The emergence of compartmental organization in olfactory bulb glomeruli during postnatal development
Abstract
The olfactory bulb glomerulus is a discrete and heterogeneous neuropil where olfactory receptor cell axons synapse with dendrites of mitral, tufted, and periglomerular neurons. To understand better the maturation of glomeruli and the spatiotemporal interactions that occur during postnatal development, we employed confocal microscopy and markers for immature and mature olfactory receptor cell axons in parallel with a marker for synaptic structure in maturing glomeruli. Sprague-Dawley rats at postnatal days 1, 6, 12, and 18 were processed for single- and double-label immunocytochemistry for olfactory marker protein (OMP), growth-associated protein (GAP-43), and synaptophysin. Mature or adult-like subcompartmental organization within the glomerulus emerged by postnatal day 12. Earlier in development immature axons entered the core of the glomerulus and moved to the periphery as they matured. However, beginning around 12 days postnatal, immature axons distributed in the periphery and moved toward the core as they matured. This change in the trajectories of axons into glomeruli suggests that different rules may be followed in establishing versus maintaining glomeruli. Double labeling with OMP and synaptophysin demonstrated strong colocalization compared with GAP-43 and synaptophysin, which showed much less colocalization, consistent with the notion that OMP is associated with more mature axons. J. Comp. Neurol. 422:297–311, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
The olfactory bulb glomerulus is the site of the first synapse in the olfactory pathway. Axons from the olfactory receptor neurons in the olfactory epithelium terminate in the glomeruli on target dendrites from mitral, tufted, and periglomerular neurons. It was recently established that the axons terminating in any one glomerulus are derived largely from olfactory receptor neurons that express the same odor receptor (Ressler et al., 1994; Vassar et al., 1994; Mombaerts, 1996). Thus each glomerulus in the olfactory bulb expresses a specific molecular phenotype based on the subset of receptor cells that innervate the glomerulus. These observations, coupled with prior functional analyses of the rat olfactory bulb (Stewart et al., 1979; Greer et al., 1982; Johnson et al., 1998, Johnson et al., 1996), have provided strong support for the notion that the glomerulus is a fundamental organizational unit in odor coding (Shepherd, 1993). Similar conclusions have been reached based on both anatomical and functional analyses of glomeruli in several species including insects (e.g., Vickers et al., 1998) and crustaceans (e.g., Schmidt and Ache, 1992; see Hildebrand and Shepherd, 1997 for review).
Initially, the glomerulus was viewed as a comparatively homogeneous structure in which primary afferent axons and target dendrites were uniformly distributed. However, more recent reconstructions of glomeruli have revealed a subcompartmental organization and segregation of synaptic circuits that was not previously recognized (Chao et al., 1997; Kosaka et al., 1997; Kasowski et al., 1999). In brief, the axons from olfactory receptor cells establish contiguous islands in the glomerulus within which they establish synapses with isolated target dendrites. In contrast, the local circuit reciprocal dendrodendritic circuits are found within dendritic bundles that are segregated from the axonal islands by glial processes. The segregation of synapses appears significant for both the continual turnover of axons within glomeruli as well as the separation of primary afferent and local circuit synapses that are both using glutamate as their primary neurotransmitter.
Analyses of early development of the glomerulus have emphasized the seminal role of primary afferents in inducing the formation of glomeruli (Graziadei and Monti-Graziadei, 1986; Valverde et al., 1992; Malun and Brunjes, 1996; Treloar et al., 1999; Bailey et al., 1999). Glomeruli are first apparent in mammals during late embryonic development and during the postnatal period increase in size and definition. There is now a general consensus that new glomeruli are not formed de novo beyond postnatal days 2–5 (Meisami and Sendera, 1993). However, functional analyses of olfactory bulb glomeruli using probes such as 2-deoxyglucose have suggested that mature, adult-like patterns of odor-induced activity are not apparent until around the second postnatal week (Greer et al., 1982). This suggests that while nascent glomeruli are present during the early postnatal period, substantive development/maturation continues for a more extended time. Support for this suggestion comes from the work of Hinds and Hinds (1976a, Hinds and Hinds, 1976b), who demonstrated that synaptogenesis in the olfactory glomeruli of mice extended into the second postnatal week. Similarly, Malun and Brunjes (1996) have shown that the elaboration of dendritic arbors in both the precoccial opossum and rat can extend well into the postnatal period.
To gain new insights into the maturation of glomeruli, we have examined the expression of growth-associated protein-43 (GAP-43) as a marker of immature olfactory receptor cell axons, olfactory marker protein (OMP) as a marker of mature olfactory receptor cell axons, and synaptophysin as a marker of synaptic structure, in developing glomeruli. Our data demonstrate that the adult-like pattern of organization within glomeruli emerges around 12 days postnatal. In addition, the data strongly suggest that the topography of glomerular innervation by newly arriving axons changes as glomeruli mature. The results provide new insights into not only the mechanisms of glomerular development, but also some of the apparent prerequisites for adult-like functioning in glomeruli.
MATERIALS AND METHODS
Animals
All protocols and animals used were reviewed and approved by the Yale Animal Care and Use Committee. Sprague-Dawley rats, postnatal day 1 (n = 4), 6 (n = 4), 12 (n = 4), and 18 (n = 5), were deeply anesthetized with an intraperitoneal injection of pentobarbital (Nembutal 65 mg/kg) and perfused through the heart with 0.1 M phosphate-buffered saline (PBS) (pH 7.4) at 4°C followed by 100–200 ml of 4% paraformaldehyde in 0.1 M PBS at 4°C. The brains were then removed and immersed in 4% paraformaldehyde for 1–2 hours at 4°C before washing in 0.1 M PBS for 24 hours at 4°C. The olfactory bulbs were then embedded in 2% agar and coronal sections cut at 50 μm on a vibratome. The sections were collected in a cryoprotectant, 30% sucrose, for storage at −20°C prior to processing for immunocytochemistry.
Alternatively, Sprague-Dawley rats at postnatal days 0 (n = 2), 6 (n = 2), 12 (n = 2) and 18 (n = 2) were processed for paraffin embedding following perfusion and dissection as described above. The olfactory bulbs were cut at 10 μm in the coronal plane and stained with thionine for histological analysis.
Immunocytochemistry
Selection of antibodies.
To identify mature olfactory receptor cell axons, we employed anti-olfactory marker protein (OMP). OMP is a cytoplasmic protein that is highly specific to the majority of olfactory receptor cell axons in the olfactory bulb (Monti-Graziadei et al., 1980; Margolis, 1985; Ring et al., 1997) and upregulates as the axons mature and establish synapses (Verhaagen et al., 1989, Verhaagen et al., 1990). Although some populations of OMP-expressing cells are found outside the olfactory bulb (Baker et al., 1989), these are unlikely to compromise the analyses because they do not project processes to the olfactory bulb glomeruli. To identify immature olfactory receptor cell axons, we employed anti-GAP-43. GAP-43 is a membrane-associated protein found in growing immature axons (Meiri et al., 1988) and is abundantly expressed in immature olfactory receptor cell axons prior to the appearance of OMP (Verhaagen et al., 1989, Verhaagen et al., 1990; Gong and Shipley, 1995; Treloar et al., 1996). It is important to note that GAP-43 expression is not limited to olfactory receptor cell axons; it may also be expressed in juxtaglomerular cells and centrifugal axons during development. However, because the majority of staining appears in olfactory receptor cell axons and because contributions from other cell populations are transient, GAP-43 proved to be the most effective marker of developing olfactory receptor cell axons. Finally, anti-synaptophysin was employed as a marker for the presence of synaptic vesicles (Jahn and Sudhof, 1994). Although it is not a definitive measure of synapse formation, synaptophysin staining does establish the presence of a synaptic vesicle-associated protein and has been effectively employed to determine the appearance (Johnson et al., 1996) and distribution (Kasowski et al., 1999) of synapses in the olfactory bulb glomerulus as well as elsewhere in the central nervous system (CNS) (e.g., Tyzio et al., 1999).
Staining protocol.
The protocol for immunocytochemistry was described previously (Kasowski et al., 1999). Briefly, the tissue sections stored at −20°C were washed with several rinses of 0.1 M PBS before immunocytochemistry processing. All immunocytochemical reactions were performed on free-floating sections. Following 2 × 10 minute rinses in 0.1 M PBS, sections were preincubated in 0.1 M PBS with 1% bovine serum albumin (BSA; Sigma, St. Louis, MO) and 0.2% Triton X-100 (Sigma) for 30 minutes at room temperature. Sections were then rinsed 5 × 7 minutes in 0.1 M PBS with 0.5% BSA at room temperature before incubating for 24–48 hours at 4°C in one of the following antibodies: goat anti-OMP (1:1000, generously provided by Dr. F. Margolis); rabbit anti-synaptophysin (1:100, Dako, Carpinteria, CA); and mouse anti-GAP-43 (1:500, Boehringer Mannheim, Indianapolis, IN). Dilutions were made in 0.1 M PBS with 0.5% BSA. Sections were then rinsed 5 × 7 minutes in 0.1 M PBS with 0.5% BSA before incubating for 1 hour at room temperature in one of the following secondary antibodies: rhodamine (TRITC)-conjugated AffiniPure donkey anti-goat IgG (H+L; Jackson ImmunoResearch Labs, West Grove, PA); fluorescein goat anti-rabbit IgG (H+L); Texas red goat anti-rabbit IgG (H+L; 1:200); and fluorescein horse anti-mouse IgG (H+L), rat adsorbed. All secondary antibodies were purchased from Vector (Burlingame, CA) and used at a concentration of 1:100, except where noted above. Processing was then repeated as above using a second set of primary and secondary antibodies before mounting on precleaned slides and coverslipping with Vectashield mounting medium (Vector). The sequence in which the primary antibodies were used in the double-labeling studies was varied to ensure that the patterns of labeling did not reflect the processing sequence.
Controls.
To control for nonspecific and artifactual staining, the primary antibodies were excluded in a series of experiments at each of the ages. In all cases, staining did not occur in the absence of the primary antibody.
Light and confocal microscopy
The cresyl violet-stained sections were assessed on an Olympus BH2 microscope, and representative images were captured at 1200 dpi into Adobe PhotoShop using a Spot Camera (Diagnostic Instruments, Sterling Heights, MI).
Mounted sections processed for immunocytochemistry were visualized, and images were collected using a Bio-Rad (Hercules, CA) 600 scanning confocal microscope (Olympus IMT2) equipped with a krypton-argon laser. The average thickness of optical sections from which images were obtained was approximately 1.0 μm. Serial reconstructions were used to evaluate multiple images viewed through the depth of a tissue sample. To minimize regional differences in organization (e.g., Ring et al., 1997) or maturation (e.g., Bailey et al., 1999) of the olfactory bulb, data acquisition and analysis focused on sections taken from midway along the rostral-caudal axis. To ensure that the edges of glomeruli were not misinterpreted as sections passing through the center of a glomerulus, all glomeruli included in the analyses and used for illustration in the manuscript were at least partially viewed through serial optical sections on the confocal microscope. The magnifications used in all of the illustrations were selected to emphasize the features of interest for each of the antibodies at each of the representative ages.
A Macintosh 7200 computer equipped with Adobe PhotoShop (3.0.1) software was used to format and present the images, which were printed on a Codonix NP1600M color printer. Image brightness and contrast were balanced for consistency within a figure, but further processing was not performed on any of the images.
RESULTS
In general, the size of glomeruli increased proportionally to the age of the animal (Fig. 1). As has been previously reported, although glomeruli can be detected during the perinatal period (Treloar et al., 1999), they are generally much smaller and more poorly defined by juxtaglomerular cells than at later ages (Greer et al., 1982; Meisami and Sendera, 1993; Malun and Brunjes, 1996).
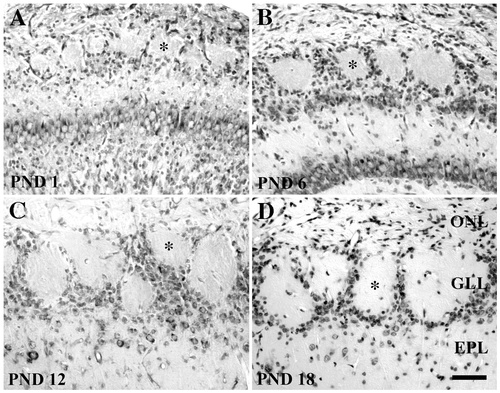
Light micrographs of cresyl violet-stained sections of the rat olfactory bulb at postnatal days 0 (A), 6 (B), 12 (C), and 18 (D). At all ages glomeruli are readily identified as spherical areas of neuropil (e.g., asterisks) surrounded by the somata of juxtaglomerular cells. However, over the course of development the glomeruli increase in cross-sectional diameter, with a corresponding increase in the number of juxtaglomerular cells. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; PND, postnatal day. Scale bar = 50 μm.
OMP selectively stains olfactory receptor cell axonal processes and was used as a marker of mature axons; GAP-43 was employed to identify immature axons in the olfactory bulb (Verhaagen et al., 1989). The synaptic vesicle-associated protein synaptophysin was utilized to distinguish synaptic specializations. At each of the ages tested, the antibodies employed exhibited distinct laminar and sublaminar patterns of staining. There was no evidence to suggest that the epitopes recognized by each of the antibodies were changing over the course of development. The characteristics of the immunostaining are described below for each of the ages examined.
Postnatal day 1
OMP.
At postnatal day 1, OMP-immunoreactive processes were evident in the olfactory nerve and glomerular layers (Fig. 2A,B). In general, the immunoreactive processes in the nerve layer appeared relatively short and discontinuous, particularly in contrast to the patterns of staining described at older ages. The OMP+ processes within the olfactory nerve layer did not appear as well-delineated axonal fascicles. Although in some cases the continuity with the glomerular layer could be detected (Fig. 2B, arrow), it was less robust than that seen at older ages, rather, OMP+ processes were broadly dispersed throughout the nerve layer in a seemingly disorderly arrangement. Similarly, OMP immunoreactivity within glomeruli demonstrated a frayed, diffuse appearance and was interspersed with punctate areas of nonimmunoreactivity distributed heterogeneously throughout each glomerulus (Fig. 2B, asterisk). There were OMP+ processes that appeared to extend beyond the inner boundaries of some glomeruli, toward the deeper regions of the olfactory bulb (Fig. 2A, arrow).
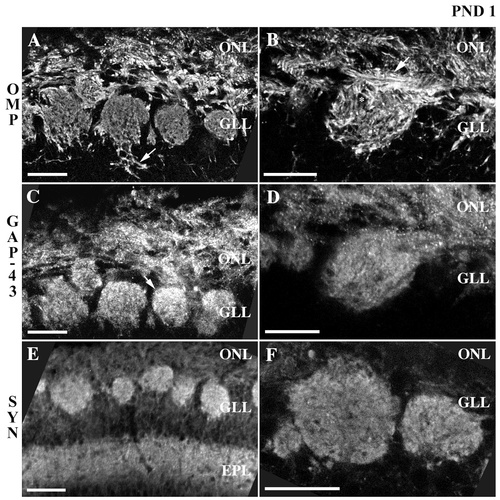
Confocal micrographs of olfactory marker protein (OMP), growth-associated protein GAP-43, and synaptophysin immunoreactivity in the superficial layers of the rat olfactory bulb at postnatal day 1. A,B: OMP immunoreactivity in the olfactory nerve and glomerular layers appears diffuse and frayed. Axonal fascicles (e.g., arrow in B), travel through the nerve layer to target individual glomeruli. Within glomeruli, areas of OMP immunoreactivity are interspersed with nonimmunoreactive puncta (e.g., asterisk in B). In A an arrow indicates OMP+ processes, which extend beyond the inner boundaries of a glomerulus, toward the deeper regions of the olfactory bulb. C,D: GAP-43 staining is dense in the nerve layer, whereas within glomeruli GAP-43 immunoreactivity often appears more dense at the base of the many glomeruli, proximal to the nerve layer (e.g., arrow in C). E,F: Synaptophysin immunoreactivity clearly demarcates individual glomeruli. A punctate pattern of synaptophysin staining is evident within glomeruli. Varying levels of synaptophysin immunoreactivity as well as nonimmunoreactive areas are also present. A similar distribution of synaptophysin staining is visible in the external plexiform layer (E). Nonimmunoreactive processes within the glomerular and external plexiform layers probably correspond to blood vessels. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; SYN, synaptophysin; PND, postnatal day. Scale bars = 25 μm.
GAP-43.
GAP-43 immunoreactivity at postnatal day 1 demonstrated a more particulate quality compared with the pattern of staining described below at older ages (Fig. 2C,D). GAP-43 staining was dense in the olfactory nerve layer, although it was difficult to resolve single axons or discrete fascicles. The GAP-43 staining appeared punctate and did not reveal the longitudinal processes seen with OMP. Within glomeruli, GAP-43 immunoreactivity exhibited a diffuse and granular pattern of distribution that was interspersed with nonimmunoreactive puncta throughout the glomerulus. In some glomeruli, GAP-43 staining exhibited a greater intensity proximal to the nerve layer (Fig. 2C, arrow), whereas in others it appeared more evenly distributed. GAP-43 immunoreactivity was absent from both the juxtaglomerular zone and the external plexiform layer.
Synaptophysin.
In general, synaptophysin immunoreactivity at postnatal day 1 (Fig. 2E,F) was comparable to that seen at older ages. Synaptophysin staining was absent from the olfactory nerve layer. Individual glomeruli, however, were readily resolved due to the presence of synaptophysin+ elements. Individual processes could not be resolved with the synaptophysin staining. The intraglomerular synaptophysin staining appeared punctate, with interdigitating areas of higher and lower immunoreactivity as well as zones within which no immunoreactivity was evident. Although synaptophysin immunoreactivity was absent from the periglomerular zone, the external plexiform layer showed a pattern of punctate synaptophysin staining interspersed with areas of nonimmunoreactivity. The synaptophysin immunoreactivity seen in the external plexiform layer is most likely associated with the presynaptic dendrites, whereas the nonimmunoreactive areas likely correspond to blood vessels.
Double labeling.
OMP/GAP-43.
Populations of GAP-43 immunoreactivity (shown in red) and OMP immunoreactivity (shown in green), as well as apparent areas of colocalization or overlap (shown in yellow) were present within the olfactory nerve layer (see Fig. 6A). However, at this age the predominant phenotype within the nerve layer was GAP-43+. OMP+ processes were evident as well, suggesting that some proportion of the axons at this age are exhibiting mature characteristics. Although some colocalization of OMP and GAP-43 immunoreactivity is possible, these areas are more likely to represent axons (0.2 μm in diameter) overlapping within the 1-μm optical sections. In the glomeruli, both OMP+ and GAP-43+ processes were evident; however, OMP staining was slightly more apparent along the periphery and outermost portions of the glomerulus (Fig. 6A, arrow), whereas GAP-43 staining appeared to remain within the more central areas of the glomerulus.
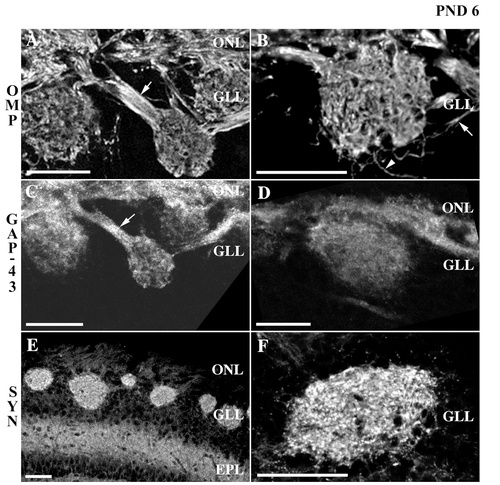
Confocal micrograph of olfactory marker protein (OMP), growth-associated protein GAP-43, and synaptophysin immunoreactivity in the superficial layers of the rat olfactory bulb at postnatal day 6. A,B: OMP staining is dense in the nerve layer where axonal fascicles (e.g., arrow in A) can be seen exiting the nerve layer to target a glomerulus. OMP immunoreactivity within glomeruli presents a more adult-like appearance. Discrete or single OMP+ processes can be seen both exiting the nerve layer to enter a glomerulus indirectly (e.g., arrow in B) and extending from a glomerulus to travel distally toward the deeper layers of the olfactory bulb (e.g., arrowhead in B). C,D: GAP-43 immunoreactivity is generally diffuse and punctate, although some GAP-43+ processes (e.g., arrow in C) can be identified. GAP-43 staining within glomeruli appears slightly more intense at the base, proximal to the nerve layer. E,F: Synaptophysin staining clearly demarcates glomeruli. Within glomeruli, a punctate pattern of synaptophysin immunoreactivity is interspersed with nonimmunoreactive areas. A similar pattern of synaptophysin staining is apparent in the external plexiform layer. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; SYN, synaptophysin; PND, postnatal day. Scale bars = 25 μm.
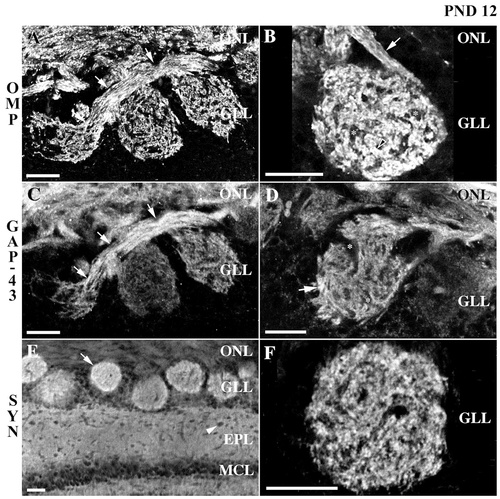
Confocal micrograph of olfactory marker protein (OMP), growth-associated protein GAP-43, and synaptophysin immunoreactivity in the rat olfactory bulb at postnatal day 12. A,B: OMP immunoreactivity is dense in the nerve layer, where fascicles of axons (e.g., arrows in A and B), exit the nerve layer to enter glomeruli. Within glomeruli, contiguous islands of OMP staining (e.g., arrowhead in B), interdigitate with nonimmunoreactive puncta (e.g., asterisks in B). C,D: The distribution of GAP-43 immunoreactivity appears more diffuse and granular than that of OMP. GAP-43 staining is dense in the nerve layer, where axonal fascicles (e.g., arrows in C) travel through the nerve layer to target a glomerulus. Within glomeruli, GAP-43+ processes (e.g., arrow in D) intersperse with nonimmunoreactive areas (e.g., asterisks in D). GAP-43 immunoreactivity within glomeruli also appears slightly stronger at the apex, proximal to the nerve layer, and in the periphery of the glomerulus. E,F: Individual glomeruli are clearly demarcated by synaptophysin immunoreactivity. The distribution of synaptophysin staining is denser along the rim of the glomerulus (e.g., arrow in E). In E an arrowhead indicates an area of nonimmunoreactivity within the external plexiform layer, where puncta of synaptophysin staining are otherwise broadly distributed throughout. These nonimmunoreactive areas probably represent blood vessels cut within the plane of the tissue. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; SYN, synaptophysin; PND, postnatal day. Scale bars = 25 μm.
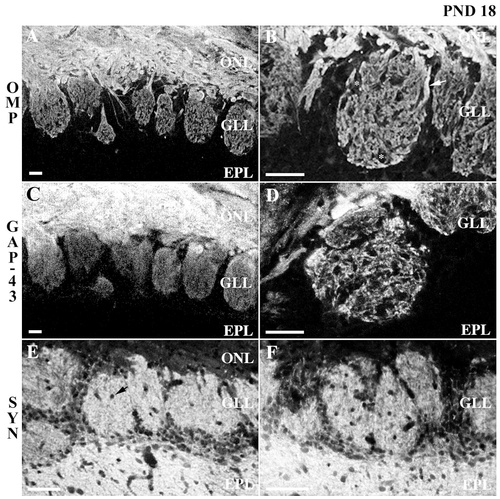
Confocal micrograph of olfactory marker protein (OMP), growth-associated protein GAP-43, and synaptophysin immunoreactivity in the rat olfactory bulb at postnatal day 18. A,B: OMP immunoreactivity appears similar to that at postnatal day 12, although the density of stained processes appears to have increased. As fascicles of axons (e.g., arrow in B) enter a glomerulus, OMP staining appears to diminish, which may reflect axon defasciculation. Within glomeruli, contiguous islands or zones of OMP staining interdigitate with discrete areas of nonimmunoreactivity (e.g., asterisks in B). C,D: GAP-43 immunoreactivity appears generally more granular than OMP. Although GAP-43 staining appears variable throughout the glomerular layer, contiguous zones of GAP-43 immunoreactivity are evident within individual glomeruli. GAP-43 staining also exhibits a higher density at the apex, proximal to the nerve layer, and in the periphery of the glomerulus. E,F: A punctate pattern of synaptophysin staining is apparent in the glomerular and external plexiform layers. The distribution of synaptophysin staining does not exhibit the contiguous zones of staining seen with OMP; however, puncta lacking immunoreactivity (e.g., arrow in E) can be seen within glomeruli. These nonimmunoreactive areas probably correspond to blood vessels. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; SYN, synaptophysin; PND, postnatal day. Scale bars = 25 μm.
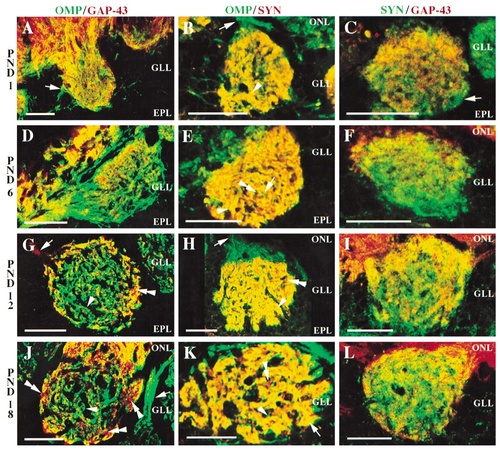
Double-labeled confocal images of olfactory marker protein (OMP), growth-associated protein (GAP-43), and synaptophysin (SYN) immunoreactivity in the rat olfactory bulb at postnatal days 1 (A–C), 6 (D–F), 12 (G–I), and 18 (J–L). A: GAP-43 staining (red) is particularly prominent in the nerve layer, with fewer OMP immunoreactive processes (green), evident at this age. Within glomeruli, OMP+ processes (e.g., arrow) appear more distinct around the periphery, whereas GAP-43+ processes appear to be distributed more densely within the core of the glomerulus. There is some evidence of colocalization (yellow) in the glomerular layer. B: OMP (green) is strongest in the nerve layer (e.g., arrow) with no evidence of synaptophysin immunoreactivity (red) or colocalization (yellow). Within glomeruli, however, colocalization is abundant. Areas of colocalization (e.g., arrowhead) interdigitate with puncta of OMP and synaptophysin staining as well as unstained areas throughout the glomerulus. C: GAP-43 only (red) is distinct in the nerve layer, with no evidence of synaptophysin staining (green). Within the glomerular neuropil puncta of single labeling for synaptophysin and GAP-43 are evident, with some evidence of colocalization (yellow). D: Areas of OMP immunoreactivity (green), GAP-43 immunoreactivity (red), and colocalization or overlap (yellow) are evident in the nerve layer. Within glomeruli, GAP-43 staining and areas of colocalization with OMP are found in the central portions. However, in the circumferential regions of the glomerulus, GAP-43 staining is largely absent and OMP staining is prominent. E: The nerve layer appears exclusively immunoreactive for OMP only (green). Colocalization with synaptophysin (yellow) is extensively distributed throughout the glomerular neuropil, where large areas of colocalization (e.g., arrowhead) interdigitate with puncta of OMP staining (e.g., arrow), synaptophysin staining (e.g., double arrowheads), as well as unstained areas. F: GAP-43 immunoreactivity (red) is the predominant phenotype in the nerve layer. Within glomeruli, GAP-43 staining is more evident in the central and more superficial, proximal to the nerve layer, regions of the glomerulus. Synaptophysin immunoreactivity (green) exhibits a punctate appearance within the glomerular neuropil, where little evidence of colocalization (yellow) with GAP-43 is seen. G: An arrow indicates a GAP-43+ process (red), which is traveling through the nerve layer to target a glomerulus. The core of the glomerulus is occupied primarily by OMP+ processes (green) (e.g., arrowhead), although puncta of GAP-43 staining (e.g., double arrowhead) can also be detected. In contrast with the pattern of staining seen at earlier ages, colocalization appears most distinct in the periphery or rim of the glomerulus. H: OMP-immunoreactive processes (green), indicated by an arrow, are prominent in the nerve layer. Within glomeruli, colocalization of OMP and synaptophysin occurs extensively. Areas of colocalization appear to rest within islands of OMP staining, whereas punctate areas of synaptophysin staining (e.g., double arrowhead) are evident within glomeruli as well. I: Colocalization (yellow) of GAP-43 (red) and synaptophysin (green) staining is most distinct at the base, proximal to the nerve layer, and in the periphery of the glomerulus. Synaptophysin immunoreactivity, however, is more robust in the central region of the glomerulus, whereas GAP-43 immunoreactivity is more apparent in the periphery and superficial areas, proximal to the nerve layer. J: Areas of OMP immunoreactivity (green) (e.g., arrow), GAP-43 immunoreactivity (red), as well as colocalization or overlap (yellow), are evident within the nerve layer, reflecting various stages of axon maturity. Within glomeruli, GAP-43 staining (e.g., double arrowheads) is more apparent in the periphery of the glomerulus, whereas OMP staining (e.g., arrowhead) remains more distinct in the core of the glomerulus. K: The nerve layer is predominantly immunoreactive for OMP alone (green). The glomerular neuropil is largely occupied by extensive areas of OMP and synaptophysin colocalization (yellow) (e.g., arrow). These areas of colocalization within glomeruli are interspersed with discrete puncta of synaptophysin immunoreactivity (red) (e.g., double arrowhead) as well as OMP immunoreactivity (e.g., arrowhead). L: GAP-43 immunoreactivity is the predominate phenotype within the nerve layer, with little evidence of synaptophysin immunoreactivity (green) or colocalization (yellow). Colocalization within glomeruli is minimal compared with that seen in OMP and synaptophysin. GAP-43 staining is strongest in the periphery of the glomerulus, whereas synaptophysin staining is more robust in the core. ONL, olfactory nerve layer; GLL, glomerular layer; EPL, external plexiform layer; PND, postnatal day. Scale bars = 25 μm.
OMP/synaptophysin.
The olfactory nerve layer appeared to be almost exclusively immunoreactive for OMP (green; Fig. 6B, arrow), with no evidence of synaptophysin immunoreactivity (red) or colocalization (yellow) within the nerve layer (Fig. 6B). In contrast, colocalization (Fig. 6B, arrowhead) was abundant within glomeruli. However, interdigitating among the areas of colocalization were puncta that were only OMP or synaptophysin immunoreactive. Although immunoreactivity was not evident in the juxtaglomerular zone, the external plexiform layer demonstrated the punctate synaptophysin staining described above (no colocalization; not illustrated).
Synaptophysin/GAP-43.
The olfactory nerve layer was strongly immunoreactive for GAP-43 but showed no evidence of labeling for synaptophysin (not illustrated; cf. Fig. 2C and E). Within the glomerular neuropil punctate areas of synaptophysin (green) and GAP-43 (red) immunoreactivity were evident (Fig. 6C). However, GAP-43 and synaptophysin showed comparatively little evidence of colocalization (yellow). The distribution of GAP-43 staining appeared slightly stronger proximal to the nerve layer and in the central portion of the glomerulus. In contrast, synaptophysin appeared more distinct in the circumferential region along the periphery of the glomerulus (Fig. 6C, arrow). The juxtaglomerular zone did not show evidence of immunoreactivity. The external plexiform layer had no evidence of GAP-43+ processes and therefore no examples of colocalization with the punctate synaptophysin immunoreactivity described above (not illustrated).
Postnatal day 6
OMP.
OMP staining in the olfactory nerve layer was very dense (Fig. 3A,B). Fascicles as well as individual axons were seen traversing the nerve layer and targeting glomeruli (Fig. 3A, arrow). Areas surrounding the fascicles devoid of OMP staining most likely represent the processes of ensheathing cells. OMP immunoreactivity within glomeruli was widely distributed, although less amorphous than that seen at postnatal day 1. In particular, the fibrous appearance seen at postnatal day 1 has been replaced with a more adult-like appearance, although the well-delineated islands or zones of OMP immunoreactivity have not yet emerged. This pattern of OMP immunoreactivity appeared consistent throughout the glomerulus. Also visible were discrete bundles or single OMP-positive processes that appeared to extend beyond the deeper boundaries of the maturing glomerulus. Some of these OMP+ processes appeared to emerge from the nerve layer to travel circuitously into a glomerulus (Fig. 3B, arrow), and others appeared to protrude from a glomerulus to course distally, toward the external plexiform area (Fig. 3B, arrowhead). OMP immunoreactivity was otherwise absent from the external plexiform layer as well as the periglomerular zone.
GAP-43.
At postnatal day 6 (Fig. 3C,D), the distribution of GAP-43 immunoreactivity exhibited a pattern that closely resembled that seen at postnatal day 1. In general, the staining exhibited a punctate appearance and was distributed in some cases along longitudinal processes. GAP-43+ processes were abundant in the olfactory nerve layer, where fascicles of axons were seen targeting a glomerulus (Fig. 3C, arrow). Within glomeruli, GAP-43 immunoreactivity appeared slightly more intense proximal to the nerve layer. Of note, the overall distribution of staining within glomeruli did not appear as zones of immunoreactivity interspersed with punctate areas of nonimmunoreactivity as observed at the older ages described below, but instead demonstrated a more diffuse pattern of staining reminiscent of that seen at postnatal day 1. The periglomerular zone and external plexiform layer were devoid of immunoreactivity.
Synaptophysin.
As was noted for postnatal day 1, synaptophysin staining clearly demarcated the glomeruli, in part due to the absence of staining in the olfactory nerve layer and periglomerular zone (Fig. 3E,F). Within the glomerular layer, however, staining was dense throughout the length of each glomerulus. A punctate pattern of synaptophysin immunoreactivity interspersed with areas of nonimmunoreactivity was seen within glomeruli. Although what appeared to be single fibers could be followed, the overall impression was one of highly intense puncta throughout the glomerulus. Although the juxtaglomerular zone showed little immunoreactivity, the external plexiform layer demonstrated a punctate pattern of staining. Scattered areas of nonimmunoreactivity probably represent blood vessels.
Double labeling.
OMP/GAP-43.
Areas of OMP immunoreactivity (green), GAP-43 immunoreactivity (red), and colocalization (yellow) were evident within the olfactory nerve layer (Fig. 6D). There appeared to be some heterogeneity, however, since in some regions of the olfactory bulb OMP staining appeared more abundant than GAP-43, whereas elsewhere in the olfactory bulb, OMP and GAP-43 staining appeared more evenly distributed (not illustrated). Within glomeruli, both OMP and GAP-43 immunoreactivity demonstrated patterns resembling those described above for each of the markers. Little or no colocalization was seen within the glomerular layer. In the periglomerular zone and external plexiform layer, we observed no evidence of OMP or GAP-43 immunoreactivity.
OMP/synaptophysin.
The olfactory nerve layer appeared to be immunoreactive for OMP only (green), with little, if any, evidence of synaptophysin (red), and no evidence of colocalization (yellow), as was noted previously at postnatal day 1 (Fig. 6E). Within glomeruli, however, large areas of colocalization were apparent throughout the neuropil. Puncta of colocalization often appeared superimposed on areas of OMP-only staining, giving the appearance of yellow/red dots on a green background. In addition, areas with no immunoreactivity and puncta of single labeling for synaptophysin (Fig. 6E, double arrowhead) and OMP alone (Fig. 6E, arrow) appeared to interdigitate with areas of colocalization (Fig. 6E, arrowhead) within the glomerulus. This pattern of staining was consistent throughout the glomerulus. The juxtaglomerular zone was devoid of staining; however, the external plexiform layer exhibited the complex distribution of synaptophysin immunoreactivity described for postnatal day 1 (not illustrated).
GAP-43/synaptophysin.
In the olfactory nerve layer, although GAP-43 immunoreactivity (red) was evident, no indication of colocalization was found since synaptophysin staining was not present in the nerve layer (see above) (Fig. 6F). Within glomeruli the distribution of synaptophysin staining had a diffuse punctate appearance, as described above. Similarly, GAP-43 staining within glomeruli in these double-label preparations was as described above. Colocalization of synaptophysin and GAP-43 was minimal in the glomerular neuropil. In those regions that did exhibit evidence of colocalization, it was most prominent in the portion of the glomerulus proximal to the nerve layer. Although the periglomerular area was devoid of staining, the external plexiform layer showed the complex pattern of synaptophysin immunoreactivity described above, with no evidence of GAP-43 staining (not illustrated).
Postnatal day 12
OMP.
At postnatal day 12, the distribution of OMP immunoreactivity appeared similar to that previously described in the adult (Kasowski et al., 1999). Staining was very dense in the olfactory nerve layer, where fascicles of axons coursed longitudinally and were seen exiting the nerve layer to enter olfactory bulb glomeruli (Fig. 4A,B, arrows). Within the nerve layer, and particularly in fascicles targeting specific glomeruli (Fig. 4B, arrow), elongated areas lacking immunoreactivity were seen interdigitating with the OMP-immunoreactive processes. As noted above, these most likely represent the ensheathing cell processes that surround olfactory receptor cell axon fascicles. Within the glomeruli, contiguous islands of OMP immunoreactivity (Fig. 4B, arrowhead) were interspersed with nonimmunoreactive puncta (Fig. 4B, asterisks). These were first seen at postnatal day 6, but became more distinctive by postnatal day 12. Staining for OMP was not apparent in the juxtaglomerular zone or in the external plexiform layer.
GAP-43.
The distribution of GAP-43 immunoreactivity was generally similar to that of OMP, although the appearance of immunoreactivity for GAP-43 was generally more granular and diffuse (Fig. 4C,D). Staining was dense in the olfactory nerve layer, where axonal fascicles could be seen running parallel to the nerve layer and passing into glomeruli (Fig. 4C, arrows). Within glomeruli, GAP-43 immunoreactivity (Fig. 4D, arrow) interspersed with discrete areas that were not immunoreactive (Fig. 4D, asterisks). The islands of GAP-43 immunoreactivity, however, appeared less well delineated that those seen with OMP, and the areas of staining within the islands had a more diffuse appearance (cf. Fig. 4A and C). GAP-43 immunoreactivity was not uniformly distributed throughout a glomerulus but appeared to exhibit a slightly greater intensity proximal to the nerve layer and along the outer rim and circumference of the glomerulus. There was no evidence of GAP-43-specific staining in the juxtaglomerular areas or in the external plexiform layer.
Synaptophysin.
As we noted above for the younger animals, the olfactory nerve layer was generally devoid of synaptophysin immunoreactivity. Individual glomeruli, however, were readily demarcated by synaptophysin immunoreactivity (Fig. 4E,F). In general, the synaptophysin staining was more diffuse than that seen with either OMP or GAP-43; synaptophysin staining was not restricted to the discrete zones or islands of contiguous staining seen with OMP and GAP-43. The punctate distribution of synaptophysin staining was not homogeneous throughout a glomerulus, rather staining appeared more dense around the periphery or rim of the glomerulus (Fig. 4E, arrow). In the external plexiform layer a similarly distinct pattern of staining was evident (Fig. 4E). Although synaptophysin immunoreactivity was densely distributed throughout the external plexiform layer, puncta of nonimmunoreactivity were broadly spread throughout (Fig. 4E, arrowhead). These areas of nonimmunoreactivity broadly distributed in glomeruli and the external plexiform layer most likely represent, at least in part, blood vessels cut both longitudinally as well as transversely within the plane of the tissue.
Double labeling.
OMP/GAP-43.
The occurrence of double labeling with markers for OMP and GAP-43 were comparable to the patterns described for each of the markers alone (Fig. 6G). The olfactory nerve layer exhibited immunoreactivity for OMP alone, GAP-43 alone (Fig. 6G, arrow), as well as areas of colocalization (not illustrated). The latter most likely reflects the superpositioning of the olfactory receptor cell axons, although it may be that in some cases both epitopes could occur within single axons during maturation. Throughout the olfactory nerve layer, these populations of immunoreactivity appeared in various combinations and densities, although all three phenotypes were generally present (not illustrated). The distribution of OMP+ and GAP-43+ processes within the glomerular neuropil appears fundamentally different from that seen at earlier ages. The core of the glomerulus appears immunoreactive predominantly for OMP (green) (Fig. 6G, arrowhead), although a few scattered GAP-43+ (red) (Fig. 6G, double arrowhead) processes can be detected. The region in which GAP-43 and OMP appear to overlap the most, however, is the periphery or rim of the glomerulus. This is in contrast to the pattern seen at younger ages, in which the core of the glomerulus was predominantly GAP-43+, whereas the more mature OMP+ processes were found in the periphery of the glomerulus. Both the juxtaglomerular zone and external plexiform area appeared to be devoid of immunoreactivity.
OMP/synaptophysin.
Colocalization of OMP and synaptophysin occurred extensively within the glomerular neuropil (Fig. 6H). These areas of colocalization appeared to rest within the islands of OMP immunoreactivity described above (Fig. 6H, arrowhead). However, other areas within these islands showed immunoreactivity for OMP alone, whereas punctate processes immunoreactive for synaptophysin alone were found within the glomerulus as well (Fig. 6H, double arrowhead). There was an absence of immunoreactivity in the juxtaglomerular zones, but the external plexiform layer exhibited the distinct pattern of synaptophysin immunoreactivity described above for the younger ages.
GAP-43/synaptophysin.
Colocalization of GAP-43 and synaptophysin was evident throughout the glomerulus but was predominant proximal to the nerve layer and in the periphery, as was the distribution of GAP-43 immunoreactivity within the glomerulus (Fig. 6I). Synaptophysin immunoreactivity, however, was more robust in the central region of the glomerulus.
Postnatal day 18
OMP.
At postnatal day 18, OMP immunoreactivity was equivalent to that described for postnatal day 12, although it does appear that the density or number of stained processes may have increased (Fig. 5A,B). Fascicles of axons were seen traveling both parallel and perpendicular to the layers of the olfactory bulb (Fig. 5A). Within the olfactory nerve, unstained areas appear as well. These may reflect the presence of immature axons (see GAP-43 below) or the ensheathing cell glia that contribute to the formation of axon fascicles. As the fascicles entered glomeruli, the immunoreactivity appeared to diminish slightly, perhaps due to the defasciculation of the axons (Fig. 5B, arrow). Within the glomeruli, the OMP immunoreactivity appeared broadly distributed within contiguous zones or islands that interdigitate with nonimmunoreactive puncta of varying sizes (Fig. 5B, asterisks). As we have previously shown, the latter most likely correspond to the dendritic and glial processes found within the glomeruli as well as transversely and longitudinally cut blood vessels (Kasowski et al., 1999). In general, OMP immunoreactivity was qualitatively uniform across the population of olfactory bulb glomeruli. The juxtaglomerular zones were devoid of OMP immunoreactivity, as were the deeper layers of the olfactory bulb.
GAP-43.
GAP-43 immunoreactivity (Fig. 5C,D) had a distribution at postnatal day 18 somewhat comparable to that of OMP. Staining was dense in the olfactory nerve layer where complexes of fascicles, as described above for OMP, were seen. As the fascicles enter glomeruli, the immunoreactivity appeared to diminish significantly. Within the glomeruli, GAP-43 staining appeared similar to OMP in that contiguous subglomerular zones appeared more heavily stained, although they lacked the sharp delineation seen with the OMP staining. Moreover, GAP-43 immunoreactivity was not consistent throughout the glomerulus but appeared to exhibit a higher density proximal to the nerve layer and in the peripheral, circumferential regions of the glomerulus. In contrast to OMP, GAP-43 immunoreactivity appeared variable across the population of glomeruli (Fig. 5C; cf. Fig. 6J). Although slight staining within juxtaglomerular areas was occasionally observed, it tended to be less than that seen in the nerve layer or glomeruli.
Synaptophysin.
Synaptophysin immunoreactivity was not found in the olfactory nerve layer (Fig. 5E,F). In the glomerular layer, however, a dense reaction product was present throughout the glomeruli. The individual zones, seen so clearly with OMP, were absent, although several puncta lacking immunoreactivity were found within the glomeruli (Fig. 5E, arrow). These unstained puncta most likely correspond to blood vessels. The juxtaglomerular zone lacked any specific immunoreactivity for synaptophysin immunoreactivity, but the deeper external plexiform layer exhibited a complex pattern of nonimmunoreactive puncta broadly distributed within an otherwise intense area of synaptophysin immunoreactivity.
Double labeling.
OMP/GAP-43.
Similar populations of immunoreactivity were seen in double-labeled images using markers for OMP and GAP-43: OMP only (green), GAP-43 only (red), and areas of colocalization/overlap (yellow) (Fig. 6J). In the nerve layer, colocalization of OMP and GAP-43 is most likely due to the superpositioning of axons. Areas of superpositioning of GAP-43+ and OMP+ processes in the nerve layer were interspersed throughout, with single labeling for OMP (Fig. 6J, arrow) and GAP-43, whose distributions appeared heterogeneous across different regions of the nerve layer. These differences in OMP and GAP-43 immunoreactivity at different regions in the bulb may reflect general variations in axon maturity in any given area within the nerve layer. Colocalization in the glomerulus remained dense proximal to the nerve layer and appeared to decrease as fascicles enter the glomeruli. Within glomeruli, staining for both OMP and GAP-43 was apparent throughout the glomerulus, although OMP immunoreactivity (Fig. 6J, arrowhead) appeared more concentrated in the center/core of the glomerulus, whereas GAP-43 (Fig. 6J, double arrowheads) appeared more concentrated in the periphery of the glomerulus. This was consistent with observations made of single labeling, described above as well as the pattern that had begun to emerge at postnatal day 12. The juxtaglomerular zone and external plexiform area appeared devoid of immunoreactivity (not illustrated).
OMP/synaptophysin.
Three populations of immunoreactivity were evident in sections double-labeled with markers for OMP and synaptophysin: OMP staining alone (green), synaptophysin alone (red), and regions of colocalization (shown in yellow) (Fig. 6K). The nerve layer expressed OMP alone; there was no evidence of synaptophysin in the nerve layer. Within glomeruli, however, large areas of colocalization were found throughout a glomerulus (Fig. 6K, arrow). These regions of colocalization were punctate in appearance and resided within the islands of OMP-positive processes. Areas of colocalization were interspersed with discrete puncta of OMP (Fig. 6K, arrowhead) and synaptophysin (Fig. 6K, double arrowhead) immunoreactivity, and the density of colocalization seemed consistent throughout a glomerulus. The juxtaglomerular zone lacked any immunoreactivity, but the external plexiform layer showed the complex pattern of synaptophysin staining described for earlier ages and appeared to be devoid of both OMP-positive processes and colocalization (not illustrated).
GAP-43/synaptophysin.
The distribution of double labeling for GAP-43 (red) and synaptophysin (green) was consistent with the patterns described above for the two markers alone at postnatal day 18 (Fig. 6L). GAP-43 immunoreactive processes were evident in the nerve layer, from which synaptophysin appeared to be entirely absent. Colocalization was evident within glomeruli, although markedly less than that seen with synaptophysin and OMP. Staining for synaptophysin alone was strongest in the core of the glomerulus, whereas staining for GAP-43 alone appeared strongest in the periphery of the glomerulus. As noted previously, staining was absent from the juxtaglomerular zone, and no evidence of colocalization of these epitopes was found in the external plexiform layer.
DISCUSSION
The most important findings to emerge from these studies include 1) the emergence of a mature or adult-like subcompartmental organization within the glomerulus by 12 days postnatal; 2) a temporal-spatial pattern of axonal maturation within the glomerulus, with the most immature axons initially found in the core, whereas later in development, they occupy more peripheral regions; and 3) the strong colocalization of OMP and synaptophysin in contrast with less frequent colocalization of synaptophysin and GAP-43. Each of these findings will be discussed in turn below.
GAP-43 and OMP proved to be effective markers of immature and mature, respectively, olfactory receptor cell axons. Although GAP-43 is a membrane constituent and OMP a cytoplasmic protein, because olfactory receptor cell axons average 0.2 μm in diameter and because we used 1.0-μm optical images to assess the data, this difference in subcellular localization seems unlikely to have biased the data. Similarly, although the presence of GAP-43 in other processes including juxtaglomerular cells and centrifugal axons had the potential to influence our interpretation of the data, this seems unlikely for the following reasons. First, the majority of GAP-43 staining in the developing glomerular layer occurs within the glomerular neuropil (cf. Figs. 2-5) where growing olfactory receptor cell axons continue to arrive. There was little evidence of GAP-43 staining in the juxtaglomerular zone in either the current or previous reports (Verhaagen et al., 1989; Treloar et al., 1999; Bailey et al., 1999). Second, expression by either developing centrifugal axons or juxtaglomerular processes is transient and diminishes as they mature over a relatively narrow time frame (Bayer, 1983). Thus, although we cannot rule out that some GAP-43 staining not associated with olfactory receptor cell axons would be found in the early postnatal period, it seems unlikely that it would confound our interpretation of the data.
Subcompartmental organization of the glomerulus
Prior studies established that the adult glomerulus exhibits a complex subcompartmental organization in which the axodendritic synapses made by olfactory receptor cell axons and the local circuit dendrodendritic synapses made by efferent dendrites are spatially segregated within the glomerulus (Chao et al., 1997; Kasowski et al., 1999). At the earliest ages tested in this study, 1 and 6 days postnatal, there was no evidence of a differential distribution of olfactory receptor cell axons within the glomerulus, rather OMP+ and GAP-43+ processes appeared homogeneously distributed throughout the glomerulus. The initial appearance of an adult-like pattern of distinct islands of OMP+ processes was at 12 days postnatal, with further refinement of immunoreactive and nonimmunoreactive zones by 18 days postnatal. These results are consistent with the results of Malun and Brunjes (1996), who showed that prior to 10 days postnatal, the glomerular arbors of mitral cell apical dendrites exhibited variable degrees of differentiation. Indeed, at postnatal day 4, examples of mitral cells with multiple apical dendrites and sparsely developed glomerular tufts were illustrated (Malun and Brunjes, 1996).
These observations suggest the possibility that the subcompartmental organization of the glomerulus emerges as the dendritic arbors mature and segregate into the dendritic bundles that then interdigitate among the islands of olfactory receptor cell axons within the glomerulus. Also consistent with this interpretation are the developmental studies of Hinds and Hinds (1976a, Hinds and Hinds (1976b), who showed that axodendritic synaptic circuits emerge initially in the glomerulus, with a later appearance and maturation of dendrodendritic circuits. Indeed, the frequency of axodendritic synapses in the perinatal period exceeds that of dendrodendritic synapses by at least an order of magnitude. Consequently, it seems reasonable to suggest that the synaptophysin staining we observed at postnatal days 1 and 6 is most likely accounted for largely by the terminals of olfactory receptor cell axons. However, as the animals mature, the density or frequency of both axodendritic and dendrodendritic synapses within the glomerulus asymptotes around 10–12 days postnatal in the mouse. This appears consistent with the maturation of glomerular organization around 12 days postnatal. The colocalization of OMP and synaptophysin staining interdigitating with smaller islands of synaptophysin alone staining is indicative of the segregation of the axo- and dendrodendritic circuits into separate compartments. Finally, this pattern of maturation is reminiscent of that reported by Oland et al. (1990) in the moth: initially protoglomeruli in the antennal lobe reflect largely the distribution of primary afferent axons; the target dendritic processes do not organize into glomerular structures until around midstage 6.
Maturation of functional measures in the olfactory bulb follows a time course similar to that reported here. Using 2-deoxyglucose, both Greer et al. (1982) as well as Astic and Saucier (1982) reported that although individual glomeruli could be detected following odor stimulation at younger ages, the definitive patterns characteristic of the adult did not appear until around postnatal day 12. Similarly, Meisami and Sendera (1993), using cytochrome oxidase staining, reported increases in both the number and size of glomeruli that appear to correspond well to the data reported here. Glomeruli more than doubled in diameter between postnatal days 1 and 25, whereas the total number of glomeruli in the bulb had stabilized by 3 days postnatal. Our data now suggest that although glomeruli may continue to increase in diameter, most likely due to the addition of new primary afferents as well as juxtaglomerular dendrites, the compartmental organization is established by 12 days. Further growth of individual glomeruli after 12 days postnatal most likely reflects the elaboration of the established adult pattern.
Maturation within the glomerular neuropil
Little is known about the spatiotemporal sequences of maturation within a glomerulus. Our prior studies of the embryonic rat olfactory bulb demonstrated that immature axons arrived first in the core of a glomerulus and were then displaced to the periphery during ensuing maturation (Treloar et al., 1999). Our current data demonstrate that this pattern of development continues through postnatal day 6. However, by postnatal day 12, the pattern inverts; the immature fibers, visualized as GAP-43+ processes, now define the outermost periphery of the glomerulus. It is notable that this pattern of maturation is also reflected in the synaptophysin staining, which tends to appear heaviest in the periphery of the glomerulus. A similar pattern of synaptophysin staining is also noted in the adult (Stone et al., 1994; Johnson et al., 1996). These data suggest that the initial formation of the glomerulus is likely to incorporate a scheme in which the most immature axons move toward the periphery as they mature, and the glomerulus grows. However, when a glomerulus becomes adult-like or mature, axons appear to enter only after coursing around the periphery of the glomerulus. This may lead, in the adult, to an initially heavier concentration of both mature and immature axons in the periphery of the glomerulus. Some support for this suggestion may be found in Johnson et al. (1996), who reported that concentrations of synaptophysin immunoreactivity were heaviest in glomeruli, where they were proximal to the olfactory nerve layer.
In a related context, Halasz and Greer (1993) and Klenoff and Greer (1998) both presented evidence of accumulations of axons in the periphery of glomeruli. Verhaagen et al. (1989) also reported heavy accumulations of GAP-43 immunoreactivity around the perimeter of glomeruli in the adult. Similarly, Holtmaat et al. (1997) showed that following overexpression of GAP-43, olfactory receptor cell axons distributed preferentially in the periphery of the glomerulus. The mechanism that may mediate this change in the approach of axons to glomeruli in adults versus neonates remains to be established. However, it may reflect alterations in the distribution and the molecular properties of the astroglia cells that both surround and invade the neuropil of glomeruli (Chiu and Greer, 1996; Bailey and Shipley, 1993; Gonzalez et al., 1993; Kasowski et al., 1999).
Synaptophysin localization
There is a general consensus that olfactory receptor cell axons lose the GAP-43 phenotype as they mature and begin to express OMP (Verhaagen et al., 1989). We previously reported that in the embryo synaptophysin was lightly expressed in the olfactory nerve when it abuts the presumptive olfactory bulb at embryonic day 17, prior to a robust expression of OMP (Treloar et al., 1999). The current data are consistent in that they too suggest only slight colocalization of synaptophysin and GAP-43, rather, synaptophysin appears most consistently colocalized with OMP expression. This simple observation is consistent with the notion that OMP expression is associated with more mature axons that have established synaptic appositions within the glomeruli. Interestingly, at the youngest ages we examined, it was difficult to dissociate the OMP and synaptophysin colocalization. It was not until postnatal day 12 that a more adult-like pattern emerged in which puncta of synaptophysin staining alone interdigitated with the islands of colocalized OMP and synaptophysin. Johnson et al. (1996) present similar findings in that their synaptophysin staining of glomeruli was more homogeneous at perinatal ages than in older animals. As has been shown in the adult, synaptophysin localization is not limited to the axodendritic synapses in the olfactory bulb; synaptophysin immunoreactivity is also found in the glomerular reciprocal dendrodendritic synapses (Stone et al., 1994; Kasowski et al., 1999). Consequently, the most plausible explanation for the findings in the current study is the emergence of the nascent dendritic bundles and their dendrodendritic synaptic circuits.
SUMMARY
In conclusion, the data presented demonstrate that the compartmental organization of olfactory receptor cell axons within glomeruli emerges slowly over the first 12 postnatal days. Compartmentalization appears to increase in concert with the upregulation of the OMP phenotype and downregulation of the GAP-43 phenotype. The appearance of puncta of synaptophysin staining, interdigitating with islands in which OMP and synaptophysin colocalized, heralded the emergence of the dendritic bundles within the glomeruli.
Acknowledgements
The authors express their appreciation to Dr. Helen Treloar, Dr. Mary Hanson, and Mr. Brian Lipscomb for helpful discussions and comments on the manuscript. This work was supported in part by NIH grants DC00210, DC03887, and NS10174 to C.A.G.




