The GTP hydrolysis defect of the Saccharomyces cerevisiae mutant G-protein Gpa1G50V
Abstract
The Saccharomyces cerevisiae haploid cell response to pheromone involves two seven-transmembrane-domain pheromone receptors that couple to a heterotrimeric G protein. The G50V mutation in the G protein α subunit (Gα), Gpa1p, is analogous to the p21ras transforming mutation Gly→Val 12, and has been extensively examined for the phenotypes it produces in yeast cells. Here we have characterized the Gpa1G50V mutant protein in vitro by examining GTPγS binding, GDP exchange, GTP occupancy and guanosine triphosphatase (GTPase) activity. Compared to wild-type (WT) Gpa1p, Gpa1G50Vp was found to have a moderately reduced GTPase activity and increased GTP occupancy, while GTPγS binding and GDP exchange were not significantly altered. The yeast regulator of G protein Signalling (RGS) protein, Sst2p, was also expressed and purified, and found to have a significantly reduced ability to stimulate the initial rate of GTP hydrolysis of Gpa1G50Vp compared to its effect on WT Gpa1p. Probing conformational transitions by a protease sensitivity assay suggested that Gpa1G50Vp did not bind the transition state mimetic GDP/AlF4− as efficiently as the WT Gpa1p. These biochemical results can explain many of the known gpa1G50V yeast cell phenotypes. Copyright © 2000 John Wiley & Sons, Ltd.
INTRODUCTION
Numerous cellular signalling pathways use heterotrimeric guanine–nucleotide binding proteins (G proteins; α, β and γ subunits) as molecular switches. Seven transmembrane-domain G protein-coupled receptors (GPCRs) transmit an extracellular signal across the plasma membrane, causing a cognate G protein alpha (Gα) subunit to exchange bound GDP for GTP, and dissociate from its Gβγ subunits. Components of this system remain in an activated state and interact with downstream effectors until the GTP is hydrolysed by the intrinsic guanosine triphosphatase (GTPase) activity of the Gα. Hydrolysis of bound GTP re-establishes the Gα–GDP form, which reassociates with the Gβγ subunits and returns the system to the inactive state (for reviews, see Bourne et al., 1990; Bourne et al., 1991; Neer, 1995; Neer and Smith, 1996). Modulators include the regulator of G protein Signalling (RGS) family members, which have been shown to act as GTPase activating proteins (GAPs) for Gα subunits (for review, see Roush, 1996; Siderovski et al., 1996; Dohlman and Thorner, 1997; Berman and Gilman, 1998; Kehrl, 1998). RGSs have been shown to inhibit G protein signalling in cells, an effect that is presumed to be a result of a shift in the G protein equilibrium toward the inactive Gα-GDP form.
The Saccharomyces cerevisiae pheromone response pathway involves a G protein system (α, β and γ subunits encoded by the GPA1, STE4 and STE18 genes, respectively) which transduces pheromone signals into cellular responses (Kurjan, 1992; Sprague and Thorner, 1992; Kurjan, 1993; Herskowitz, 1995). Activation of this pathway in haploid MATa and MATα cells results in arrest at G1 of the cell cycle and transcriptional activation of numerous genes involved in mating. The Gβγ subunits have been shown to be positive regulators of the response; cells with deletions in STE4 or STE18 cannot respond to pheromone and cannot mate, hence the original designation of the gene mutations as ‘sterile’. The main role of the alpha subunit, GPA1, is to negatively regulate the pheromone response pathway, since deletions and mutations cause phenotypes associated with activation of the pathway. The phenotypic observations may be translated to a model of the guanine nucleotide hydrolysis cycle. The negative regulation of the pathway by Gpa1p is thought to occur via simple binding of the βγ subunits, preventing their ability to activate the response pathway. In this resting state, Gpa1p–GDP is bound to the βγ subunits (Ste4p/Ste18p), and pheromone signalling is off. Upon binding of pheromones (α-factor or a-factor) to pheromone receptors (Ste2p or Ste3p), Gpa1p-GDP exchanges its GDP for GTP, and Ste4/Ste18p is released, activating the downstream pheromone response pathway. This signal is propagated through a MAP kinase cascade, which ultimately impinges on the transcription factor Ste12p. Upon hydrolysis of the GTP bound to Gpa1p, Gpa1p–GDP reassociates Ste14p/Ste18p, and the system is returned to its resting state. Other roles for Gpa1p in signal modulation have been suggested, in addition to Gβγ sequestration, but remain controversial (Miyajima et al., 1989; Stone and Reed, 1990; Kurjan et al., 1991; Stratton et al., 1996; Xu and Kurjan, 1997). The roles of other components, such as the phosphatase MSG5 (which acts at the level of the MAP kinase) (Doi et al., 1994; Zhou et al., 1999), MPT5 (Chen and Kurjan, 1997), and SGV1 (Irie et al., 1991) in pheromone response and recovery remain poorly understood. Moreover, some of these proteins may be sensitive to the activation state of Gpa1p.
The SST2 gene, originally identified by mutations that increased the sensitivity of haploid cells to pheromone (Chan and Otte, 1982a, b), encodes a member of the RGS family of proteins, and has been shown to act as a GAP for Gpa1p (Apanovitch et al., 1998). Mutations in SST2, like those in GPA1, have been shown to cause defects in mating and in pheromone response, which would be expected for a protein that regulates their GTP hydrolysis activity of Gpa1p. But some of the effects of Sst2p on pheromone response and recovery likely involve its interactions with proteins other than Gpa1p; in one case it was isolated through its interaction with Sst2p in a two-hybrid screen (Chen and Kurjan, 1997).
All G proteins contain functionally homologous protein sequence motifs, one of which is referred to as the phosphate binding loop, or P-loop [G(X)4GKS/T sequence]. The P-loop includes the Gly 12 residue of human p21ras, which produces a transformed phenotype in cell lines and where mutations have been found in a large number of malignancies (Gibbs et al., 1984; Seeburg et al., 1984; Barbacid, 1987). Biochemically, the p21Ras-G12V mutant protein was shown to display a greatly reduced GTPase activity, which was not capable of being activated by ras GAP (Trahey and McCormick, 1987). Interestingly, not all mutations analogous to the G12V mutation of p21ras have produced the same effects on heterotrimeric G protein alpha subunits. GTP hydrolysis of the Giα1G42V protein was shown to be reduced 30-fold when compared to wild type Giα1 and, similar to the p21ras-G12V, was not activated in the presence of the GAP RGS4 (Raw et al., 1997). However, GTP hydrolysis of the GsαG49V mutant was not shown to be severely compromised, exhibiting a kcat that was 25% of WT (Graziano and Gilman, 1989).
Yeast cells harbouring the gpa1G50V allele have been shown to exhibit several unique phenotypes (Miyajima et al., 1989; Kurjan et al., 1991; Xu and Kurjan, 1997). They grow more slowly than WT cells, and some cells are large and aberrantly shaped. These phenotypes are similar to what has been seen in gpa1 deletion strains, although the G50V phenotypes are less severe than null phenotypes. They also show a very high basal expression level of a pheromone inducible reporter gene, FUS1–lacZ, and a greatly reduced mating efficiency. Finally, the pheromone spot phenotype is unusual in that the halo produced by a pheromone spot on a lawn of gpa1G50V cells is larger than a WT halo, and the halo is turbid, while WT halos are clear. The larger halo size would suggest that gpa1G50V cells are more sensitive to pheromone, since cells further away from the centre of the spot are responding to lower pheromone concentrations. However, the turbidity of the halo indicates either that the cell cycle arrest is incomplete, or that recovery from cell cycle arrest is improved. Since knowledge of the specific biochemical defects of the Gpa1G50V protein is crucial to a more complete understanding of these phenotypes, we expressed and purified WT and G50V Gpa1 proteins, and compared their activities in vitro. We also purified the pheromone response RGS protein, Sst2p, to examine its ability to stimulate Gpa1p and Gpa1G50Vp GTP hydrolysis.
METHODS
Chemicals and enzymes
Chemicals were purchased from Fisher (Pittsburgh, PA), Sigma (St Louis, MO), Biorad (Hercules, CA), Boehringer-Mannheim (Germany), and Amresco (Solon, OH). Restriction enzymes and ligases were purchased from Boehringer-Mannheim (Germany) and New England Biolabs (Beverly, MA). ExpandTM high fidelity polymerase for PCR was purchased from Boehringer-Mannheim (Germany), and [γ-32P]GTP (6000 ci/mmol), [35S]GTPγS (1250 ci/mmol) and [3H]GDP (11 ci/mmol) were purchased from New England Nuclear (Boston, MA).
Construction of Gpa1 and Sst2 overexpression vectors
General molecular biology techniques used in the manipulation of DNA and bacterial cells are described in Ausubel et al. (1987). Escherichia coli (E. coli) strain TG1 was used for plasmid maintenance and subcloning. Methods used in the maintenance and transformation of yeast cells are described in Ausubel et al. (1987; Rose et al., 1990).
Gpa1 expression vector
The template DNA used for PCR was the pRS313–1.9 plasmid, which contains the complete GPA1 gene and has been previously described (Kallal and Kurjan, 1997). The N-terminal primer, LKNDH, added an NdeI site, an initiation methionine, and six histidine (His6) residues to Gpa1, and the C-terminal primer 3′BE added a BamHI site at the original site of the 3′ EcoRI site. LKNDH primer sequence: GGA ATT CCA TAT GCA TCA CCA TCA CCA TCA CAT GGG GTG TAC AGT GAG C. The 3′BE sequence is listed in Kallal and Kurjan (1997). The 50 µl PCR reactions contained the manufacturer's (Boehringer-Mannheim, Germany) recommended buffer, 1.75 mM MgCl2, 50 ng of template DNA, 1 µM primers and were cycled 30 times at 93°C for 30 s, 45°C for 30 s and 68°C for 2 min. The GPA1 PCR product was digested with NdeI and BamHI and inserted into pET3a (Novagen) that had been digested with the same enzymes. The clone was sequenced in its entirety to ensure a wild-type sequence. To construct the G50V mutation in GPA1, T3 (ATT AAC CCT CAC TAA AG) and G50V (ACC TGA CTC AAC GGC ACC TAA) primers were used to amplify a 50V DNA fragment from 5 ng pSK(His6)–Gpa1 template (5 ng). The pSK(His6)–Gpa1 template plasmid was constructed by inserting the XbaI–BamHI fragment from PET3a(His6)–GPA1 into pBluescript SK+ (Stratagene, La Jolla, CA.) using the same enzymes. An overlapping PCR reaction was then performed using T3 and 3′BE primers, and a mixture of 50V PCR fragment and a full-length GPA1 1.9 kb EcoRI gel purified restriction fragment as template (50:1 ratio; approximately 100 ng:2 ng). The resulting PCR product was digested with XbaI and BlpI (also known as EspI) and ligated into the larger gel-purified fragment of pSK(His6)–Gpa1 that had been digested with the same enzymes. Candidates were sequenced across the region of the clone encoding amino acid 50, the unsequenced region of the clone 3′ to the HindIII site (HindIII–BamHI from pSK(His6)–Gpa1) was replaced with that fragment from a wild-type clone, and the entire (His6)–Gpa1G50V fragment from this pSK(His6)–Gpa1G50V was removed and put into PET3a using XbaI and BamHI.
Sst2 expression vector
The SST2 open reading frame, plus approximately 150 bp 3′ to the stop codon, was amplified using the same parameters listed above for GPA1, except that a final concentration of 2.25 mM Mg was used in the PCR reactions, and 500 ng yeast genomic DNA was used as template. The N-terminal primer, 5′SSTHH, added an NcoI site, an initiator methionine, and six histidine residues to SST2. The C-terminal primer, 3′ SST2, added a BamHI site. Primer sequences were as follows: 5′ SSTHH, CGC GGA TCC ATG GCG CAC CAC CAC CAC CAC CAC GGC ATG GTG GAT AAA AAT AGG; 3′ SST2, AGG GGA TCC AAG CGC TCA CGT TAG TCA. The PCR product and pET11d vector (Novagen) were digested with NcoI and BamHI, and ligated together. Several cloned SST2 constructs were sequenced entirely to confirm a wild-type SST2 sequence.
Similar PCR reactions and subcloning were performed on strain IH993 (kindly provided by C. Brenner, Thomas Jefferson University; MATα sst2–1 met1 his6 can1 cyh2) which contains the sst2–1 allele of SST2 (Chan and Otte, 1982a, b). Three separate reactions were performed and three individual subclones were sequenced entirely. All of the sequences were identical and contained the specific alterations of the sst2–1 coding sequence described.
pGal plasmids and complementation tests
To construct galactose-inducible (His6)–Sst2 and (His6)–Gpa1 expression plasmids for yeast complementation tests, extra restriction sites were added to a pGal vector (Kang et al., 1990) using an XbaI, NcoI, SacI linker with overhangs compatible for ligation into the HindIII and BamHI sites of pGal, creating pGalXNS. The three-site linker was created using complementary oligos, XNSW; AGC TTT CTA GAC CAT GGA GCT CG, and XNSC; GAT CCG AGC TCC ATG GTC TAG AA. The (His6)–Gpa1 and (His6)–Sst2 constructs in PET3a and PET11d (above) were cut out and inserted into pGalXNS with XbaI and BamHI, creating pGal(His6)–Gpa1 and pGal(His6)–Sst2. BssHII sites between codons 73 and 137 in the SST2 open reading frame and in Pet11d were used to separate the I73T and G137E mutations by substituting this fragment from the sst2–1 allele into the WT allele and vice versa. The Pet11d I73T and G137E clones were then inserted into pGalXNS, using XbaI and BamHI. Mating and pheromone spot tests were performed as previously described (Kallal and Kurjan, 1997). Overnight liquid cultures used for lawns in the SST2 complementation tests were grown in minimal dropout media selecting for the pGalURA3 plasmid. For the pGal(His6)–Gpa1 complementation tests, all cultures were grown in galactose (2% galactose, 1% raffinose, 0% glucose) minimal drop-out media. The WT yeast strain used was W3031A (MATa), which contains leu2, his3, trp1 and ura3 mutations, and the mating tester strain N4352A (MATα) have been previously described (Kallal and Kurjan, 1997). The MATa gpa1::LEU2 strain and the MATasst2::LEU2 strain used in the complementation tests were gifts from Janet Kurjan, University of Vermont (Dietzel and Kurjan, 1987a, b).
Purification of Gpa1p and Sst2p
The hexa-histidine-tagged expression constructs for Sst2 and Gpa1 were transformed into the E. coli strain BL21pLysS (Novagen). 1 litre LB cultures supplemented with 100 µg/ml ampicillin and 20 µg/ml chloramphenicol were inoculated with 10–25 ml of an overnight culture, and grown at 37°C for several hours to an OD600 of approximately 1. IPTG was then added to 1 mM, and the culture was incubated for several additional hours at 37°C for Gpa1p, and 30°C for Sst2p. Cells were centrifuged, washed in 50 mM Hepes, pH 8, 10% glycerol, 300 mM NaCl (cell washing buffer) plus protease inhibitors (1 µg/ml leupeptin, 1 µg/ml pepstatin, 0.5 mM PMSF), frozen at −80°C, thawed, refrozen and thawed twice more, and spun at 40 000 rpm for 1 h to prepare extracts. For Gpa1p, proteins precipitated in 40% ammonium sulphate were resuspended in cell washing buffer without NaCl, and loaded onto Ni-chelate columns (His-bind resin, Novagen). For Sst2p, the cell lysate was loaded directly onto the Ni column. Proteins were eluted with a 0–250 mM imidazole gradient. Fractions were analysed by SDS–polyacrylamide gel electrophoresis (PAGE), and desired fractions were pooled and loaded onto MonoQ columns. Proteins were eluted from the MonoQ column with a 0.1–2.5 M NaCl gradient. Fractions were again examined by SDS–PAGE, and selected MonoQ fractions were then dialysed against 50 mM Hepes pH 8, 1 mM DTT, 0.1 mM EDTA (Protein Storage Buffer), plus 50% glycerol for Gpa1p, and stored at 4°C or −20°C until use. All chromatography was performed on Pharmacia FPLC systems. Protein concentrations were determined using the Bio-Rad Protein Assay (Biorad, Hercules, CA).
Biochemical analysis
Methods used in GTP hydrolysis, GTPγS binding, GDP exchange, and GTP occupancy, are approximately as described in (Graziano and Gilman, 1989; Berman et al., 1996; Raw et al., 1997). Brief protocols and unique aspects of procedures are as follows.
Nucleotide hydrolysis
For steady-state GTPase assays, Gpa1 protein was mixed with 5 µM γ[p32]-GTP (approximately 100cpm/fmol) in GTPase reaction buffer (25 mM Hepes, 25 mM NaCl, and 1 mM DTT) containing 10 mM MgSO4. The reactions were incubated at the temperatures indicated in the figure legends. Reactions were stopped with 10-fold volumes of 50 mM Na-phosphate (pH 2)/10% activated charcoal, centrifuged for 10 min, and supernatant samples containing 32Pi were counted in a scintillation counter (Beckman). For initial rate (single turnover) experiments, Gpa1 protein was incubated with 0.5 µM [γ-32P]GTP (approximately 100cpm/fmol) in GTPase reaction buffer con‒taining 5 mM EDTA. The GTP was allowed to bind to Gpa1p for 40 minutes at 4°C, and then the reaction was brought to the temperature at which the assay was to be performed. A zero time sample was taken, and then Sst2p (or protein storage buffer), 10 mM MgSO4, and 150 µM cold competitor GTP were added at the beginning of the time course. Samples were withdrawn for each time point, stopped and processed to measure released phosphate as described above. Single turnover results were curve-fitted to B=Beq(1−e−kt) as described in (Raw et al., 1997) to estimate kcat values.
Nucleotide binding, exchange and occupancy experi‒ments
For [35S]GTPγS binding, 100 nM Gpa1p or Gpa1G50Vp was mixed with 0.4 µM [35S]GTPγS (approximately 300cpm/fmol) in GTPase reac‒tion buffer containing 10 mM MgSO4. Samples were withdrawn at various times and mixed with 5 mls cold wash buffer (25 mM Hepes pH 8, 100 mM NaCl, 5 mM MgSO4), filtered over nitrocellulose filters (HAWP 025, Millipore, Bedford, MA), and washed three times with 5 ml cold wash buffer. Filters were then counted in a scintillation counter. For GDP exchange assays shown, 100 nM protein was incubated with 0.2 µM [3H]GDP (approximately 30 cpm/fmol) for 40 min on ice in GTPase reaction buffer containing 10 mM MgSO4. The mixture was then warmed to room temperature, and exchange was started by the addition of 10 µM GTP. Time point samples were withdrawn, filtered and counted as for [35S]GTPγS binding assays.
Protease digestion assays
For initial experiments (not shown), gels were silver stained, and 100–500 ng Gpa1 protein per gel lane was incubated with 100 µM GDP, GTPS, or GDP plus 10 mM NaF and 60 µM AlCl3 (‘GDP/AlF4−’) for 1 h at 30°C. EndoproteinaseLysC (LysC) was added at concentrations ranging from 0.0025 ng/µl to 10 ng/µl. For the experiments shown, 1 µg Gpa1 protein per gel lane was incubated with nucleotides using the same conditions listed above, followed by a 20 min incubation at 37°C after the addition of 0.3 or 2 ng/µl endoproteinaseLysC. Samples were then loaded onto SDS polyacrylamide (10%) gels, and after electrophoresis, stained with Coomassie Blue.
RESULTS
Hexahistidine-tagged GPA1 and SST2 complement deletions in vivo
To simplify purification of Gpa1 and Sst2 proteins, a methionine and a six-histidine (His6) tag was added to the N-terminus of each protein (see Methods). To test the ability of the His6 tagged versions of SST2 and GPA1 to complement deletions of these genes, the (His6)–Gpa1 and (His6)–Sst2 constructs were inserted into a galactose-inducible yeast expression vector (see Methods), and transformed into gpa1 or sst2 deletion strains. Since a deletion in GPA1 prevents growth of haploid cells, the gpa1Δ strain harbours a plasmid containing the MATα allele, pTrpMATα, to allow growth of the strain (Kurjan et al., 1991). Transformants were then tested in two ways for (His6)–Gpa1 function. First, cells were grown in rich galactose-containing media overnight, diluted and plated for growth of single colonies. Out of 56 isolates tested for pTrpMATα loss, all of the gpa1Δ cells originally containing only the pTrpMATα plasmid were still TRP+, indicating a growth requirement for the plasmid. In the cells transformed with the pGal(His6)–Gpa1 plasmid, only 25 out of 64 colonies tested contained pTrpMATα, a 61% loss. This result indicates that the (His6)–Gpa1 plasmid complements the growth defect in the gpa1Δ cells. Secondly, several pGal(His6)–Gpa1 transformants that had lost pTrpMATα were then tested for their ability to mate. Results reveal that (His6)–Gpa1 supports mating in haploid cells, confirming its ability to function in yeast cells (Figure 1A). These complementation results are interesting, since our N-terminally tagged protein is unlikely to be N-terminally myristolylated because a free methionine is required for this modification. Studies with the Gpa1 myristoylation site mutant, Gpa1–G2A, indicated that it did not complement the gpa1 null mutant growth defect, reportedly due to membrane mislocalization (Song et al., 1996; Stone et al., 1991). It is possible that our overexpression vector produces sufficient intracellular protein levels to enable Gpa1p to reach the plasma membrane and couple to Ste4p/Ste18p, or that yeast cells proteolytically process the (His6)–Gpa1 construct, which is followed by appropriate myristolylation. Regardless, many mammalian G proteins tagged at their N-termini have been shown to bind and hydrolyse GTP normally, suggesting that the (His6) tag is unlikely to affect the nucleotide binding and hydrolysis properties of Gpa1p.
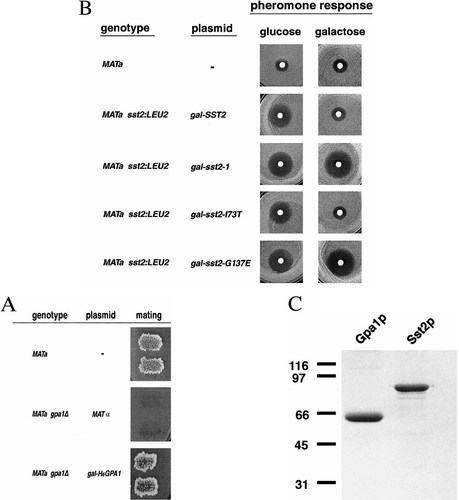
Complementation of the hexa-his tagged constructs and purification of proteins. (A) Mating complementation of the His6Gpa1 clone under the control of a galactose-inducible promoter. A gpa1 deletion strain, containing a MATα plasmid, was transformed with pGal–His6Gpa1, cured of the MATα plasmid, and patches were printed onto lawns of MATa mating tester strains. Growth on the diploid-selective minimal plate indicates mating. (B) Pheromone spot tests were performed on sst2–1 mutant strains containing the indicated plasmids. 0.3 ml of cells at an OD600=0.2 was spread onto URA drop-out plates containing either glucose or galactose and raffinose, they were briefly allowed to dry, and 8 µg α-factor in a 4 µl volume was spotted onto a filter disc on the centre of each plate. Plates were incubated for 30 h at 30°C. (C) Coomassie Blue-stained SDS–PAGE of purified Gpa1p, Gpa1G50Vp and Sst2p. Molecular weight marker sizes are shown to the left of the gel. Approximately 1.5 µg of each protein was loaded onto the gel.
Similarly, the pGal(His6)–Sst2 plasmid was transformed into a yeast strain containing a gene disruption in SST2, and tested for its ability to complement the abnormal pheromone response phenotype exhibited by sst2 mutant yeast strains. Since deletion of SST2 is not lethal to yeast cells, a pheromone spot test was performed under conditions where (His6)–Sst2 was not expressed from the plasmid (on glucose), and on galactose plates where the expression of (His6)–Sst2 is driven from a gal-inducible promoter. Typical pheromone spot tests indicated that expression of (His6)–Sst2 restored wild-type pheromone response to the sst2 mutant strain in a galactose-dependent manner (Figure 1B).
To examine the sequence defect of mutant sst2–1 allele (Chan and Otte, 1982a), which results in a pheromone response defect that is similar to that of a null allele, we isolated the Sst2 gene from the sst2–1 yeast strain (see Methods). The sst2–1 allele was found to consist of three amino acid alterations when compared with the published Sst2p ORF sequence (Dietzel and Kurjan, 1987a): I73T, G137E and D616H. The D616H alteration likely represents a polymorphism, since it was present in our wild-type W3031A strain. When the I73T and G137E mutations were separated and tested individually, only the G137E mutation was shown to be the causative mutation (Figure 1B). We intended to examine the biochemical activity of Sst2–1 and Sst2G137E proteins, but attempts to purify either the Sst2–1p or Sst2G137Ep suggested that these proteins were unstable in E. coli; no proteins of the appropriate size could be visualized in samples of bacterial extracts or column fractions on Coomassie Blue-stained SDS polyacrylamide gels. Since the sst2–1 mutant protein has been detected in yeast cell extracts (Dohlman et al., 1995), it is unclear why we were unable to express this protein in E. coli cells.
Expression and purification of Gpa1p and Sst2p
The purification of His6-tagged Gpa1p and Sst2p resulted in near-homogeneous preparations of these proteins (Figure 1C). Single major protein bands migrating at the expected size of 54 kDa were visible in Gpa1 and Gpa1G50V-purified protein fractions, and a band migrating at 80 kDa was visible in the purified Sst2 protein fractions. The level of protein purity as estimated from Coomassie Blue-stained SDS–polyacrylamide gels was at least 95% for Gpa1p and 90% for Sst2p. During purification, protein fractions were assayed for GTPase and GAP activities, both to identify protein-associated activities as well as to eliminate contaminating activities. After Ni-chelate column chromatography, the preparation of Sst2p contained a small amount of GTP hydrolysing activity, which was separated away from Sst2p during the MonoQ chromatography step (data not shown).
Steady state GTPase activity, GDP exchange, and GTP occupancy
Steady state hydrolysis of GTP by purified Gα subunits is generally a measure of the rate of GDP exchange, usually the rate-limiting step in the absence of activated receptors. Experiments with Gpa1p indicated the GTP hydrolysed was proportional to the amount of Gpa1p added to the assay, and steady-state GTP hydrolysis was linear over time with a range of concentrations of Gpa1p (Figure 2A). The specific activity of Gpa1 calculated from a number of individual preparations was approximately 2 nmol GTP hydrolysed/min/mg Gpa1p at 30°C. This is comparable to steady-state activities reported for mammalian Gα subunits such as Gαi and Gαo, (Voiekov et al., 1986; Linder et al., 1990; Berman et al., 1996; A. Pronin personal communication). The Gpa1G50V protein steady-state hydrolysis was approximately 30% of the WT level (Figure 2B). Per molecule of Gpa1p, the turnover rates were calculated to be approximately 0.16/min for WT Gpa1p, and 0.051/min for Gpa1G50Vp. Our studies indicate a WT Gpa1p steady-state hydrolysis activity that is approximately four-fold above that previously reported for Gpa1p at the same assay temperature (Apanovitch et al., 1998).
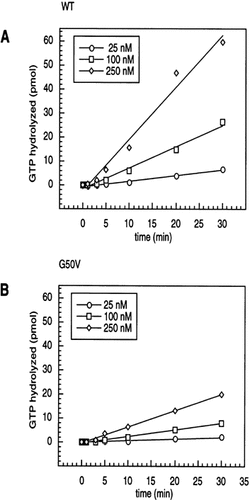
Steady-state GTPase activity of Gpa1p (A) and Gpa1G50Vp, (B). GTPase time courses were performed with the indicated concentrations of Gpa1p at 30°C in the presence of 5 µM [γ-32P]GTP. Samples were stopped at the various time points on ice and processed (see Methods), and released 32Pi was quantitated in a scintillation counter. Data represent the average of duplicate samples. Experiments were replicated twice.
Since the complete GTP hydrolysis cycle involves GTP binding, GTP hydrolysis, and GDP exchange, partial reactions were examined to identify the particular defect(s) associated with the G50V mutant. First, binding of a non-hydrolysable radiolabelled analogue of GTP, [35S]GTPγS, was examined (Figure 3A). The rate of GTPγS binding appears similar for the WT and G50V proteins, although the G50V protein reproducibly bound nucleotide at a slightly faster rate. In these and other experiments (Figure 3C), the longer incubations of both proteins with [35S]GTPγS resulted in equal amounts of total nucleotide bound for equivalent amounts of protein, suggesting that only the rate of binding was different, not the total amount of nucleotide bound. The GDP exchange rates were also generally similar between WT and G50V proteins (Figure 3B). However, the Gpa1G50V protein exchanged the [3H]GDP for GTP slightly faster than WT Gpa1p: 0.11–0.12/min for Gpa1p and 0.14–0.18/min for Gpa1G50Vp. Comparison of the Gpa1p exchange rate (0.11–0.12/min) to the steady-state hydrolysis rate (0.16/min) for the WT protein reveals that these numbers are similar, suggesting that the exchange rate is limiting for the Gpa1p in the steady-state hydrolysis reaction, as has been shown for other Gα subunits. It is likely that the small difference between the steady-state hydrolysis and GDP exchange represents the different incubation temperatures at which the assays were performed (30°C for steady-state hydrolysis, and room temperature for GDP exchange). In contrast, the steady-state hydrolysis rate for Gpa1G50Vp was measured at 0.051/min, while GDP exchange was normal at 0.14–0.18/min. These results suggest that GDP exchange is not limiting in this case and that the GTP hydrolysis step itself has been affected in this mutant.
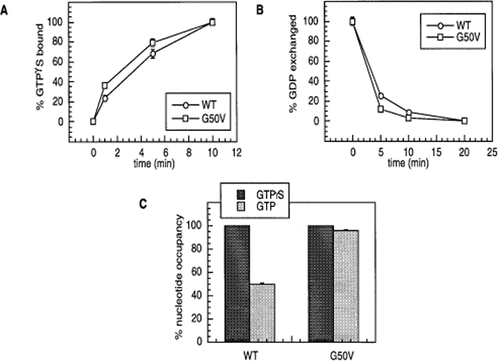
Nucleotide binding, exchange and occupancy of Gpa1p and Gpa1G50Vp. (A) Time course of Gpa1 protein binding to [35S]GTPγS; 100 nM protein was mixed with 0.4 µM [35S]GTPγS at room temperature, and samples were withdrawn at the indicated times. Samples were then filtered, washed, and the [35S]GTPγS bound to filters was quantitated by scintillation counting. Data shown represent the average±SEM of duplicate samples in a representative experiment, which has been replicated twice. (B) GDP exchange was measured by incubation of 100 nM protein with 0.2 µM [3H]GDP in GTPase reaction buffer containing 10 mM MgSO4 for 40 min at 4°C, and then warmed to room temperature. To start the exchange, GTP was added to 10 µM, and samples were withdrawn at the indicated times and filtered, as described above for [35S]GTPγS binding. Data represent the average±SEM of duplicate samples; these results have been replicated twice. (C) Nucleotide occupancy experiments: protein was mixed in reaction buffer containing 10 mM MgSO4, and split into two aliquots. To one, [35S]GTPγS was added to 2 µM, and to the other [γ-32P]GTP to 2 µM. Samples were then incubated at room temperature for 20 min, stopped in cold wash buffer, filtered, washed, and the radioactivity counted, as described in (A) and (B). Data represent the average of duplicate samples, ±SEM, and have been replicated three times.
Independent assessment of the relative amounts of [35S]GTPγS and [γ-32P]GTP bound by Gα subunits at equilibrium allows quantitation of their fractional nucleotide occupancies by GTP. In these experiments, Gpa1 proteins were divided into two aliquots and incubated in one case with [35S]GTPγS and in the other with [γ-32P]GTP. Incubation was allowed to proceed long enough so that equilibrium was presumably reached, but not long enough to hydrolyse a significant portion of the GTP in the sample incubated with [γ-32P]GTP. The protein samples were then bound to filters, washed, and the filter-bound radioactivity was quantitated (Figure 3C). The Gpa1G50Vp exhibits a higher GTP occupancy than the Gpa1p in this assay (96%, compared with 50% for WT). Since a reduced ability to hydrolyse GTP would result in more GTP remaining bound to the protein, these data also suggest that GTP hydrolysis by Gpa1G50Vp is reduced.
GTP Hydrolysis and Effect of Sst2p
To directly measure the catalytic rate of GTP hydrolysis (kcat), the reaction must be measured under conditions where the assay is not limited by GDP exchange. One way to accomplish this experimentally is to measure only one round of GTP hydrolysis (see Methods). Under these conditions, it becomes possible to quantitate the effect of the RGS protein, Sst2p, on the catalytic rate of GTP hydrolysis by Gpa1p. Since a single round of hydrolysis occurs rapidly at temperatures of 20°C or above (not shown), assays were performed at 4°C (Figure 4), similar to protocols used in assessing the effects of other RGS proteins. We found that the unstimulated rates of catalysis for both Gpa1p and Gpa1G50Vp were slow in the absence of Sst2p. The estimated kcat at 4°C for Gpa1p and Gpa1G50Vp were approximately 0.21/min and 0.043/min, respectively (see Methods). Multiple representative experiments gave similar results, the WT Gpa1p rate appearing somewhat faster than that reported by Apanovitch et al. (1998). These data revealed a catalytic defect in Gpa1G50Vp, although its activity is approximately 20% of the wild-type, and thus constitutes only a moderate defect.
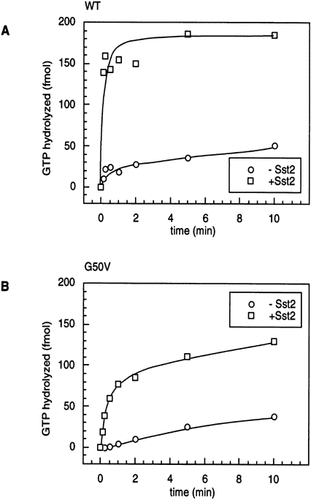
Stimulation of initial rate of GTP hydrolysis by Sst2p. (A) 25 nM Gpa1p, or (B) 25 nM Gpa1G50Vp, was incubated with 0.5 µM [γ-32P]GTP for 40 min at 4°C in GTPase reaction buffer containing 5 mM EDTA. A time 0 sample was removed, and MgSO4, cold GTP and Sst2p were added to 10 mM, 150 µM and 100 nM, respectively, at the beginning of the reaction. Samples were taken at the indicated times, stopped and processed (see Methods), and released Pi was quantitated. Gpa1G50Vp/Sst2p experiments have been replicated at least four times and Gpa1p/Sst2p experiments have been replicated at least six times. The GTP hydrolysed in these assays represents a single round of GTP hydrolysis.
For Gpa1p, the Sst2p stimulated GTPase activity is extremely rapid, even at 4°C (Figure 4A). The rate of GTP hydrolysis by Gpa1G50Vp is also stimulated by Sst2p, but to a lesser extent. The precise degree of kcat stimulation by Sst2p was very difficult to estimate, not only because of the speed of the reactions, but also because the GAP activity of purified Sst2p was extremely unstable; a single freezing cycle, or storage of the protein for more than 7 days at 4°C, resulted in an almost complete loss of activity. This was not due to protein degradation, since SDS–PAGE and Coomassie Blue staining revealed full-length protein in inactivated fractions. Regardless, freshly prepared Sst2p reproducibly stimulated the kcat of Gpa1p at least 30–100-fold (Figure 4A; 80-fold). This result is comparable to levels of stimulation that have been observed with mammalian RGS proteins, as well as for Sst2p (Apanovitch et al., 1998, and references therein). Sst2p reproducibly stimulated the kcat of Gpa1G50Vp only six-fold to 20-fold (Figure 4B; 14-fold), indicating that the Gpa1G50Vp is not as efficiently stimulated by Sst2p as the wild-type Gpa1p.
Protease digestion assay: detection of conformational shifts
Gα subunits are commonly subjected to partial proteolysis with trypsin to detect different conformational states; such assays performed in the presence of various guanine nucleotides display different digestion patterns. In general, Gα proteolytic products can be protected to varying degrees in the presence of activating nucleotides such as GTP, GPPNP, GTPγS, and GDP/AlF4− when compared with similarly treated samples in the presence of GDP. Attempts to probe conformational transitions in Gpa1p using trypsin were unsuccessful, although presumed target cleavage sites in the switch II region are present.
Since proteolysis provides a convenient method for probing conformational transitions, we examined the possibility that another protease might be useful in these assays. Based on the amino acid sequence, proteases that would recognize sites in the Gpa1p switch II region (amino acids 321–333) were identified, and several were tested. We identified Endoproteinase LysC (LysC) as a useful protease, and proteolytic treatment conditions were optimized. In these assays, Gpa1p exhibited a much greater sensitivity to LysC digestion in the presence of GDP (Figure 5, panel A, lanes 4–6) than in the absence of nucleotide (panel A, lanes 1–3). In addition, the activating nucleotides GDP/AlF4− and GTPγS prevented complete digestion of a 30 kDa band at higher concentrations of protease (panel A, lanes 9 and 12) compared to the GDP treatment, where the band was completely absent (panel A, lane 6). However, when Gpa1G50Vp was subjected to the same analysis, the 30 kDa band was not protected in the presence of GDP/AlF4− at the higher protease concentration (lane 9 in panel B vs. panel A). This result indicates that Gpa1G50Vp has a reduced ability to bind AlF4−. Because the 30 kDa band was still protected in the Gpa1G50Vp sample incubated with GTPγS (panel B, lane 12), the protein appeared competent to reach the active conformation required for binding this nucleotide. Since GDP/AlF4− is thought to mimic the GTPase reaction transition state, and GTPγS the active state, reduced binding to AlF4− would be expected in a protein that is catalytically deficient.
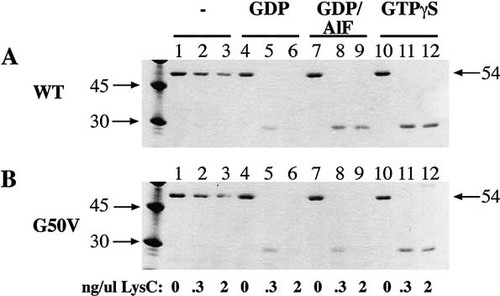
Conformational shift of Gpa1p and Gpa1G50Vp. 1 µg of protein for each gel lane was incubated with GTPase reaction buffer containing 10 mM MgSO4 and the indicated guanine nucleotide at 100 µM. LysC was then added to 0, 0.3, or 2 ng/µl, samples were incubated at 37°C for 20 min, run on SDS–polyacrylamide gels, and stained with Coomassie Blue. The 45 kDa and 30 kDa size markers are indicated on the left side of each gel, and size of full-length Gpa1p is 54 kDa, indicated on the right.
DISCUSSION
The complementation of a gpa1 deletion by our (His)6–Gpa1 construct was surprising, based on the idea that N-terminal myristoylation might be blocked by the (His)6 tag and by analogy to studies with the G2A myristoylation site mutant (Song et al., 1996). We consider the possibility that our levels of overexpression were higher than those that have been tested for the Gpa1–G2A mutant, or that our (His6)–Gpa1 construct is proteolytically cleaved, removing the histidine tag and exposing the N-terminus, which could then be myristoylated. It is also possible that the G2A myristoylation mutant does not complement for reasons other than its lack of the myristoyl moiety, although the Gpa1–G2A mutant was shown to correctly bind Ste4 in a nucleotide-sensitive manner in vitro with GST fused to its C-terminus (Song et al., 1996). We consider it likely that our His6-tagged Gpa1 protein can associate correctly to Ste4, whether or not it is myristoylated. In addition, our results and those of others (Apanovitch et al., 1998), indicate that the N-terminally tagged Gpa1 interacts with its RGS protein Sst2p, and that this interaction appropriately increases the Gpa1p GTP hydrolysis. Because many G proteins have been purified as N-terminally tagged proteins and been shown to behave similarly to their untagged counterparts in biochemical analyses, we expect that our results closely reflect the properties of native Gpa1p.
The LysC protease conformational-transition assay represents a useful tool in the assessment of the conformation transitions of Gpa1p in the presence of various guanine nucleotides. The correlation of reduced GTP hydrolysis and reduced ability to bind AlF4− suggests that the Gpa1G50V mutant protein is defective in its ability to reach a GTP hydrolysis transition state. In addition, Sst2p, like some other RGS proteins, has been shown to bind preferentially to its GDP/AlF4− activated Gα subunit (Apanovitch et al., 1998). A reduced ability of Gpa1G50Vp to reach the transition state would decrease Sst2p binding, reducing its ability to stimulate GTP hydrolysis. Likewise, we find that Gpa1G50Vp is not stimulated as efficiently by Sst2p as is the WT Gpa1p (Figure 4).
The LysC digestion results obtained with Gpa1p and the G50V mutant (Figure 5) are essentially identical to those obtained with tryptic digests of Giα1 and the analogous mutant protein, Giα1G42V; the mutant protein was not resistant to protease digestion after incubation with GDP/AlF4− (Raw et al., 1997). Similar tryptic digestion results have been obtained with other Gα subunits, such as Gαs (Graziano and Gilman, 1989). Preliminary results indicate that the LysC protease detects conformation changes in Gα subunits other than Gpa1p (not shown). Hence, LysC could be used in supplementing tryptic digest results, or in other situations where trypsin digestion is not informative.
Crystallographic studies of the Giα1G42V protein indicate that the substituted valine sterically displaces the catalytic Q204 of the switch II region into an incompetent position, explaining the reduced GTPase activity observed in this mutant (Raw et al., 1997). This is similar to proposed consequences of the G12V substitution in p21ras, although the Ras crystal structures were less ordered in the switch II region, and results were not as conclusive (Pai et al., 1989; Krengel et al., 1990; Prive et al., 1992). The analogous valine at position 50 in Gpa1p would be expected to cause similar steric hindrance to the analogous catalytic Q323. However, since only a moderate reduction in catalytic activity was observed (Figure 4), our biochemical results suggest that the Gpa1p structure is not as drastically affected by the valine substitution as is Giα1.
With the characterization of the Gpa1G50V protein, known yeast cell phenotypes can be at least partially explained by the biochemical defects. The growth defect, cell morphology, high basal Fus1–lacZ expression, and larger halo size in pheromone spot tests are all consistent with a GTPase defect, as has been suggested (Miyajima et al., 1989; Kurjan et al., 1991; Xu and Kurjan, 1997). Since GTP hydrolysis defects cause G proteins to remain longer in their activated GTP-bound form, these types of mutations are activating mutations, and are often dominant or semi-dominant. Some of the phenotypes exhibited by gpa1G50V yeast strains are partially dominant, such as the mating defect and the expression of the pheromone-induced reporter gene fus1–lacZ, which would be consistent with an activating mutation. However, the pheromone spot phenotype and the growth defect were shown to be recessive to the wild-type allele, complicating the interpretations and suggesting that the phenotypes observed in gpa1G50V strains might represent multiple functions of Gpa1. In any case, a lower rate of GTP hydrolysis would lock Gpa1 in its activated state, and the pheromone response pathway would be hyperactive. Arrest at G1 of the cell cycle, cell shape changes and induction of pheromone specific transcripts are all results of activation of the pheromone response pathway, which phenotypically correlate well with the Gpa1G50Vp GTPase defect.
The severely reduced mating efficiency and the turbidity of the pheromone spot, however, have been difficult to explain in gpa1G50V cells. Interestingly, the mating defect in gpa1G50V yeast cells can be genetically suppressed separately from the pheromone spot phenotype (Xu and Kurjan, 1997), further suggesting that some of the gpa1G50V defects represent separate functions of Gpa1p. One plausible explanation for the turbidity of the pheromone spot is that Gpa1G50Vp may partially bind Ste4p/Ste18p, the Gβγ subunits, in an agonist-independent manner. If some Ste4p/Ste18p remained bound to Gpa1G50Vp in the presence of pheromone, cell growth might be permitted, resulting in the observed turbidity inside the halo. This ability to grow in the presence of pheromone as a result of partial Ste4p/Ste18p binding could also explain the ability of certain gpa1 alleles to ‘stimulate’ recovery from G1 arrest in the presence of pheromone (Jahng et al., 1988; Miyajima et al., 1989; Stone and Reed, 1990; Kurjan et al., 1991; Stratton et al., 1996; Xu and Kurjan, 1997). An inability to dissociate normally from Gβγ has been shown for the GαsG226A mutant protein in vitro, which also has conformation transition defects (Miller et al., 1988). And although Gβγ binding has not been directly examined for the GαsG49V mutant (analogous to G50V in Gpa1p), a defect in Gβγ dissociation has been suggested to explain the observed reduction of adenylyl cyclase activity in membranes, compared to the activation observed using purified components (Graziano and Gilman, 1989; Masters et al., 1989). Examination of Gpa1p binding to its βγ subunits in the presence of various guanine nucleotides would provide a direct test of this hypothesis. In addition, although many of the gpa1G50V phenotypes can be explained in terms of altered interactions with Ste4/18, it is possible that Ste4/18 independent functions are altered in gpa1G50V yeast strains. Since the gpa1G50V growth defect and basal transcriptional induction of fus1–lacZ are eliminated in a ste4 mutant strain (Xu and Kurjan, 1997), it seems clear that these phenotypes are due to activation of pathway through Ste4. There is no way to assess STE4 independent effects on mating and recovery in ste4 deletion strains, since they do not mate or respond to pheromone; it remains possible that there are other modulators of the pathway that act through Gpa1 or in conjunction with this pathway to affect recovery from pheromone and/or mating.
The ability to combine genetics with biochemistry in the study of Gpa1, Sst2 and other pheromone response proteins will further define their roles in coordinating G1 arrest, recovery and mating. Development of the necessary biochemical tools for this system allows the study of isolated G protein subunits and their regulators, and will provide unique insight into mechanisms of G protein-coupled receptor signal regulation.
Acknowledgements
We thank A. Pronin, M. Germann, C. Brenner and J. Krupnick, for helpful discussions, H. Alder and the Kimmel Cancer Institute Nucleic Acids Facility for synthesis of oligonucleotides and sequencing, J. Kurjan for the sst2::LEU2 strain and pGal vector, C. Brenner for the sst2–1 strain, and J. Benovic for support.




