-institutional Therapeutics Discovery Institute (Tri-I TDI) is an excellent example of this new type of PPP. Tri-I TDI was established in 2013 to expedite the discovery of new therapeutics based on technologies from three medical institutes, the Memorial Sloan Kettering Cancer Center, The Rockefeller University, and Weill Cornell Medicine, and in collaboration with an industrial partner. Takeda contributes to this venture, a team of experienced chemists and pharmacologists which carries out the optimization of potency and pharmacology properties of hit and lead compounds originally discovered by academic researchers. Through this type of partnership, Takeda provides expertise not typically found within the academic labs particularly those associated with medical school but essential to the exploration of the translational potential of early-stage academic discoveries. A March 2019 press release announced a successful demonstration of the potential of this type of collaborative venture. A series of chemical leads were identified by a Weill Cornell Medicine researcher as modulators for sphingosince-1-phosphate. The structures were optimized for pharmacology properties and activity within the Tri-I TDI, resulting in the identification of an advanced lead compound that is efficacious in preclinical proof-of-concept studies. The IP was then licensed to Bridge Medicines, a start-up launched by the Tri-I TDI partners, to continue preclinical development, with the goal of entering clinical trials for one or more of the target indications.
3.2 Indiana Clinical and Translational Sciences Institute
The progress of new ideas through the development process, either to the market as therapeutic products or as implementations into health and disease management, faces many barriers. The NIH established the National Center for Accelerating Translational Sciences (NCATS) and Clinical and Translational Science Awards (CTSAs) with the goal to “transform the translational science process so that new treatments and cures for disease can be delivered to patients faster.” As the national platform for achieving this goal, the CTSA funding opportunity charged applicants to “create local ‘Hubs’ which support innovations in methodology, training and career development, as well as high quality clinical and translational research locally and nationally.” The Indiana Clinical and Translational Sciences Institute (Indiana CTSI) was created in 2008 as a statewide colaboratory to accelerate clinical and translational research across the three state research universities, Indiana University, Purdue University, and the University of Notre Dame along with partners within the corporate sector, the regional health care systems, and local foundations. The mission of the Indiana CTSI is to serve as the statewide catalyst for translational research, improve human health across Indiana, and promote the application of new paradigms that are capable of being implanted nationally. The Indiana CTSI is structured with the specific aims to promote collaborative research, provide resources and services to support clinical and translational research, offer education and training development programs for next-generation translational researchers, partner with the local community, and integrate with the national CTSA network.
The Purdue University Institute for Drug Discovery has expanded a program originally offered through the University's Center for Cancer Research to provide specific advisement to projects with translational potential in therapeutics development. On a semiannual basis, a drug evaluation committee (DEC) is assembled with diverse team of experts including current and former large pharmaceutical company executives, research clinicians, serial biotechnology company entrepreneurs, and experienced academicians. The DEC provides expert reviews of projects presented by members of Purdue's faculty. In addition to scientific and technical advice, these discussions often include drug development topics not typically considered by academics including potential market size, current market competition, clinical trial indications, potential clinical trial recruitment challenges, chemistry manufacturing controls, and other manufacturing challenges. The Institute for Drug Discovery considers the activities of the DEC, an essential part of their successful effort to advance several drug candidates in their drug pipeline. It not only provides the PIs valuable advice enabling them to focus on critical milestones, but this type of expert analysis can also be used to justify internal funding, develop a proper IP strategy, and hone SBIR/STTR applications.
By including representatives from multiple institutions, the IDDA Advisory Committee can be a clearinghouse for drug discovery and development resources available through Indiana-CTSI partners and, when necessary, identify and expand current capabilities by initiating new, external partnerships. The MTP has connected with drug discovery research programs and their PIs across the state primarily through two annual seed grant programs: the annual IDDA Support Program and the MTP Pre-Clinical Service Award. The applications are simple to prepare, two pages in length, and focus on background, current status, and commercialization potential. By not requiring an upfront proposed budget, the program provides an opportunity for the review process to help shape specific aims. In the author's experience, the success of seed grant programs to support academic research is frequently underwhelming. Typically, the amount of funding is less than what is truly needed to complete the project, and reasonable participation rates require active recruitment of applicants. More consequentially, the funding is typically allocated at the discretion of the PI and thus may not be ultimately utilized in the productive way. This is particularly true for translational biomedical research projects where key milestones and data collection efforts that are needed for the applied aspects of a project are at times at odds with support for additional basic research goals. Results needed for publication and basic research grant applications are different than data that would help support a successful SBIR/STTR application. Thus, new MTP projects are first assessed for clinical, biological target, and chemical relevance by the IDDA advisory committee. The diverse experiences of the committee, including representatives from Eli Lilly with significant drug discovery and development experience, are extremely helpful for this critical first step and play an important role in identifying immediate needs and the development of a project budget. Whether or not selected for a second stage discussion, each project is assigned an IDDA project manager to directly assist the PI(s), provide valuable feedback, track the project for key milestones, and routinely assess future needs. A second round of evaluation is carried out through short presentations and interactive discussion. This provides an opportunity for the review committee to ask questions, evaluate the current plans, and offer guidance in generating the most appropriate specific aims. A strong subset of projects is provided funding with the expectations of directing the support toward key milestones recommended by the review committee.
Despite similarity in structure, the goals of the two seed grant programs are distinct and complementary in their effort to support translational research. The IDDA Support Program provides small grants to facilitate collaborative translational research and partnerships related to drug discovery through support for team building across diverse disciplines, the identification of complementary expertise across the Indiana CTSI, and, when necessary, the creation of new, external partnerships. The IDDA advisory committee is motivated by a desire to connect complementary biological and chemical expertise. From a drug discovery perspective, this includes the design and development of new assays based on the identification of a novel biological target, the identification of potential hits from an in silico screen or actual hits from a high-throughput screen, or the validation of target within a particular disease or the confirmation of the activity from a screening hit through follow-up experimentation. When possible, funding is provided to research service cores within the Indiana CTSI consortium and not directly to the PIs associated with the project.
The MTP contains a number of programmatic innovations that complement the broad translational goals of the other programs within the Indiana CTSI. These benefits include routine feedback from the advisory committee's pharmaceutical experts, implementation of a project management paradigm, and financial support for CRO-generated preclinical data. Drug discovery projects supported by the MTP benefit from combined intellectual resources and alternative perspectives provided by the IDDA advisory committee which complement the PI who is often inspired, ultimately supported, by objectives rooted in more basic research discovery. Drug discovery is very much an iterative process where a number of steps must be accomplished sequentially, and each step is informative to future decision-making. A project management approach tracks the progress of the project and helps to ensure the project remains on a trajectory through the translational pipeline toward a potential clinical benefit (182). Over the past seven years, the MTP has been able to connect and provide mentoring advice to researchers and research teams associated with more than 70 projects across the Indiana-CTSI-associated institutions. More than 25 projects have received some financial assistance, but these and several more have been placed into our project management portfolio for routine oversight. Three projects have received a second award for significant progress made along the drug discovery pathway and maintain momentum.
3.2.1 Novel Protein–Protein Interaction (PPI) Inhibitors that Target Cancer Signaling Pathways
Indiana-CTSI researchers utilized in silico screening to identify potential classes of compounds that could affect the transcription factor TEAD interactions with YAP, a coactivator that has been implicated as an oncogene and is amplified in a number of human cancers. The IDDA support program provided funds to screen hundreds of compounds in an in vitro assay. The results of this screen provided four compounds that bound to TEAD and inhibited the interaction. Based on these results, a second round of funding was considered to synthesize a small library of compounds related to these hits and characterize their activity in biochemical and cellular assays of TEAD activity. The IDDA advisory also suggested that the library be screened for selectivity with a number of known PPIs. The results of these studies serve as an important basis for not only an NIH R01 application but also for the next stage of the drug discovery pathway preliminary preclinical data collection and animal model studies (183).
3.2.2 Development of Small-Molecule Inhibitors for Novel Cancer Therapeutic Target PRMT5
Indiana-CTSI researchers discovered protein arginine methyltransferase 5 as an activator of NF-kB, a factor shown to be constitutively activated in colon cancer. In 2014, the IDDA support program provided funds for the development of a high-throughput screen of PRMT5-specific AlphaLISA technique and identified a number of hits (184). Biochemical studies validated a few lead compounds, and additional funds were provided in 2015 to generate a small library of analogues based on the most active lead. Current efforts have begun to explore the activity of the prepared derivatives in colon cancer cellular assays. The results of these studies serve as an important basis for not only an NIH R01 application but also for the next stage of the drug discovery pathway preliminary preclinical data collection and animal model studies.
3.2.3 New Therapeutic Lead Compounds for Neurodegenerative Diseases Associated with Aberrant Proteostasis
Indiana-CTSI researchers have shown that a bacterial-derived, natural product corrects the aberrant cholesterol-storage phenotype associated with the lysosomal storage disease, Niemman-Pick Type C, and specific forms of AML. The researchers access gram quantities of the material through fermentation of Streptomyces chromofuscus. Through an award from the MTP Service Grant Program, the lead compound has been evaluated for solubility, in vitro metabolic stability, P-gp assessment, and murine pharmacokinetic profiling. These results suggest that the natural product is a strong lead compound and worthy of testing in animal models of both diseases and helped secure an NIH grant to study the therapeutic potential of the molecule (173).
3.2.4 Novel Lead Compound for Diabetic Foot Ulcers
Indiana-CTSI researchers have developed potent and highly selective inhibitors of matrix metalloproteinase-9 (MMP-9) which are water soluble. Inhibition of MMP-9 has been shown to lower inflammation and enhance angiogenesis. Topical application of the lead compound has been shown to accelerate diabetic wound healing in mouse models of the disease. Diabetic foot ulcers (DFUs) are a significant health problem. An award from the MTP Service Grant Program was leveraged to obtain additional funding from the University of Notre Dame and support IND-enabling toxicological studies (185).
4 Additional Collaborative Opportunities
Despite the advent of combinatorial chemistry and high-throughput screening in the 1990s, which enabled the preparation and screening of large, diverse compound libraries, the number of drug candidates in clinical trials did not scale proportionally (186). Major factors contributing to this fact were that screening libraries were limited in quality and chemical diversity. Over the past 20 years, the process has evolved from simply a number game to specific advances in the quality of biological assays and the creation of mutually beneficial research partnerships even between pharmaceutical companies. Compound sharing is becoming routine in drug discovery as an efficient way to rapidly couple increased chemical diversity to biological screening opportunities (187). Recent examples include agreements between Sanofi, Syngenta, and AstraZeneca (AZ) to exchange 240 000 compounds, and AZ has also partnered with Bayer to provide access to each other's entire compound library. Molecular contributions for a European consortium of seven companies as well as compounds from several academic centers have created the Joint European Compound Library (JECL). Unfortunately, academic institutions in the United States are more reticent about compound sharing due to, somewhat unfounded, concerns about IP. Thus, efforts to create a statewide compound resource never got traction. More recently, however, Notre Dame's Warren Center for Drug Discovery established a new relationship with the Boston University – Center for Molecular Diversity (BU-CMD) as a part of a larger consortium of molecular providers. Under the arrangement, the BU-CMD will curate unique chemical entities from chemistry sources within six academic institutions. The compounds would be made available to a complementary network of screening facilities and expand their own compound collection. Intellectual property concerns are alleviated through the use of material transfer agreements and consideration of the relationship as one-on-one collaborations despite the larger programmatic organization. While some of our core facilities have purchased commercial libraries, a majority of the unique compounds currently sit idle on shelves or in freezers of a number of laboratories and represent a largely, untapped resource for drug discovery. Compound sharing across institutions can be mutually beneficial and benefit from the utilization of common protocols for compound and chemoinformatics data storage.
5 Summary
Due to its simultaneous contributions to fundamental biological discoveries and accompanying training next-generation researchers, ADD should remain an important contributor to the field. With the dramatic increase in cost and time to develop new therapies, the pharmaceutical industry has contracted particularly in the support for early-stage biomedical research. Academic scientists are well positioned to fill this gap, but unfortunately, funding for academic research has waned, until very recently, over the past two decades. However, universities have responded by tapping into alternative sources of financial support including foundations and philanthropy in order for ADD to expand their role to a larger portion of the development pathway. In order to ensure the generation of a sustainable platform and establish a more reliable return on investment, support structures from ADD must complement the skills and technology from what is typical to individual research laboratories. Biological experts can provide critical evaluation of proposed screening assays and provide insight into target validation for specific indications. Chemical expertise is useful in ensuring the proper selection of screening libraries and the optimization of compound structures to provide leads with drug-like properties. While computational tools have long been utilized for structure-based design, the application of newer artificial intelligence and machine-learning approaches to compound property prediction can significantly help academic researchers predict PAINS (188), toxicological profiles (189), and ADME profiles (190). Expertise in both of these areas need to be complemented by pharmaceutical industry experience. Industry experts can focus projects toward key translational goals and away from conflicting academic priorities. Universities can access these needed skills by creating policies that promote mutually beneficial partnerships with the pharmaceutical industry. Seed grants should be used to engage researchers and garner information about the status of current projects which would assist in developing a needs assessment strategy. When funding is provided, support should be focused on obtaining key pharmacological data that helps shepherd the project from academic research lab into early-stage start-up entities. There is no doubt that the current environment provides expanding opportunities for academic contributions to the development of new therapies despite the increased risk placed on early-stage research.
References
Finding quality chemical matter from academic screening sets can pose some unique challenges. These sets are usually not proprietary but are obtained from commercial vendors. The proportion of promiscuous, unstable, and reactive compounds is higher than the typical pharma proprietary library, whose collections have been designed for physicochemical properties and stabilities which are more druglike. Even in relatively clean libraries, these undesirable compounds rise to the top due to activity across multiple assays. Complex solution behavior of these collections, including very low aqueous solubilities and aggregation, must also be dealt with to select reliable leads for optimization. We have come to these conclusions after conducting target-based high-throughput screening for over a decade on scores of targets. More recently, we conducted an accelerated screening program of about two dozen novel targets over a three-year period using a library of around 200 000 diverse compounds from commercial vendors. This experience has resulted in a workflow to address the liabilities of poor screening matter, avoid wasting effort on risky compounds, and focus on the most promising leads. As more academic laboratories engage in industry collaborations around drug discovery, it is important that the academic laboratory has thoroughly vetted the quality of the chemical matter they bring to the negotiating table. The disclosure of well-researched discovery leads from academia will go a long way to encourage these collaborations and mend the somewhat sullied reputation of academic discovery lead matter (191).
The building and selection of a new screening library should include the computational filtering for undesirable functionality and properties. Compounds which are unstable in DMSO, aqueous buffer, or air should be flagged with suitably constructed SMARTS queries (192). Examples of the sort of functionality in the design of these SMARTS queries include compounds such as nonalkali metals, extended fused aromatics, and functionality with extremely high or low pKa values which preclude their cell permeability. The exact nature of these filters would depend on the investigators' needs and biases, and there are published SMARTS filters available (193). In total, we have designed over 300 SMARTS queries which can reduce vendors screening offerings by half. These SMARTS filters include published lists of pan-assay interference compounds (PAINS) (194, 195). These queries for undesirable chemical functionality can be followed by filtering for custom physicochemical properties such as molecular mass, topological polar surface area, and calculated octanol/water partition coefficient as examples. Vendors are aware of drug-like physicochemical properties and PAINS and may offer collections prefiltered for these properties. However, we have found that after full SMARTS and properties filtering, a vendor's collection can be reduced to as low as 20% of the initial offering. We typically conduct this filtering in MOE (196), but most computational software suites can either directly filter using SMARTS queries or one can develop a KNIME protocol (197) to filter and calculate the physicochemical properties.
In the case of the existing libraries which have not been prefiltered, this analysis occurs in the hit follow-up or triage phase of the screening program where questionable chemical matter is flagged for a critical review by the medicinal chemist (198). Hits meeting a predetermined activity threshold are computationally filtered using PAINS and SMARTS queries. We assign a three-tiered ranking system to all hits consisting of green, yellow, and red categories, corresponding to no known issues with green compounds, problematic but fixable functionality with yellow compounds, and red compounds regarded as unsuitable for follow-up due to reactivity, stability, or known promiscuous behavior. This “stoplight” analysis can be an effective method to communicate the complexity of high-throughput screening to faculty collaborators unfamiliar with the complexities of selecting quality lead matter. A final assessment of potential promiscuity is conducted by searching individual hits and their substructures in online bioactivity databases such as SciFinder (199), ChEMBL (200), UniProt (201), and Binding DB (202). This final check of reported activities is important since actives in our assays often show up as frequent hitters in these databases. In many instances, the mechanism of promiscuous activity is not obvious, and an online check of these databases can alert one to a potential new PAINS. Once this triage analysis is complete, we can focus on the best chemical matter and begin assessing the next critical property which is solubility.
The assessment of complex solubility behavior in screening is difficult to predict computationally, and we therefore rely on physical measurements. Compounds must be soluble throughout the dose–response titration. Behaviors such as failure to reach 100% inhibition or steep Hill slopes (203) are warnings that activity may be solubility limited, or aggregation may be responsible for activity. In addition to these readouts, we consider any potential lead compound having a calculated logP octanol/water of greater than 3 to be suspect, and the aqueous solubility and tendency to form aggregates should be determined.
Our method to determine problematic solubility and aggregation behavior starts with a kinetic solubility determination. Plate-based nephelometers are commercially available for high-throughput determinations, but we find a simple low-tech single-cell nephelometer or turbidimeter to be superior in sensitivity and ease of operation. In this method, a stock solution of the compound of interest in DMSO is added incrementally to a large stirred cell containing PBS or assay buffer. We typically prepare these stock solutions at 10–20 mM. The addition of the compound DMSO solution to the buffer spans a range of 1–100 μM solubilities, and the final DMSO concentration is kept below 5%, or whichever value reproduces the assay conditions. The reflectance at 90° to the incident light is monitored by a nephelometer or turbidimeter, of which several are commercially available and inexpensive. A plot of reflectance vs. concentration gives a straight line while the compound concentration is soluble but rapidly curves upward as precipitating or aggregating particles causes increasing reflectance. Figure 23 shows three types of solubility behaviors and their characteristic plots. The kinetic solubility of beta-estradiol illustrates the near ideal behavior for a well-behaved solute approaching its aqueous solubility limit at a literature value of approximately 13 μM. We could have confidence in any measured activity higher than this solubility threshold. By contrast, Compound A does not achieve a level baseline which indicates solubility, and the reflectance rises from the first addition of DMSO solution. We cannot have confidence that either a single-point activity value or a dose–response activity would be meaningful. We cannot determine the nature of the particles, whether they are aggregates or a solid precipitate, however. We would not have confidence that any activity values are reliable for this compound and would normally choose to flag this compound as low priority or disqualify it for follow-up altogether. Aggregation itself does not disqualify a compound as a potential drug; in fact, several marked compounds have been shown to form aggregates (204). However, in an academic discovery lead finding campaign where promising leads must be selected based on screen activity from among several hit classes, the presence of aggregation or solubility limited behavior, which would consume resources in the search for a “fix” which may never materialize.
Aggregation is characterized by a critical aggregation concentration, CAC, which defines the phase change from solute to the inhibitory aggregation state. The presence of a defined CAC as determined by DLS is a strong evidence of colloidal aggregation (205). Finally, Triton X-100 displays a typical behavior of surfactants which show maximum reflectance just below its critical micelle concentration (CMC). Reflectance then drops following the completed phase transition to micelles at concentrations above the CMC. The Triton X-100 stock solution was from a 10% aqueous stock rather than a DMSO solution. Since surfactants such as Triton X-100 are recommended to disrupt aggregation in a biochemical assay, they are typically used at concentrations higher than their CMC. The loss of compound inhibitory activity on addition of surfactants is another hallmark of aggregation-based inhibition (206).
The white light source from these instruments may be filtered to pass only the red light (610 nm) to avoid fluorescent interference. The use of a stirred cell, as opposed to a plate reader for these measurements, allows higher accuracy, longer path lengths for greater sensitivity, and more reproducible values. Obviously, the design of the nephelometer must allow the inclusion of a magnetic stir bar in the cell with no obstruction of the light path. Some instrument models do not allow this. However, the design of a nephelometer is straightforward, and an instrument could be easily constructed in collaboration with an analytical or engineering department in most universities.
The determination of solubility via nephelometry is not without its caveats. The compound should be relatively free of insoluble matter such as silica gel. Aggregates may be forming, which are beyond the sensitivity of the nephelometer. Compounds which can form micelles give a characteristic initial upward inflection followed by return to baseline at the CMC. In addition, in our hands, some compounds will aggregate at submicromolar concentrations forming sub-micron-sized particles while simultaneously precipitating larger particles, suggesting these are two independent phase transitions from solute. The nanoparticle formation and aggregation may be further characterized after initial nephelometry using dynamic light scattering. Compound A (Figure 24) was analyzed with DLS starting at the low 4 μM concentrations where the nephelometry reflectance was weak. DLS scattering showed a population centering around 80 nm and much larger micron-sized particles at 1–10 μm. Population analysis shows these larger particles increasing dramatically with higher concentrations (not shown).
The workflow we have presented addresses the two most pressing issues we have experienced in assessing high-throughput screening campaigns, unsuitable chemical functionality and problematic solubility. These problems can be effectively addressed with a minimum of effort and cost in an academic setting where resources are often limiting.
References
Historically, pharmaceutical companies were known to be quite generous with supporting academic research. Companies often awarded unrestricted grants that served to support, either directly or indirectly, postdocs in top-tier academic laboratories. The hope was that those trained scientists would eventually find their way into highly competitive roles within their own industrial laboratories. For the interested reader, a number of editorials and case studies provide historical snapshots of academic collaboration trends over time (207-215). Government-based sources of funding for academic research have always been competitive, but historic funding levels by national agencies such as the NIH and NSF continue to decrease, which has led to significantly increased pressure on academics to find alternative sources for funding their research (216). Despite long-standing concerns over reproducibility of data generated in academic laboratories (217, 218), many scientists across the pharmaceutical and biotech industry are routinely collaborating with academic colleagues when it comes to developing enabling tools and knowledge such as in vitro and in vivo models and new technologies for screening and imaging. Some of this partnering is being conducted under the banner of open innovation (219). There is a good deal of partnering conducted in a relatively informal manner at no to minimal cost, but most is conducted only after having established formal material transfer agreements and/or research agreements. In an effort to minimize transaction costs and perhaps create more of a free flow of information and materials, some companies have worked to establish formal institutional relationships with academic institutions and/or research institutes, taking advantage of natural geographical relationships or with the strategic intent of expanding their geographic footprint (219).
1 Conception of LRAP
As the business model within the pharmaceutical industry has continued to evolve, limited resources have been increasingly diverted to support portfolio-enabling priority work, and unrestricted grants appear to be rarely offered in the present environment. In 2010, a group of senior scientists at Eli Lilly and Company sought to reverse this trend in part. They successfully recommended to Dr. Jan Lundberg, the then president of Lilly Research Laboratories, that limited financial resources be set aside in reserve to promote academic partnerships and scientific development of Lilly's best scientists. One of the work streams led by Drs. Jim Stevens and Stephen Burley (distinguished Lilly fellows – investigative toxicology and structural biology, respectively, at the time) worked to propose a new approach to partnering for Lilly called the Lilly Research Award Program (LRAP). The goal of the LRAP program was to support excellent precompetitive science independent of Lilly's direct portfolio-facing work. The hope was that funded research would focus on discovery, development, and technology/methodology-related areas that might serve to augment innovative science at Lilly. Peer-reviewed publications and external scientific presentations of the collaborative research were the main expected outcomes, but if the work were to lead to patentable inventions, a free nonexclusive license to leverage the work was anticipated to be included in the overall agreement.
A critical first step was to work with Lilly's legal team to help define an agreement that would serve to be seen by most academic institutions as an equitable and easily executable research agreement. It was also important to limit the resources required by Lilly legal to get the agreements executed in a reasonably rapid period of time with a very limited amount of negotiation. Lilly had precedent for building contract efficiencies after establishing alignment with the Association of University Tech Transfer Managers (AUTM) to craft an equitable special Material Transfer Agreement that proved to be foundational for Lilly's premier open innovation initiative. That collaborative program was Lilly's phenotypic drug discovery (PD2) program, which evolved and expanded to include other opportunities for crowdsourcing and collaboration. It was subsequently renamed the open innovation drug discovery (OIDD) program (220, 221).
2 LRAP Process
To efficiently initiate the LRAP process with a potential principal investigator, a concise letter of intent (LOI) was crafted, and a process was established for LRAP to have the LOI signed by the appropriate institutional representative. The LOI informs the target institution and academic principal investigator of the key details of the LRAP process and eventual agreement if awarded. This includes (i) a deadline for reaching an agreement once the award is granted, (ii) granting of a nonexclusive license to Lilly of any intellectual property generated as a result of the collaboration, and (iii) appropriate recognition of Lilly scientists' contributions to the research in any publications.
After an internal call for proposals is issued, scientists at Lilly work with their selected academic principal investigator(s) to submit a brief research summary (∼1 page) proposal to the LRAP team. The primary purpose of the research summary document is to ensure that the scope of the submission will adhere to the precompetitive guidelines of research under LRAP. Feedback is provided at that time to help guide the scientists on scope (e.g. is it exciting, and will it be doable?), and any potential legal questions are addressed early. A signed LOI and full proposal is then submitted to the LRAP team. Three internal scientific peers are selected to review the proposal, and one is identified as the primary reviewer. Notably, the authors are blinded to the expert reviewers selected. The reviewers are asked to make a recommendation to advance the proposal (or not) for a full committee review. If a proposal is advanced for review, the primary reviewer is asked to present the proposal's strengths and weaknesses (15 min including Q&A) to the Scientific Committee, which is composed of senior scientists (not Senior Management) from across Lilly. The committee review is conducted similar to the approach used in an NIH study section. Scores from the committee review are used to inform the final decisions for funding. For proposals that are not funded, by rule the authors can elect one time to resubmit a reworked proposal that sufficiently takes into account written feedback from the review process.
Once an agreement is signed, an initial payment (25%) is made to get the project going. At the end of year 1 of an agreement, the Lilly investigator and collaborator prepare a short summary progress report that serves to inform a decision on authorizing a second payment (50%). A final summary report is required prior to issuing the final agreed payment (remaining 25%). Some projects have been cancelled due to insufficient progress on the science, and others have been rescoped based on breaking science. Sometimes, given delays in project progression or just interest in continuing the collaboration, no-cost extensions to the original agreement have been executed.
Since the initiation of the program in 2011, nearly 600 proposals have been reviewed in approximately 15 funding rounds, and at the time of writing about 140 have been fully funded, constituting a commitment that totals >$35M to date. Proposals have been initiated from all of Lilly's key R&D sites, all therapeutic areas, and functional areas ranging from discovery to development, including regulatory and global health outcomes. More than 80 institutions have been engaged in LRAP agreements. Many of the agreements have been signed in <90 days and with minimal negotiation on the details of the agreements. The program is managed by a small group of volunteers who make up the LRAP operations team, and their combined effort represents approximately 0.5 full-time employee commitment. At the time of writing, we have logged more than 70 peer-reviewed publications (just from the first 11 LRAP rounds) that have issued across a broad range of disciplines citing the collaborative work conducted under the banner of the LRAP program. A select set of seven example publications are cited below that demonstrates the type of precompetitive work being conducted through this unique approach to industry–academia collaboration. The first example (222, 223) was a collaboration to characterize the binding modes of various ligands to several GPCR targets (M1, M2, and M5 muscarinic acetylcholine receptors) via X-ray crystallography. The second example (224) highlights some of the outcomes of a project seeking to develop new in silico-based approaches to improving process chemistry efficiency as part of Lilly's investment in Green Chemistry production approaches. The final example highlights a collaborative effort to better predict, via in silico methods, the potential existence of novel (e.g. more stable) polymorphic crystal forms of drug candidates that might prove to complicate pharmaceutical development and commercialization (225-228).