Orbitofrontal sulci of the human and macaque monkey brain
Abstract
The present study investigated the orbitofrontal sulci in 100 normal adult human cerebral hemispheres by using magnetic resonance images that were transformed into the standardized proportional stereotaxic space most commonly used, that of Talairach and Tournoux (Talairach and Tournoux [1988]. Co-planar stereotaxic atlas of the human brain. New York: Thieme). The patterns formed by the individual sulci were then examined and compared with those of the less convoluted macaque monkey brain. Four sulci forming a similar sulcal pattern were identified in both species. The olfactory sulcus occupies the most medial position forming the lateral border of the gyrus rectus. Lateral to this, the medial, lateral, and transverse orbital sulci form a pattern often resembling an “H,” “X,” or “K.” These sulci divide the orbitofrontal cortex into four major gyri: the medial, lateral, anterior, and posterior orbital gyri. Three major types of sulcal pattern were identified in both species based on the arrangement of these orbital sulci. Additional sulci were observed in the human brain, creating more complex patterns. Probability maps were constructed for the four main orbitofrontal sulci of the human brain. These maps provide a statistical description of the variability of the location of the orbitofrontal sulci within the three-dimensional coordinate system of Talairach and Tournoux (Talairach and Tournoux [1988]. Co-planar stereotaxic atlas of the human brain. New York: Thieme). Because these maps may be directly compared with any image transformed into the same standardized space, they provide a valuable tool for identifying and describing the location of functional or structural changes in the orbitofrontal region of the human brain. J. Comp. Neurol. 422:35–54, 2000. © 2000 Wiley-Liss, Inc.
The ventral surface of the frontal lobe, traditionally referred to as the orbitofrontal cortex, extends from the frontal pole rostrally to the anterior perforated substance caudally. The frontal operculum and the ventromedial margin of the cerebral hemisphere form its lateral and medial borders, respectively. Cytoarchitectonic analyses have revealed five major subregions, including frontal polar area 10, area 11 anteriorly, area 13 caudally, area 14 medially, and area 12 laterally (Walker, 1940; Barbas and Pandya, 1989; Carmichael and Price, 1994; Petrides and Pandya, 1994). In the macaque monkey, three major orbital sulci have been identified: a shallow olfactory groove hidden by the overlying olfactory tract; a longer, deeper medial orbital sulcus; and a smaller lateral orbital sulcus that is more variable. In addition, smaller sulci may extend from the medial orbital sulcus to form more complex patterns (Connolly, 1936; Walker, 1940; Bonin and Bailey, 1947). Although there is general agreement regarding the principal orbitofrontal sulci in the macaque monkey, a systematic analysis of the sulcal patterns in the orbitofrontal region has not been performed.
Greater variability exists among the sulcal and gyral patterns of the human orbitofrontal cortex. The olfactory sulcus occupies the most medial position and runs in a straight rostrocaudal direction underneath the olfactory tract. It delimits the innermost orbital convolution, first called the “gyrus rectus” by Valentin in 1841 (Meyer, 1971). However, lateral to this, some texts simply describe the remaining sulci as “orbital sulci” and state that they often form an “H-shaped” pattern (e.g., Williams et al., 1989), whereas others use the term “orbital gyri” and do not label the orbitofrontal sulci at all (e.g., Carpenter, 1985; Netter, 1986; Martin, 1996).
Gratiolet (1854) first used the term scissure en H to describe an “H-pattern” of sulci on the orbitofrontal surface (Meyer, 1971). Since then, although reference is often made to an “H-pattern of sulci,” discrepancies exist with respect to the labeling of the various sulci forming the “H-pattern.” Classical authors (Weisbach, 1870; Eberstaller, 1890; Economo and Koskinas, 1925; Bailey and Bonin, 1951), as well as more recent investigators (Ono et al., 1990), have attempted to identify and label the sulci of the human orbitofrontal cortex. Weisbach (1870) and Eberstaller (1890), and later Economo and Koskinas (1925), use the term sulcus orbitalis transversus to refer to the entire posterior component of the “H-shaped pattern,” including the horizontal connecting link and the posterior medial and lateral limbs of the “H-pattern.” According to this usage, the sulcus orbitalis transversus extends in an arc-like manner across the posterior orbital region, delimiting the posterior orbital gyrus. The anterior medial and lateral extensions of the “H-pattern” are referred to by Weisbach (1870) as the sulcus orbitalis medius and externus, by Eberstaller (1890) as the ramus medialis and lateralis (of the sulcus orbitalis), and by Economo and Koskinas (1925) as the sulcus orbitalis medialis and lateralis. Ono and colleagues (1990) use the term transverse orbital sulcus to describe only the horizontal sulcus in the mid-orbitofrontal region joining the longitudinal sulci observed there. By contrast, Bailey and Bonin (1951) use the term arcuate orbital sulcus to describe that part of the “H-pattern” that is the connecting link. They refer to the medial and lateral longitudinal components of the “H-pattern,” including both the anterior and posterior limbs, as the medial and lateral orbital sulci, respectively.
Duvernoy (1991), in a major departure from previous descriptions of the human orbitofrontal sulci (Weisbach, 1870; Eberstaller, 1890; Economo and Koskinas, 1925; Bailey and Bonin, 1951; Ono et al., 1990) and terminology in other primate brains (Connolly, 1936; Walker, 1940; Bonin and Bailey, 1947), refers to the olfactory sulcus as the medial orbital sulcus. Moreover, he uses the term lateral orbital sulcus to refer to a sulcus found on the ventrolateral, rather than the orbital, surface of the brain. This major inconsistency arises because Duvernoy chose to use the terms medial and lateral orbital sulci, not to describe parts of the “H-pattern” of orbitofrontal sulci, but rather to refer to sulci medial and lateral to the “H-pattern.” Although earlier investigators describe an “H-pattern” of sulci, they consider it to be the complex formed by the medial orbital sulcus, the lateral orbital sulcus, and the transverse connecting link (Economo and Koskinas, 1925; Bailey and Bonin, 1951; Ono et al., 1990).
As can be seen from the above short review, there are four major problems regarding current descriptions of the human orbitofrontal sulci. First, apart from the olfactory sulcus, the other orbital sulci have not been consistently identified, nor have their location, pattern, and incidence been described. For instance, it is not clear which orbitofrontal sulci are constant and which are highly variable. Second, the patterns formed by the arrangement of specific sulci and the variability of these patterns have not been clearly documented. Third, although investigators refer to a general “H-shaped pattern,” they apply inconsistent names to the sulci comprising the “H.” A universal nomenclature is critical if the results of research involving this region are to be compared across investigations. Finally, there has been no quantitative investigation of the major orbitofrontal sulci and their variability assessed within a standardized stereotaxic space. To interpret and discuss the location of activity changes in a given brain region obtained in functional neuroimaging studies (e.g., positron emission tomography and functional magnetic resonance imaging), it is necessary to make reference to constant, reliable anatomic landmarks. Moreover, these landmarks should be defined within a standardized reference system that would allow their location and variability to be described quantitatively by using stereotaxic coordinates.
The present study addressed the above problems. It examined the sulci of the human orbitofrontal cortex, the pattern formed by individual sulci, and the incidence of variability in this pattern. The human orbitofrontal sulcal pattern was then compared with that of the less convoluted macaque monkey and a classification system, based on comparable sulci in the two species, was provided. In addition, probability maps were constructed which provide a quantitative description of the location and variability of the various human orbitofrontal sulci within the standardized, three-dimensional, proportional coordinate system of Talairach and Tournoux (1988), the one most often used in modern functional neuroimaging studies.
Abbreviations
-
- Fr
-
sulcus fragmentosus
-
- HR
-
horizontal ramus of the Sylvian fissure
-
- IOS
-
intermediate orbital sulcus
-
- IOSl
-
lateral intermediate orbital sulcus
-
- IOSm
-
medial intermediate orbital sulcus
-
- LOS
-
lateral orbital sulcus
-
- LOSc
-
caudal portion of lateral orbital sulcus
-
- LOSr
-
rostral portion of lateral orbital sulcus
-
- MOS
-
medial orbital sulcus
-
- MOSc
-
caudal portion of medial orbital sulcus
-
- MOSr
-
rostral portion of medial orbital sulcus
-
- Olf
-
olfactory sulcus
-
- POS
-
posterior orbital sulcus
-
- POSl
-
lateral posterior orbital sulcus
-
- POSm
-
medial posterior orbital sulcus
-
- SF
-
Sylvian fissure
-
- TOS
-
transverse orbital sulcus
MATERIALS AND METHODS
Subjects
Human.
Magnetic resonance scans of 50 human brains were examined. Subjects were healthy volunteers drawn from the Montreal area population acquired as part of the International Consortium for Brain Mapping project (Mazziotta et al., 1995). The sample consisted of 22 females (mean age, 24.84 years, SD 5.28) and 28 males (mean age, 25.42 years, SD 5.29). All subjects were right-handed, had a negative history of neurologic and/or psychiatric disorders, and gave informed consent.
Monkey.
The orbital surfaces of 50 adult monkey brains (Macaca mulatta) were analyzed. The ventral surface of each brain was photographed, and the sulcal patterns identified by examining the external morphology of each hemisphere.
Magnetic resonance imaging of human brains
All MR scans were performed on a Phillips Gyroscan 1.5-T superconducting magnet system. By using a 3-D fast-field echo-acquisition sequence, 160 contiguous 1 mm T1-weighted images (Tr = 18 msec, Te = 10 msec, flip angle 30 degrees) were collected in the sagittal plane. Each MR volume was then transformed into the standardized stereotaxic space of Talairach and Tournoux (1988) by an automatic registration procedure that uses a 3-D cross-correlation approach to match a single MR volume with the intensity average of 305 MR brain volumes previously aligned into stereotaxic space (Collins et al., 1994). This transformation of 3-D MRI volumes into stereotaxic space effectively normalizes the images for inter-individual differences in brain size. The thickness of the interpolated sagittal, axial, and coronal slices was 1 mm. After the transformation, all volumes were resampled on a 1 mm3 isotropic grid. The anterior commissure (AC) is the origin of the coordinate system used (Talairach and Tournoux, 1988). In standardized Talairach proportional space, coordinates are expressed in millimeters (mm). The mediolateral (or left-right) axis is defined by using the x-coordinate (positive = right hemisphere), the rostrocaudal (anterior-posterior) axis by the y-coordinate (positive = rostral to anterior commissure), and the dorsoventral (superior-inferior) axis by the z-coordinate (positive = superior to a horizontal line drawn through anterior and posterior commissures).
Segmentation of intrasulcal gray matter
Individual orbitofrontal sulci were identified on MR scans by using an interactive 3-D imaging software package, which allows the scans to be viewed and marked simultaneously in the coronal, horizontal, and sagittal planes of section (MacDonald, 1996) (Fig. 1). The gray matter voxels belonging to the banks of each sulcus were marked by means of a mouse driven cursor. When the cursor is moved to any point in a given section (e.g., coronal), the sections in the other two planes (e.g., sagittal and horizontal) that run through that point are automatically displayed. A given sulcus was followed through each 1-mm brain section in all three planes enabling the investigator to determine with accuracy the extent and direction of each sulcus. After the overall intensity of the scans was set by visual inspection to give adequate gray/white contrast, all the gray matter voxels extending from the brain surface to the depth of any given sulcus were marked.
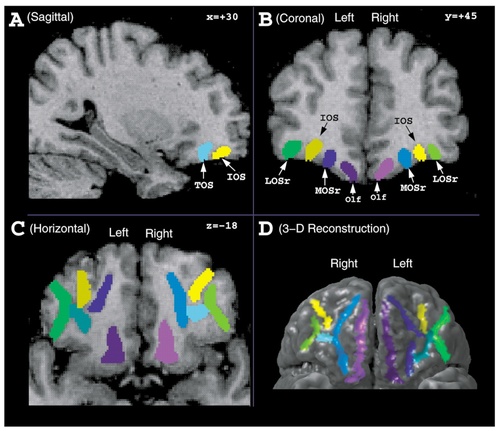
The orbitofrontal sulci were viewed and marked simultaneously on T1-weighted MRI images in sagittal (A), coronal (B), and horizontal (C) planes of section by using a program for the display and segmentation of brain structures (MacDonald, 1996). Three-dimensional surface reconstructions were also created for each hemisphere (D). The stereotaxic coordinates are expressed in millimeters in the standardized Talairach proportional space. x, medial-to-lateral distance relative to the midline (positive = right hemisphere); y, anterior-posterior distance relative to the anterior commissure (positive = anterior); z, superior-inferior distance relative to the anterior commissure-posterior commissure line (positive = superior). For abbreviations, see list.
Surface renderings and probability mapping
Three-dimensional surface reconstructions of individual brains were obtained by using an automatic, model-based, surface deformation algorithm (MacDonald et al., 1994). The sulcal labels, which were marked on contiguous two-dimensional sections, could then be transferred to and viewed on the 3-D renderings of the orbitofrontal cortex, allowing for an accurate inspection of sulcal patterns (Fig. 1D).
Three-dimensional stereotaxic probability maps were created for each sulcus by determining the incidence of labeled voxels across subjects. More specifically, at a particular 3-D stereotaxic location, the number of times a given voxel belonged to the structure of interest was divided by the number of subjects under study to obtain a probability value. These probability maps represent a 3-D image of the likelihood that any voxel in stereotaxic space will be classified as including the intrasulcal gray matter of a given sulcus, thereby allowing one to quantify spatial variability of these anatomic structures. The probability maps have been superimposed on an average MRI of 305 subjects that had been transformed into Talairach space (Evans et al., 1992). The Talairach grid system overlies each probability map enabling one to identify readily the stereotaxic x, y, and z locations for each sulcus.
RESULTS
Main human orbitofrontal sulci
Four human orbitofrontal sulci were consistently identified and the corresponding gray matter voxels were marked: the olfactory (Olf), medial (MOS), lateral (LOS), and transverse (TOS) orbital sulci (Fig. 1). The olfactory sulcus occupies the most medial position, running in a rostro-caudal direction and providing the lateral border of the gyrus rectus (Fig. 2). Most often, this sulcus occupies a more lateral position caudally and veers medially as it moves rostrally. For this reason, the gyrus rectus is quite wide caudally and narrows in the rostral direction. In the majority of cases (61%), the rostral part of the olfactory sulcus ends parallel to the ventromedial convexity (Fig. 2A) but it may also veer medially (32%; Fig. 2B,C) or laterally (7%; Fig. 2D). In some instances, the anterior portion of this sulcus wraps around the ventromedial convexity and ends on the medial surface (Fig. 2C). The posterior part of the olfactory sulcus projects laterally and often forms a hook (Fig. 2E–H). This hook may be small, ending in the medial orbital gyrus (36%; Fig. 2F), or quite long, extending into the posterior orbital gyrus between MOSc and LOSc (8%; Fig. 2G). In some cases, the lateral extension of the olfactory sulcus anastomoses with MOSc (7%; Fig. 2H).
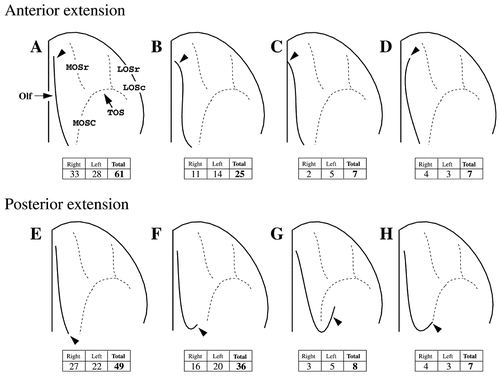
Variations in the anterior (A–D) and posterior (E–F) extensions of the olfactory sulcus. For abbreviations, see list.
Lateral to the olfactory sulcus, there are two longitudinally directed sulci: the medial and lateral orbital sulci, which can be viewed as having rostral and caudal portions. Located approximately in the center of the orbitofrontal cortex, the transverse orbital sulcus runs horizontally between the medial and lateral orbital sulci. It thereby separates the rostral portions of the medial orbital (MOSr) and lateral orbital (LOSr) sulci from their caudal portions, MOSc and LOSc. In all cases, one or two longitudinal sulci, the intermediate orbital sulcus/sulci (IOS), were found between the MOSr and the LOSr. In addition, other less consistent sulci were often present: (1) the sulcus fragmentosus (Fr), which is small and sometimes fragmented, located between Olf and MOS; and (2) one or two longitudinal sulci lying posterior to TOS in between MOSc and LOSc.
Human orbitofrontal sulcal patterns
Three main orbitofrontal pattern types were defined based on the continuity of the medial and lateral orbital sulci (Figs. 3, 4). Differences in the orientation of the medial and lateral orbital sulci produced variations of these basic pattern types (Fig. 3; Types I–III, A–F).
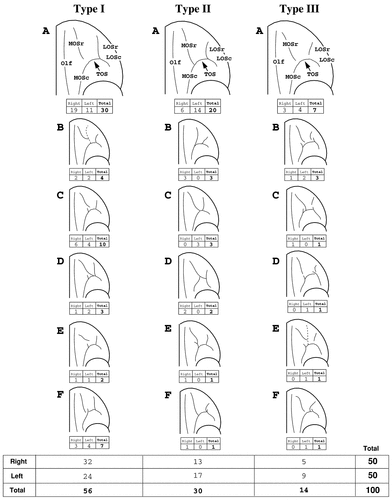
The main orbitofrontal sulcal patterns of the human brain (Types I–III) and their variability (A–F). Incidences for each pattern are presented for the right and left hemisphere. For abbreviations, see list.
Examples of the orbitofrontal sulcal patterns in three human brains. For abbreviations, see list. Scale bar = 1 cm.
Type I.
The Type I pattern, observed in 56% of hemispheres, is the most common pattern formed by the medial, lateral, and transverse orbital sulci (Figs. 3, 4, Type I). In this pattern, LOSr is connected with LOSc and, in most cases, forms a continuous lateral orbital sulcus. However, the separate portions of the medial orbital sulcus, MOSr and MOSc, are clearly distinct. Six variations of the Type I pattern were observed (Fig. 3; Type I, A–F).
In 61% of Type I cases, MOSc ends at the level of TOS and MOSr emerges slightly rostral to this point (Type IA and IB). Most often (54%), MOSr is a free sulcus lying longitudinally, rostral to MOSc (Type IA). In some cases (7%), MOSr emerges from an intermediate orbital sulcus and projects anteromedially (Type IB). In the remaining 39% of Type I cases, the anterior part of MOSc does not end at the TOS, but extends rostral to it (Types IC, ID, IE, and IF). In Type IC, MOSr begins just rostral and/or lateral to the anterior extension of MOSc. In Type ID, the posterior part of MOSr is again situated lateral to the anterior extension of MOSc but in these cases, MOSr joins with TOS caudally. In Type IE, MOSr projects sharply in the medial direction lying almost horizontally. Finally, in Type IF, the posterior part of MOSr is located medial to the anterior extension of MOSc. Overall, the Type I pattern was seen more often in the right hemisphere (57%) than in the left (43%).
An example of Type IC is shown in Figure 5. Caudally, Olf occupies the most medial position and MOSc is the first major sulcus lateral to Olf (section 2h). LOSc is found lateral to MOSc and inferior to the horizontal ramus of the Sylvian fissure (HR). These two sulci can be followed rostrally where they come together at the surface and join with TOS (section 2g). Just past TOS, LOS continues and may now be regarded as LOSr (section 2f). At this level, the sulcus fragmentosus emerges lateral to Olf and MOSc is still visible, having extended slightly rostral to TOS. MOSc ends at y = +38 and, at y = +40, MOSr begins. In this section, an intermediate orbital sulcus also begins to emerge from LOSr and, at y = +44 and y = +47, four longitudinal sulci can be clearly identified.
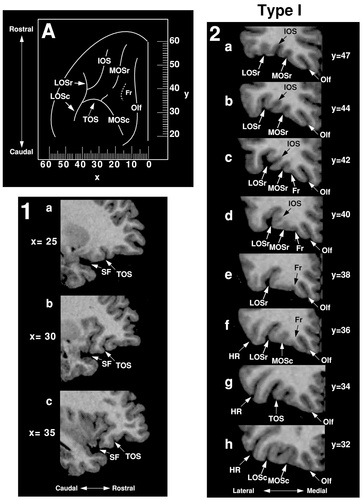
Magnetic resonance images illustrate the Type I pattern in sagittal (1) and coronal (2) sections. The transverse orbital sulcus is most clearly seen in sagittal sections (1, a–c), whereas the longitudinal sulci (Olf, MOS, LOS, and IOS) are most clearly seen in coronal sections (2, a–h). The Type I pattern has been reconstructed on the orbitofrontal surface (A) and the corresponding Talairach coordinates are shown, the x-axis representing the mediolateral dimension (negative numbers = left hemisphere) and the y-axis the rostrocaudal (positive numbers = rostral to the anterior commissure). For abbreviations, see list.
Type II.
The commonly described “H-pattern,” formed by the joining of MOS, LOS, and TOS, has been designated Type II (Figs. 3, 4, Type II). The rostral and caudal portions of the medial and lateral orbital sulci are connected to one another and are joined by the horizontally oriented TOS. This pattern was observed in 30% of hemispheres and was seen more frequently in the left (57%) than in the right (43%). In 83% of Type II cases, MOS and LOS occupy far medial and lateral positions, respectively, and are connected by a long TOS (Fig. 3, Types IIA, IIB, IIE, and IIF). In 17% of Type II cases, TOS is relatively short, bringing MOS and LOS close together (Types IIC and IID).
Type IIA, the prototypical “H-pattern,” was observed in 20% of hemispheres. It composed the majority of Type I cases (67%) and was found more often in the left hemisphere (70%) than in the right (30%). Type IIB (10%) differs slightly in that the rostral extensions of MOS and LOS veer laterally as they project anteriorly (Type IIC). In some instances, MOS, LOS, and TOS form patterns resembling the letter “X” (10%; Type IIC) or “K” (7%; Type IID). In one hemisphere, MOSr emerges from MOSc but is not continuous with it (Type IIE). Similarly, in one hemisphere, LOSr emerges from LOSc but is not continuous with it (Type IIF).
An example of the H-pattern, Type IIA, is shown in Figure 6. Caudally, MOSc is the first major sulcus lateral to Olf (sections 2g and 2f). LOSc is situated lateral to MOSc and inferior to HR. MOS and LOS can be followed rostrally to y = +36 where they join with TOS. MOS and LOS continue rostrally past TOS, thereafter referred to as MOSr and LOSr, respectively. At y = +41, an IOS emerges between MOS and LOS and, at y = +44, four longitudinal sulci, Olf, MOS, IOS, and LOS, can be clearly identified.
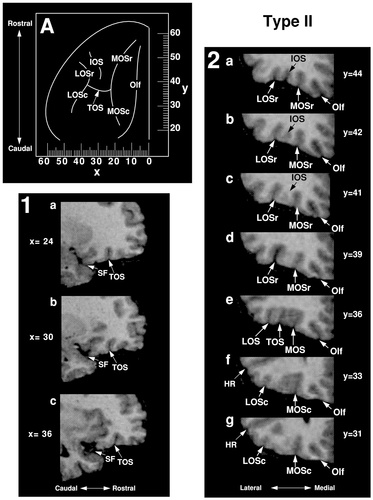
Magnetic resonance images illustrate the Type II pattern in sagittal (1) and coronal (2) sections. The Type I pattern has been reconstructed on the orbitofrontal surface (A), and the corresponding Talairach coordinates are shown, the x-axis representing the mediolateral dimension (negative numbers = left hemisphere) and the y-axis the rostrocaudal (positive numbers = rostral to the anterior commissure). For abbreviations, see list.
Type III.
In Type III, the rostral and caudal portions of both MOS and LOS are separate (Figs. 3, 4). Type III was observed in 14% of the hemispheres and more often in the left (64%) than in the right (36%). Six variations of this pattern were observed (Fig. 3, Type III, A–F).
In 50% of the Type III cases, the caudal portions of the medial and lateral orbital sulci join with the TOS and do not extend above it (Type IIIA). In 36% of the Type III cases, the anterior part of both MOSc and LOSc extends slightly rostral to TOS (Types IIIB, IIIC, IIID). In these cases, the caudal part of MOSr lies either medial (Type IIIB and IIIC) or rostral to (Type IIID) the anterior extension of MOSc. In Type IIIC, the caudal part of MOSr joins with TOS. Furthermore, the caudal part of LOSr is located either medial (Type IIIB), rostral (Type IIIC), or lateral (Type IIID) to the anterior extension of LOSc. In one left hemisphere, the anterior part of MOSc again extends slightly rostral to TOS, and MOSr emerges from an intermediate orbital sulcus (Type IIIE). Finally, in one right hemisphere, LOSr joins posteriorly with TOS, medial to the anterior extension of LOSc (Type IIIF).
An example of the Type III pattern is shown in Figure 7. Caudally, MOSc is the first major sulcus lateral to Olf (sections 2h, 2g, and 2f). The sulcus fragmentosus is also observed between Olf and MOSc. LOSc is located lateral to MOSc and inferior to HR. MOSc and LOSc join with TOS at y = +33 (section 2e). IOS emerges from TOS and continues rostrally (section 2d). At y = +37, MOSr and LOSr can be seen beginning on either side of the IOS and, at y = +39 and y = +42, four longitudinal sulci can be clearly identified.
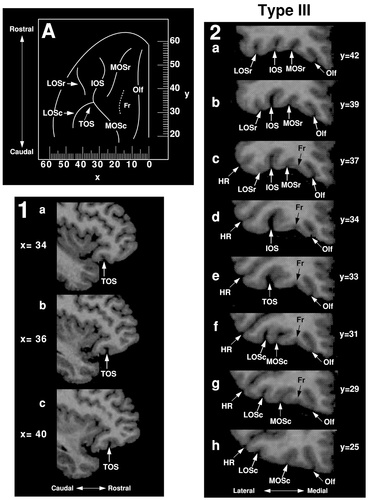
MRI images illustrate the Type III pattern in sagittal (1) and coronal (2) sections. The Type I pattern has been reconstructed on the orbitofrontal surface (A) and the corresponding Talairach coordinates are shown, the x-axis representing the mediolateral dimension (negative numbers = left hemisphere) and the y-axis the rostrocaudal (positive numbers = rostral to the anterior commissure). For abbreviations, see list.
Other human orbitofrontal sulci
More complex patterns are formed by the addition of the intermediate orbital sulcus, the posterior orbital sulci, and the sulcus fragmentosus (Fig. 8).

Human orbitofrontal sulcal patterns formed by the addition of intermediate orbital sulci (A), posterior orbital sulci (B), and the sulcus fragmentosus (C). In A–C, the additional sulcus illustrated is represented by a dotted line.
Intermediate orbital sulcus/sulci.
In all hemispheres, one or two longitudinal sulci were found anterior to TOS in between MOSr and LOSr (Fig. 8A). In 81% of the cases, one deep, highly visible sulcus was present and may be thought of as an intermediate orbital sulcus (IOS) (Fig. 8A, 1-6). When one IOS was present, most often it joined caudally with TOS and, in seven of these cases, it forked anteriorly into medial and lateral branches (Figs. 8A–1,2). In other cases, IOS was situated anterior to TOS as a free sulcus (Fig. 8A–3) or emerged from either MOSr (Fig. 8A–4) or LOSr (Fig. 8A–5) to form a “Y”-shape, or from both MOSr and LOSr to form a triangulated pattern (Fig. 8A–6).
In 19% of the hemispheres, two intermediate orbital sulci were present, one occupying a more medial position (IOSm) and the other a more lateral one (IOSl) (Fig. 8A,7-10). When this occurred, several variations were observed. Most often, IOSm was joined caudally with TOS and was the deeper, more pronounced sulcus and IOSl was a shallower sulcus located quite anteriorly (Fig. 8A–7). In other cases, IOSl was joined caudally with TOS and IOSm was a free sulcus lying anterior to TOS (Fig. 8A–8). Other patterns formed by the two intermediate orbital sulci are also shown (Fig. 8A–9,10).

The three main orbitofrontal patterns of the macaque monkey (Types I–III) and their variability (Type II, a–c). Incidences for each pattern are presented for the right and left hemisphere. For abbreviations, see list.
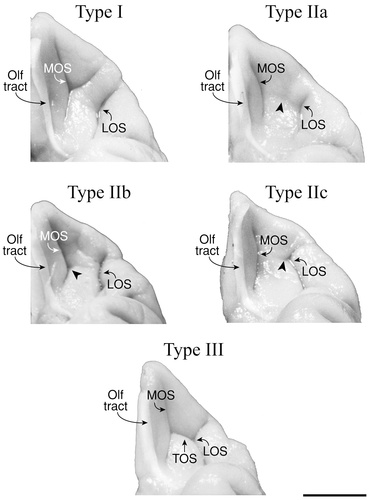
Examples of the orbitofrontal sulcal patterns (Types I–III) in five macaque monkey brains. For abbreviations, see list. Scale bar = 1 cm.
Examples of the intermediate orbital sulcus/sulci are shown is Figure 4. In the Type I and II examples, two intermediate orbital sulci (IOSm and IOSl) are present, located between MOSr and LOSr. In the Type I case, IOSm joins with TOS, whereas IOSl does not. In the Type II case, both IOSm and IOSl are free sulci lying rostral to TOS. In the Type III example, one IOS is present and is connected caudally with TOS.
Posterior orbital sulcus/sulci.
In 77% of the hemispheres, either one or two longitudinal sulci were found posterior to TOS in between MOSc and LOSc. In 81% of these cases (62/77), one deep, highly visible sulcus was present often running the entire extent of the posterior orbital region (Fig. 8B–1,2). In some instances, this sulcus emerged from the posterior orbital convexity and ran rostrally (Fig. 8B–1), whereas in others, it was connected with TOS and extended caudally (Fig. 8B–2). In 19% of these cases (15/77), two posterior orbital sulci (POS) were observed, one medially (POSm) and the other laterally (POSl). When this occurred, either both sulci emerged from the posterior orbital convexity and extended rostrally (Fig. 8B–3) or one sulcus was connected to TOS and the other emerged from the posterior orbital convexity (Fig. 8B–4).
Examples of the posterior orbital sulcus/sulci are shown is Figure 4 (Types I and II). In the Type I example, a posterior orbital sulcus can be seen extending caudally from the transverse orbital sulcus. In the Type II example, two posterior orbital sulci are present; the medial one (POSm) emerging from the posterior convexity and projecting rostrally and the lateral one (POSl) emerging from LOSc and running caudally.
Sulcus fragmentosus.
In 10% of the hemispheres, a small sulcus, the sulcus fragmentosus (Fr), was present as a slight indentation lying longitudinally approximately mid-way between Olf and the base of the orbital depression (Fig. 8C). This sulcus, when present, was composed of one or two short components. When two fragments were observed, one occupied a more rostral position and the other a more caudal one. Overall, this small sulcus was found more frequently in the left hemisphere (70%) than in the right (30%). Examples illustrating the sulcus fragmentosus are shown (Figs. 4, 5, 7).
Macaque orbitofrontal sulci
The orbitofrontal cortex of the macaque monkey contains three major sulci: the olfactory sulcus (Olf), the medial orbital (MOS), and the lateral orbital sulcus (LOS). Often, a fourth sulcus is observed traversing the mid-orbitofrontal region. The olfactory sulcus, often hidden from direct view by the olfactory tract, occupies the most medial position. MOS lies lateral to Olf at the base of the orbital depression and is the longest and most consistent of the orbital sulci. It provides a border between the medially situated gyrus rectus and the remainder of the orbitofrontal cortex. LOS is smaller and more variable than MOS. Most often it occupies a posterolateral position and is longitudinally oriented. In most hemispheres, either a rudimentary or a well-developed transverse sulcus was also observed. This sulcus may (1) emerge from MOS or LOS, (2) extend anterolaterally from MOS to form a “Y” shape, or (3) anastomose horizontally with MOS and LOS to form an “H” shape. Slight variations in the arrangement of these sulci produced three major sulcal patterns in the 100 hemispheres examined (Fig. 9, Types I–III).
Macaque orbitofrontal sulcal patterns
Type I.
Type I, observed in 64% of the hemispheres, is the most common sulcal pattern found in the macaque monkey orbitofrontal region (Fig. 9). This pattern consists of three longitudinal sulci: the olfactory and medial and lateral orbital sulci. In addition, a horizontal sulcus extends from MOS in an anterolateral direction to form a “Y” shape. An example of the Type I pattern is shown in Figure 10. In this example, the overlying olfactory tract hides the olfactory sulcus. Type I was observed more frequently in the left hemisphere (53%) than in the right (47%).
Type II.
Type II was observed in 24% of the monkey hemispheres and is the simplest pattern formed by the orbitofrontal sulci (Fig. 9). This pattern consists of three longitudinal sulci: the olfactory and medial and lateral orbital sulci. In addition, a rudimentary transverse orbital sulcus was observed, located between the medial and lateral orbital sulci. In some cases, this slight horizontal indentation did not join with either the MOS or LOS (Type IIa), whereas in others, it extended from either the MOS (Type IIb) or LOS (Type IIc) in the mid-orbital region. This pattern was found slightly more often in the right hemisphere (54%) than the left (46%).
Three examples of the Type II pattern (a–c) are shown in Figure 10. The olfactory tract and the underlying olfactory sulcus occupy the most medial position. Just lateral to Olf, at the base of the orbital depression, lies the long, longitudinally oriented MOS. Lateral to MOS is the smaller LOS. Additionally, a small, horizontal indentation may be found in the mid-orbitofrontal region between MOS and LOS (Fig. 10, arrowhead).
Type III.
The Type III pattern comprised 7% of the monkey hemispheres. In these cases, MOS and LOS were joined to each other by a horizontally oriented sulcus to form an “H” pattern similar to the one seen in the human brain (Fig. 9). This pattern was observed slightly more often in the right hemisphere (57%) than in the left (43%). An example of this pattern is shown in Figure 10.
Probability maps of the human orbitofrontal sulci
Probability maps were created for the olfactory, medial, lateral, and transverse orbital sulci (Figs. 11-13). Probability values are displayed by means of a color scale ranging from 0.1 and 0.9. Thus, all the points of these maps belong to the banks of the sulci for at least five subjects from the sample studied (0.1 = 5 of 50) and the highest probability value shown includes at least 45 subjects (0.9 = 45 of 50). For example, the white area found in the map of the olfactory sulcus corresponds to a probability value of 0.9 or higher, indicating that, in at least 90% of the subjects, the olfactory sulcus occupied these coordinates.
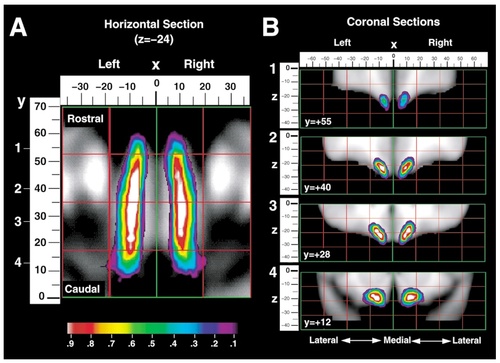
A: Probability map for the location of the olfactory sulcus shown in horizontal section, through z = −24. The mediolateral (x) position in millimeters is shown above the image with x = 0 marking the interhemispheric plane, negative numbers representing the left hemisphere and positive numbers representing the right hemisphere. The rostrocaudal (y) dimension in millimeters is shown to the left of the image with y = 0 marking the vertical plane drawn through the anterior commissure and positive numbers representing a region rostral to this plane. B: Four coronal sections of the probability map, at y = +55, y = +40, y = +28, and y = +12 are also presented with the x-dimension shown above and the dorsoventral (z) dimension shown on the left.
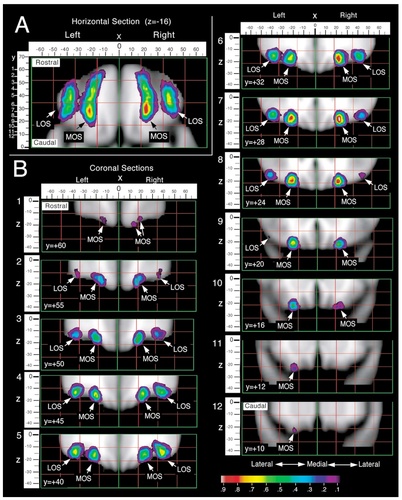
Probability maps for the location of the medial (MOS) and lateral orbital (LOS) sulci, including both the rostral and caudal portions of each sulcus. A: A two-dimensional horizontal section of the probability map is shown, though z = −16, with the mediolateral (x) dimension shown above the image and the rostrocaudal (y) dimension to the left. B: Coronal sections of the probability map illustrate the location of the medial and lateral orbital sulci at various levels along the rostrocaudal plane.
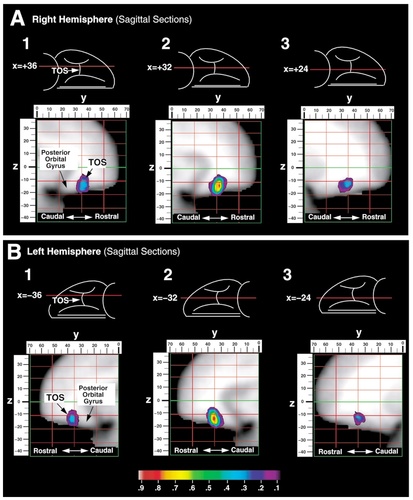
Probability map for the location of the transverse orbital sulcus (TOS) in the right (A) and left (B) hemispheres. Three sagittal sections are taken through this sulcus at x = 36, x = 32, and x = 24 in both the right and left hemispheres (positive x-value = right hemisphere). The rostrocaudal (y) dimension is shown above each image with y = 0 marking the vertical plane drawn through the anterior commissure and positive numbers representing a region rostral to this plane. The dorsoventral (z) dimension is to the left of each image with z = 0 marking the horizontal plane taken through the anterior and posterior commissures. Sagittal sections (1–3) taken at three levels along the mediolateral dimension are shown for each hemisphere.
Gender differences in spatial distribution of sulci
The present study examined possible gender differences in the probability maps of each orbitofrontal sulcus. The spatial distributions of each orbitofrontal sulcus in males and females were compared by means of a z-test (Worsley et al., 1996). No significant differences were found between male and female sulcal probability maps, indicating that the spatial distribution of each orbitofrontal sulcus is similar for males and females.
DISCUSSION
The goal of the present study was to achieve a better understanding of the sulcal anatomy of the human orbitofrontal cortex. Specifically, the aim of this study was to identify constantly appearing sulci on the human orbitofrontal surface, the patterns formed by these sulci, and to provide a statistical description of the variability of the location of each major orbitofrontal sulcus in the standardized stereotaxic proportional space of Talairach and Tournoux (1988), which is the one most often used in functional neuroimaging studies. In addition, the human orbitofrontal sulcal patterns were compared with those of the less convoluted macaque monkey brain, and a classification system was provided based on comparable sulci in the two species.
Main orbitofrontal sulci of the human and the macaque monkey brain
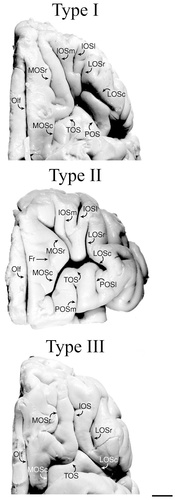
In the human brain, four main sulci were consistently identified on the orbitofrontal surface: the olfactory, medial, lateral, and transverse orbital sulci (Fig. 14A). The olfactory sulcus occupies the most medial position in all brains studied. Lateral to this, there are two longitudinal sulci (the medial and lateral orbital sulci) and a horizontal sulcus (the transverse orbital sulcus). Located at the base of the orbital depression, the medial orbital sulcus is the first major sulcus lateral to the olfactory sulcus. In the majority of cases (71%), the medial orbital sulcus consists of separate rostral and caudal portions whereas, in the remaining cases (29%), these portions fuse to form a continuous sulcus. The lateral orbital sulcus occupies the most lateral position on the orbital surface and it, too, may be thought of as having a rostral and a caudal portion. In the majority of cases (85%), these portions fuse to form a continuous sulcus.
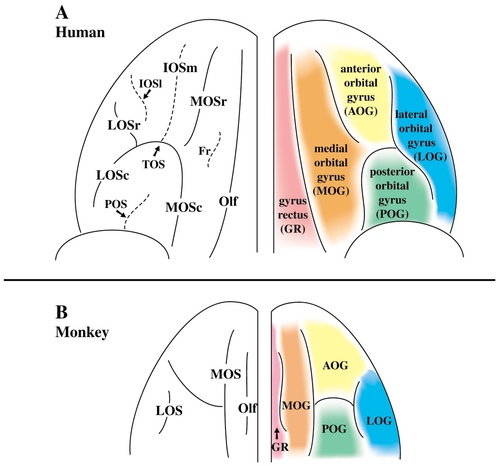
Summary of the orbitofrontal sulci and gyri in the human (A) and macaque monkey (B) brain. The most common sulcal pattern is shown on the left for each species, and the gyri formed by these sulci are shown on the right. For abbreviations, see list.
Despite minor variations, a common pattern was formed by these four sulci, essentially dividing the orbitofrontal cortex into five major gyri (Fig. 14A). The first gyrus, often called the gyrus rectus, is delineated laterally by the olfactory sulcus and medially by the rostral sulcus on the ventromedial surface. The remaining orbital sulci often create a pattern resembling an “H,” “K,” or “X.” Although discontinuity of sulci often exists in these basic patterns, the medial, lateral, and transverse orbital sulci essentially divide the remaining orbital surface into four major gyri. These are 1) the medial orbital gyrus created by the olfactory and the medial orbital sulcus; 2) the anterior orbital gyrus delimited by the transverse orbital sulcus and the rostral portions of the medial and lateral orbital sulci; 3) the posterior orbital gyrus formed by the transverse orbital sulcus and the caudal portions of the medial and lateral orbital sulci; and 4) the lateral orbital gyrus delimited medially by the lateral orbital sulcus and extending laterally around the ventrolateral convexity.
Four sulci were also observed on the orbital surface of the macaque monkey brain. The olfactory sulcus occupies the most medial position. Lateral to this, the orbital sulci consist of 1) a longitudinal medial orbital sulcus; 2) a longitudinal lateral orbital sulcus; and 3) either an incipient or a proper transverse sulcus in the mid-orbital region. The arrangement of the medial, lateral, and transverse orbital sulci creates three basic pattern types on the orbital surface of the macaque monkey brain. In all three types, the medial and lateral orbital sulci are longitudinal sulci running parallel to one another. The difference between these pattern types is found in the orientation or the depth of the sulcus lying between the medial and lateral orbital sulci.
In the simplest pattern, there is a small, horizontal depression in the mid-orbital region between the medial and lateral orbital sulci (Fig. 9; Type II). In some cases, this incipient transverse orbital sulcus emerges from the mid-point of either the medial (Type IIb) or the lateral (Type IIc) orbital sulcus whereas in other cases, it does not connect to either sulcus (Type IIa). In the second major pattern, a proper transverse orbital sulcus connects the medial and lateral orbital sulci to form an “H” shape, resembling the pattern seen in the human brain (Fig. 9, Type III). Finally, in the most common pattern, the medial orbital sulcus branches at its mid-point and forks anterolaterally to form a “Y” shape (Fig. 9, Type I). When this occurs, the lateral orbital sulcus is a short longitudinal sulcus located caudal to the lateral branch of the “Y.”
Although a less variable pattern is present in the macaque brain, comparable sulci form a fundamentally similar sulcal pattern on both the human and the macaque orbital surfaces. In both species, an olfactory sulcus is easily identifiable, forming the lateral border of the gyrus rectus. Lateral to the olfactory sulcus, the cortex slopes at approximately a 45-degree angle. At the base of this orbital depression, in both the macaque and human brain, lies the medial orbital sulcus. This sulcus, together with the olfactory sulcus medially, essentially creates a medial orbital gyrus. Occupying the most lateral position of the orbital surface in both species, the lateral orbital sulcus runs parallel to the medial orbital sulcus. The lateral orbital gyrus extends from the lateral orbital sulcus around the ventrolateral convexity. The medial and lateral orbital sulci together create a mid-orbital region that may further be subdivided, by either an incipient or a proper horizontal sulcus, to form anterior and posterior orbital gyri. In the human brain, a well-defined transverse orbital sulcus is present, connecting the medial and lateral orbital sulci. In some macaque monkey brains, the anterior and posterior orbital gyri are separated by a proper transverse orbital sulcus, as in the human brain. In others, however, the anterior and posterior gyri are delineated either by an incipient transverse orbital sulcus in the form of a horizontal depression or by a lateral branch forking off the medial orbital sulcus (Fig. 9).
Functional correlations/applications
Functional neuroimaging studies have demonstrated neuronal activity in the orbitofrontal cortex in relation to sensory processes such as olfaction (Zatorre et al., 1992; Zald and Pardo, 1997; Dade et al., 1998) and gustation (Small et al., 1997a,Small et al., 1997b, 1999; Zald et al., 1998). In addition, activity changes in the orbitofrontal cortex have been associated with certain pathologic states such as obsessive compulsive disorder (OCD) (Baxter et al., 1987, Baxter et al., 1988; Nordahl et al., 1989; Benkelfat et al., 1990; Swedo et al., 1992). Although these studies have reported altered activity in the orbitofrontal cortex, it has been difficult to relate these changes to specific orbital subregions for lack of understanding the patterns of anatomic landmarks. For this reason, investigators have used general terminology (e.g., “orbital frontal activation”) to describe the location of their activity changes (Baxter et al., 1987, Baxter, et al., 1988; Nordahl et al., 1989; Benkelfat et al., 1990; Swedo et al., 1992; Zatorre et al., 1992; Small et al., 1997a,Small et al., 1997b, Small et al., 1999; Zald and Pardo, 1997; Dade et al., 1998; Zald et al., 1998).
Investigations with modern functional neuroimaging methods most often express their results in the Talairach coordinate system (Talairach and Tournoux, 1988). The utilization of this standardized proportional stereotaxic space enables accurate communication of research and clinical findings in a common quantitative framework, permitting the pooling and comparison of information across studies and the detection of new anatomical-functional correlations. Unfortunately, the Talairach atlas provides information in this standard space for only one hemisphere of a single brain (a 64-year-old female) that was variably sampled (with 2–6 mm spacing). The effective use of this stereotaxic space requires statistical statements of the variability in the location of different brain structures within it (i.e., probabilistic maps) that are necessary to account for individual differences in brain shape and size (Evans et al., 1994; Mazziotta et al., 1995). Probability maps have already been created for the cingulate and paracingulate sulci (Paus et al., 1996), the primary auditory cortex (Penhune et al., 1996), and the pars opercularis of the inferior frontal gyrus (Tomaiuolo et al., 1999).
The present study provides probabilistic maps that quantify the neuroanatomic variability of the human orbitofrontal sulci. The probability maps presented here are part of a large-scale, multicenter project (ICBM) currently under way to construct a 3-D digital atlas of the human brain (Mazziotta et al., 1995). The goal of this project is to quantify neuroanatomic variability in the form of stereotaxic maps, where each voxel expresses the likelihood of finding a particular structure at that location. By coregistering these orbitofrontal sulcal maps with any image of brain structure or function that has been similarly transformed into Talairach space, the relative location of a given lesion or activity change may now be statistically assessed. Furthermore, these maps provide a common reference for studies of the orbitofrontal cortex, allowing accurate exchange and facilitating comparison of data between studies.
Acknowledgements
The authors express their gratitude to Demetrios J. Sahlas, M.D., for his thorough and careful review of an early draft of the manuscript and to Georges LeGoualher, Ph.D., for his valuable assistance in generating the probability maps. The assistance of the staff members of the McConnell Brain Imaging Center, the Neurophotography Department, and the librarians of the Montreal Neurological Institute is gratefully acknowledged.




