Topographical organization of projections from the subiculum to the hypothalamus in the rat
Abstract
The projections from the subiculum to the hypothalamus were comprehensively examined in the rat by using the anterograde Phaseolus vulgaris leucoagglutinin (PHA-L) and retrograde cholera toxin B subunit (CTb) methods. Tracing of efferents with PHA-L indicated that the medial preoptic region received projection fibers from the temporal two-thirds of the subiculum, whereas the anterior, tuberal, and mammillary regions received those from the full longitudinal extent of the subiculum. The subicular projections to the anterior and tuberal hypothalamic regions were also found to be organized in a topographical manner such that the temporal-to-septal axis of origin in the subiculum determined a ventromedial-to-dorsolateral axis of termination in the medial zone of the hypothalamus: Massive labeled fibers from the temporalmost part of the subiculum terminated in the subparaventricular zone and its caudal continuum around the dorsal and medial aspects of the ventromedial nucleus, and those from progressively more septal parts terminated in progressively more dorsolateral parts of the medial zone. In addition, the temporal-to-septal axis of origin in the subiculum tended to determine a medial-to-lateral axis of termination in the preoptic region as well as a ventral-to-dorsal axis of termination in the mammillary region. Furthermore, the temporal-to-septal axis of origin in the septal two-thirds of the subiculum corresponded to a ventrolateral-to-dorsomedial axis of termination in the medial mammillary nucleus. The topographical projections from the subiculum to the medial zone of the hypothalamus were confirmed by CTb experiments, representatively in the subicular projections to the anterior hypothalamic region. These results suggest that different populations of neurons existing along the longitudinal axis of the subiculum may exert their influences on the execution of different hypothalamic functions. J. Comp. Neurol. 419:205–222, 2000. © 2000 Wiley-Liss, Inc.
The subiculum constitutes a major output structure of the hippocampal formation, which has been implicated in various behavioral and endocrine functions as well as in the process of learning and memory (for review see Swanson et al., 1987). On the other hand, the hypothalamus constitutes an anatomofunctional unit essential for integration of autonomic, endocrine, and behavioral responses, all of which are critical for homeostasis and reproduction (for review see Swanson, 1987). Therefore, it is of great interest to determine the organization of the subiculohypothalamic projections.
Autoradiographic studies have indicated that the prominent subiculohypothalamic projections are those to the mammillary complex and originate from the septal portion of the subiculum and that efferent fibers from the temporal portion are distributed in and around the ventromedial hypothalamic nucleus (Swanson and Cowan, 1975a, 1977; Meibach and Siegel, 1977; Krettek and Price, 1978). The subiculohypothalamic projections have been further analyzed by using an extremely sensitive anterograde tracer, Phaseolus vulgaris leucoagglutinin (PHA-L). Fibers from the septal portion of the subiculum are distributed densely in the mammillary nuclei and sparsely in the ventrolateral part of the hypothalamus (Witter et al., 1990), whereas the temporal portion of the subiculum sends projection fibers to the medial parts of the hypothalamus where they are more widely distributed than those previously reported (Canteras and Swanson, 1992; Cullinan et al., 1993).
However, the subiculohypothalamic fibers from the subicular region caudal to the caudal end of the Ammon's horn have never been examined by using PHA-L, and previous observations concerning their distribution in the hypothalamus are controversial. Meibach and Siegel (1977) injected 3H-leucine into this subicular region and observed the terminal field of labeled axons exclusively in the mammillary complex. However, several fluorescent retrograde studies showed that neurons sending their axons to the medial parts of the hypothalamus were situated in this region of the subiculum (Namura et al., 1994; Naber and Witter, 1998). Furthermore, previous reports suggest that the subiculohypothalamic projections are topographically organized, but there have been no comprehensive studies on it. In the present study, we therefore examined the projections from various parts of the subiculum to the hypothalamus, using the anterograde PHA-L and retrograde cholera toxin B subunit (CTb) methods.
Abbreviations
-
- ac
-
anterior commissure
-
- AH
-
Ammon's horn
-
- AHN
-
anterior hypothalamic nucleus
-
- Arc
-
arcuate hypothalamic nucleus
-
- BST
-
bed nucleus of the stria terminalis
-
- CA1
-
field CA1 of Ammon's horn
-
- CA2
-
field CA2 of Ammon's horn
-
- DA
-
dorsal hypothalamic area
-
- DG
-
dentate gyrus
-
- DMH
-
dorsomedial hypothalamic nucleus
-
- f
-
fornix
-
- fr
-
fasciculus retroflexus
-
- G
-
gelatinosus thalamic nucleus
-
- LH
-
lateral hypothalamic area
-
- LM
-
lateral mammillary nucleus
-
- LPA
-
lateral preoptic area
-
- mcht
-
medial corticohypothalamic tract
-
- MM
-
medial mammillary nucleus
-
- mp
-
mammillary peduncle
-
- MPA
-
medial preoptic area
-
- MPO
-
medial preoptic nucleus
-
- mt
-
mammillothalamic tract
-
- opt
-
optic tract
-
- PH
-
posterior hypothalamic area
-
- PT
-
paratenial thalamic nucleus
-
- PVH
-
paraventricular hypothalamic nucleus
-
- PVT
-
paraventricular thalamic nucleus
-
- Re
-
reuniens thalamic nucleus
-
- Rt
-
reticular thalamic nucleus
-
- Sd
-
dorsal subiculum
-
- Si
-
intermediate subiculum
-
- sm
-
stria medullaris
-
- st
-
stria terminalis
-
- StHy
-
striohypothalamic nucleus
-
- Sub
-
subiculum
-
- SubI
-
subincertal nucleus
-
- SuM
-
supramammillary nucleus
-
- Sv
-
ventral subiculum
-
- VMH
-
ventromedial hypothalamic nucleus
-
- ZI
-
zona incerta
MATERIALS AND METHODS
Experiments were carried out on male Wistar rats weighing 250–320 g. All surgical procedures were performed with general anesthesia via intraperitoneal injection of chloral hydrate (350 mg/kg). The Principles of laboratory animal care (NIH publication No. 86-23, revised 1985) were followed in all respects for the animals used in this study. The atlas of the rat brain by Paxinos and Watson (1997) was used to determine coordinates for stereotaxic injection of tracers.
Anterograde labeling experiments
PHA-L (Vector Laboratories, Burlingame, CA) was injected into the subiculum in 38 rats. In each rat, a single injection was made iontophoretically through a glass micropipette filled with a 2.5% solution of PHA-L dissolved in 0.01 M phosphate buffer (pH 7.3). The driving current (5–6 μA, 200 msec, 2 Hz) was delivered for a period of 30–40 minutes. After 9–12 days survival, the rats were deeply reanesthetized and perfused transcardially with 100 ml of saline, followed by 500 ml of a solution composed of 8% formalin and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3), and then with 200 ml of 10% sucrose in the same buffer. After perfusion, the brains were removed and placed in a cold solution of 20% sucrose in the same buffer. Subsequently, the brains were cut serially into frontal sections of 50 μm thickness on a freezing microtome. The sections were washed in phosphate-buffered saline (pH 7.3; PBS), incubated in PBS containing 0.2% Triton X-100 and 3% normal goat serum (PBS-goat) for 60 minutes, and incubated overnight in PBS-goat containing rabbit anti-PHA-L (E-Y Labs Inc., San Mateo, CA) diluted at 1:1,000. Subsequently, the sections were washed in PBS and incubated in PBS-goat containing biotinylated goat anti-rabbit IgG (Vector) diluted at 1:200 for 4 hours. After washing in PBS, the sections were incubated in PBS containing avidin-biotin-peroxidase (Vector) diluted at 1:100 for 1 hour. After washing in PBS, the sections were incubated in 25 ml of 0.1 M phosphate buffer (pH 7.3) containing 10 mg diaminobenzidine (DAB) and 10 μl of 30% H2O2.
Retrograde labeling experiments
CTb (List Biological Labs Inc., Campell, CA) was injected into the hypothalamus in 7 rats. In each rat, a single injection was made iontophoretically through a glass micropipette filled with a 0.5% solution of CTb dissolved in 0.05 M PBS. The driving current (5–6 μA, 200 msec, 2 Hz) was delivered for a period of 7–10 minutes. After 7–10 days of survival, transcardial perfusion of the rats under deep anesthesia and cutting of the brains were performed in accordance with the above-noted procedures. Brain sections were then washed in PBS, incubated in PBS containing 0.2% Triton X-100 and 3% normal rabbit serum (PBS-rabbit) for 60 minutes, and incubated overnight in PBS-rabbit containing goat anti-CTb (List Biological Labs Inc.) diluted at 1:10,000. After washing in PBS, the sections were incubated in PBS-rabbit containing biotinylated rabbit anti-goat IgG (Vector) diluted at 1:200 for 4 hours. After washing in PBS, the sections were incubated in PBS containing avidin-biotin-peroxidase diluted at 1:100 for 1 hour. After washing in PBS, the sections were incubated in 25 ml of 0.1 M phosphate buffer (pH 7.3) containing 10 mg DAB and 10 μl of 30% H2O2.
After immunohistochemical demonstration of PHA-L and CTb, the sections were mounted onto gelatinized slides, coverslipped with Canada balsam (Wako), and examined under the microscope (Nikon Optiphoto-2). PHA-L-labeled axons, CTb-labeled cells, and injection sites were plotted by using a camera lucida attached to the microscope. Coverslips were then removed, and the sections were counterstained with 1% cresyl violet for cytoarchitectural landmarks.
RESULTS
Terminology
The hippocampal formation is grossly an elongated structure having a long C-shaped axis reffered to as the septotemporal axis, and along the axis the subiculum wraps the Ammon's horn from behind. On the basis of this topology, the most septal part and the most temporal part of the subiculum have been referred to as the dorsal subiculum and the ventral subiculum, respectively (Swanson and Cowan, 1977). It is also likely that the dorsal subiculum and the ventral subiculum, respectively, correspond to the subicular parts dorsal and ventral to the field CA1 of Ammon's horn (Witter et al., 1990; Cullinan et al., 1993). Groenewegen et al. (1987) further divided the subiculum into the dorsal, intermediate, and ventral parts, although they did not mention the grounds for such distinctions. We interpret the intermediate part as a subicular region caudal to the caudal end of the field CA1. In the present study, the subiculum is therefore divided into three parts: ventral (Sv), intermediate (Si), and dorsal (Sd), as shown in Figure 1.
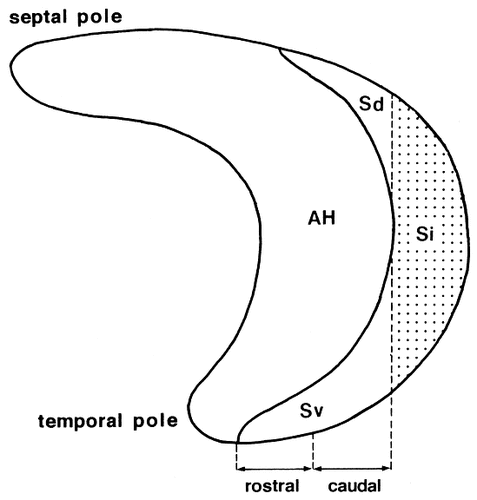
Lateral view showing the extent of the AH, Sd, Si (stippled area), and Sv.
Anterograde tracing with PHA-L
In 14 of 38 rats in which PHA-L injection was made into the subiculum, the injection site was centered on the subiculum, with little or no involvement of adjacent structures (Fig. 2). Therefore, the results obtained from these 14 rats were used to analyze the subiculohypothalamic projections. These projections were largely ipsilateral, with the exception of the projections to the mammillary region, which were bilateral.
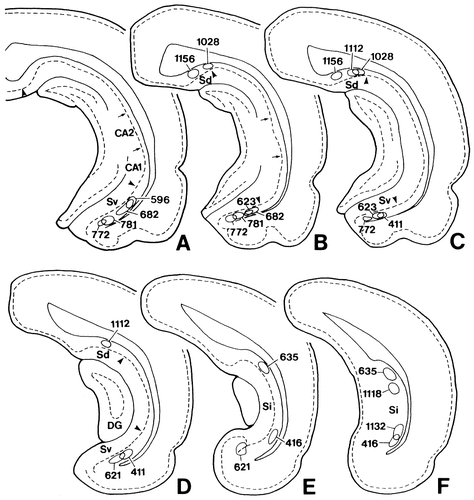
Line drawings of frontal sections arranged rostrocaudally (A–F) showing the sites of PHA-L injection into the subiculum. Each circle indicates the injection site. Each numeral indicates the case number. Arrows and arrowheads indicate the border between the CA1 and CA2 and the border between the CA1 and the subiculum, respectively.
Sv-hypothalamic projections.
In case 772 and case 781, PHA-L was injected into the medial part of the rostral Sv, although the deposit of PHA-L slightly intruded into the caudal Sv in case 772 (Fig. 2A–C). These two experiments showed substantially the same anterograde labeling in the hypothalamus, so the results of case 772 are described in detail below. Many labeled fibers diverging mainly from the medial corticohypothalamic tract (mcht) were distributed in the hypothalamic regions, although some labeled fibers from the precommissural fornix traveled ventrally to reach the medial preopotic area (MPA) at the level just rostral to the anterior commissure; only a few labeled fibers were detected in the postcommissural fornix. Labeled fibers leaving the mcht ran ventrally or ventrolaterally through the paraventricular hypothalamic nucleus (PVH), giving rise to a few terminal boutons within the PVH and periventricular nucleus, and were moderately distributed in the caudal part of the MPA including the medial preoptic nucleus (MPO) and the striohypothalamic nucleus (Figs. 3A, 4A). The suprachiasmatic nucleus contained only a few labeled axons. In the anterior hypothalamic region, a dense plexus of labeled fibers and terminals was seen in a vertical narrow area between the ventrolateral border of the PVH and the dorsomedial border of the anterior hypothalamic nucleus (AHN; Figs. 3B, 4B); this area corresponds to the subparaventricular zone (SPVZ) of Watts et al. (1987). Some labeled fibers terminated in the most dorsomedial part of the AHN, whereas few labeled fibers were found in the PVH. The plexus of labeled fibers and terminals in the SPVZ extended caudally into the tuberal region and surrounded the ventromedial hypothalamic nucleus (VMH); many labeled fibers and terminals were distributed around the medial and dorsal aspects of the VMH, and some labeled axons were seen around the lateral and ventral aspects of the nucleus (Figs. 3C, 4C). Although the labeling within the VMH itself was weak at this level, some labeled axons were distributed in the central and ventrolateral subdivisions of the nucleus at more caudal levels. Moderate numbers of labeled axons were distributed throughout the entire dorsomedial hypothalamic nucleus (DMH; Fig. 3C). A few labeled fibers were observed to pass through the rostrolateral part of the arcuate nucleus (Arc). In the more caudal sections, the posterior hypothalamic area contained a few labeled fibers. In the mammillary region, a small number of labeled fibers terminated in the ventral premammillary nucleus. Sparse labeling was present in the posterolateral part of the Arc, whereas its posteromedial part was free from labeling. The tuberomammillary nucleus contained only a few labeled fibers. More caudally, moderate numbers of labeled axons were seen in the medial part of the supramammillary nucleus (SuM) and in the cell-poor zone between the medial mammillary nucleus (MM) and the Arc (Fig. 3D). The ventral aspect of the caudal part of the MM was surrounded by only a few labeled fibers.

Projection drawings arranged rostrocaudally (A–D) showing the distribution of labeled fibers (fine lines) and terminals (fine dots) in the hypothalamus after PHA-L injection into the rostral part of the Sv (case 772; Fig. 2).
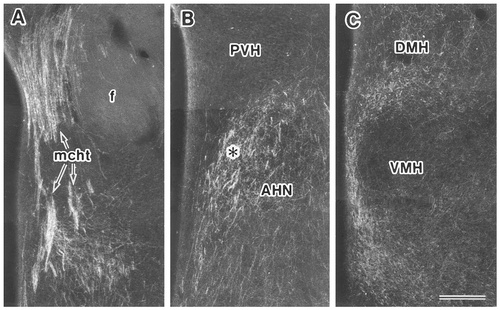
Photomicrographs showing the distribution of labeled fibers and terminals in the preoptic (A), anterior (B), and tuberal (C) hypothalamic regions after PHA-L injection into the rostral part of the Sv (case 772). Asterisk in B indicates the SPVZ (after Watts et al., 1987; Watts and Swanson, 1987). Scale bar = 200 μm.
In case 682, the injection site was placed in the lateral part of the rostral Sv (Fig. 2A,B). The routes taken by labeled fibers as well as their target areas in the medial hypothalamus were similar to those in case 772 and case 781. However, denser labeling was found in the region ventral to the SPVZ, and labeled axons surrounding the VMH were more abundant around the ventral aspect than around the dorsal and medial aspects. The same distribution pattern of labeled axons was also found in case 596 in which PHA-L was injected into the lateral portion of the rostralmost part of the Sv, although the labeling was less dense (Fig. 2A).
In case 411, the injection site was centered on the midmediolateral part of the caudal Sv (Fig. 2C,D), whereas, in case 621, it was placed in the medial part of the caudal Sv and involved part of the presubiculum as well (Fig. 2D,E). However, because the distribution patterns of labeled axons were very similar in these cases, the results of case 411 are representatively described below. Labeled fibers descending through the septal region from the precommissural fornix were distributed in the rostral part of the MPA, whereas labeled fibers coursing mainly through the mcht and additionally through the postcommissural fornix were distributed in the caudal part of the MPA including the MPO (Fig. 5A). Few labeled fibers were present in the suprachiasmatic nucleus. In the anterior hypothalamic region, a dense plexus of labeled fibers, most of which diverged from the fornix, was found in an area involving the dorsal half of the SPVZ and the dorsomedial quadrant of the AHN (Figs. 5B, 6A). Some of these fibers coursed dorsally and terminated in the surrounding structures of the PVH, such as the area just medial to the fornix, the zona incerta, and the ventral part of the reuniens thalamic nucleus. Moderate numbers of labeled axons emerging from the dense plexus or from the fornix also spread ventrally or ventrolaterally through the AHN and its vicinities. At the tuberal level, labeled fibers and terminals formed a dense plexus around the dorsal aspect of the VMH and extended into the rostral part of the DMH (Figs. 5C, 6B); when followed caudally, the plexus around the dorsal aspect of the VMH was still found, although the caudal part of the DMH was almost free from labeling. The medial aspect of the VMH was surrounded by a small number of labeled fibers, whereas few labeled fibers existed around the ventral and lateral aspects of the VMH. Only a few labeled fibers were detected in the VMH itself. Some labeled fibers ran for some distance dorsally through the DMH and reached the dorsal hypothalamic area. The zona incerta, the subincertal nucleus and the ventral part of the reuniens thalamic nucleus contained a few labeled fibers. In the more caudal sections, small numbers of labeled axons were seen in the posterior hypothalamic area. In the mammillary region, a few labeled fibers were observed in the ventral premammillary nucleus and the posterolateral part of the Arc. More caudally, sparse labeling was detected in the medial part of the SuM. Labeled fibers that appeared to leave the postcomissural fornix were distributed in the ventrolateral portion of the rostral part of the MM as well as in the cell-poor zone between the MM and the Arc (Fig. 5D). The ventral surface of the caudal part of the MM was surrounded by relatively few labeled fibers. In case 623, in which the center of the injection site was situated more rostrally than that of case 411, PHA-L deposit intruded considerably into the rostral Sv (Fig. 2B,C); the resultant distribution of labeled axons was similar to that in case 772 and case 781.
Projection drawings arranged rostrocaudally (A–D) showing the distribution of labeled fibers (fine lines) and terminals (fine dots) in the hypothalamus after PHA-L injection into the caudal part of the Sv (case 411; Fig. 2).
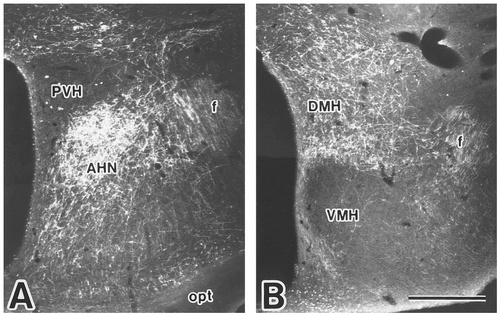
Photomicrographs showing the distribution of labeled fibers and terminals in the anterior (A) and tuberal (B) hypothalamic regions after PHA-L injection into the caudal part of the Sv (case 411). Scale bar = 500 μm.
Si-hypothalamic projections.
In case 416 and case 1132, PHA-L was injected into the ventral part of the Si (Figs. 2E,F, 7). The results obtained in case 416 are representatively described in detail; the resulting distribution of labeled axons was very similar in these two cases. Many labeled fibers coursed through the precommissural and postcommissural portions of the fornix, whereas only a few labeled fibers appeared in the mcht. Labeled fibers diverging from the precommissural fornix were distributed in the rostral part of the MPA, whereas those from the postcommissural fornix were distributed in the caudal part of the MPA, particularly in its lateral half (Fig. 8A). In the anterior hypothalamic region, a dense plexus of labeled axons diverging from the postcommissural fornix was observed in an area between the PVH and the fornix; the plexus involved the dorsolateral part of the AHN (Figs. 8B, 9A). A moderate number of labeled fibers from the fornix coursed dorsally or dorsomedially to innervate thalamic nuclei, such as the medial part of the reticular nucleus, the zona incerta, and the nucleus reuniens. On the other hand, a moderate number of fibers traveled ventromedially or ventrolaterally and were widely distributed in the ventral regions of the hypothalamus. In the tuberal region, labeled axons diverging from the fornix formed a dense terminal field just medial to the fornix, which extended medially to innervate the lateral part of the DMH (Figs. 8C, 9B). Some of these fibers took a dorsomedial course and reached the dorsal hypothalamic area and the reuniens thalamic nucleus. The VMH was surrounded by a few labeled fibers. The posterior hypothalamic area contained a moderate number of labeled axons. In the mammillary region, the dorsal and ventral premammillary nuclei, the tuberomammillary nucleus, and the Arc contained few labeled fibers. More caudally, a few labeled fibers with terminal boutons were observed in the medial part of the SuM and the cell-poor zone between the MM and the Arc (Fig. 8D). Moderate terminal labeling was present bilaterally in the rostroventral part of the MM. Very sparse labeling was found in and around the caudal part of the MM.
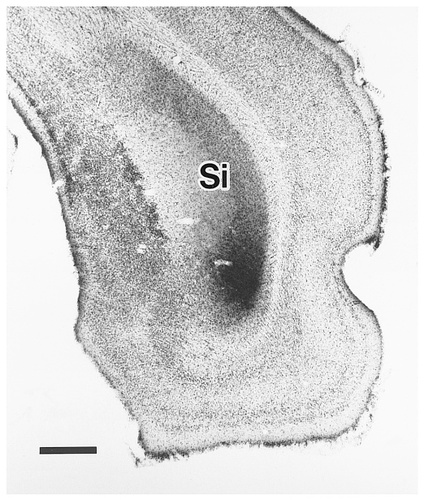
Photomicrograph showing a site of PHA-L injection into the ventral part of the Si (case 416). Scale bar = 500 μm.

Projection drawings arranged rostrocaudally (A–D) showing the distribution of labeled fibers (fine lines) and terminals (fine dots) in the hypothalamus after PHA-L injection into the ventral part of the Si (case 416; Fig. 2).
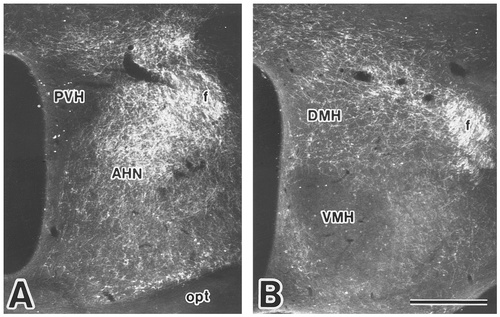
Photomicrographs showing the distribution of labeled fibers and terminals in the anterior (A) and tuberal (B) hypothalamic regions after PHA-L injection into the ventral part of the Si (case 416). Scale bar = 500 μm.
In case 635, PHA-L was injected into the dorsal part of the Si (Fig. 2E,F). Labeled fibers running through the precommissural and postcommissural portions of the fornix but not through the mcht were distributed in the hypothalamic regions. At the rostral level of the preoptic region, only a few labeled fibers diverging from the precommissural fornix were detected in the MPA; at the caudal level, some labeled fibers were observed to diverge from the postcommissural fornix and to run ventrolaterally into the lateral preoptic area through the bed nucleus of the stria terminalis (Fig. 10A). In the anterior hypothalamic region, labeled axons leaving the fornix formed a dense terminal field between the fornix and the AHN (Figs. 10B, 11A). Some labeled fibers from the fornix were directed laterally into the lateral hypothalamic area or ventrally into the ventral hypothalamic region. The zona incerta contained some labeled fibers. In the tuberal region, a less dense plexus of labeled axons was confined to an area between the subincertal nucleus and the DMH (Figs. 10C, 11B). The posterior hypothalamic area contained only a few labeled fibers. In the mammillary region, a small number of labeled fibers which diverged from the fornix ran in a dorsomedial direction and gave rise to a few terminal boutons in the SuM (Fig. 10D). In the rostral part of the MM, moderate numbers of labeled axons were distributed bilaterally in its ventral half, with an ipsilateral predominance. A few labeled fibers were also detected in the caudoventral part of the MM. In case 1118, in which the injection site was placed more ventrally than in case 635, more labeled fibers were distributed in the MM, particularly in its caudal part, than in case 635.
Projection drawings arranged rostrocaudally (A–D) showing the distribution of labeled fibers (fine lines) and terminals (fine dots) in the hypothalamus after PHA-L injections into the dorsal part of the Si (case 635; Fig. 2).
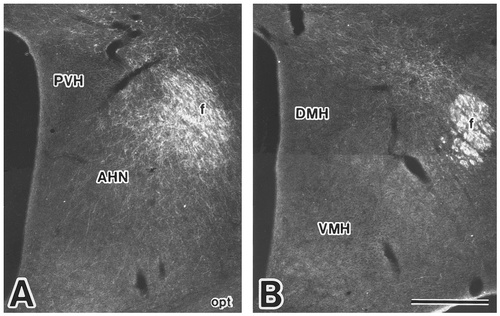
Photomicrographs showing the distribution of labeled fibers and terminals in the anterior (A) and tuberal (B) hypothalamic regions after PHA-L injection into the dorsal part of the Si (case 635). Scale bar = 500 μm.
Sd-hypothalamic projections.
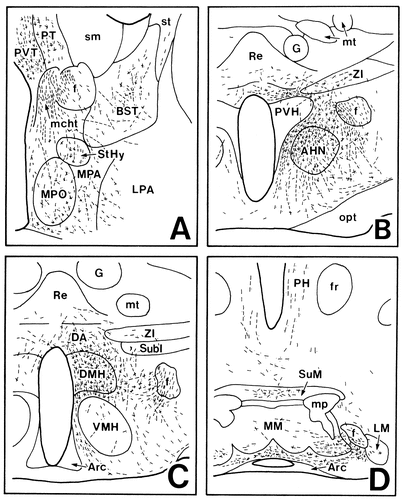
In case 1028 and case 1112, PHA-L was injected into the lateral part of the Sd, although the injection site in case 1028 involved the dorsal end of the field CA1 of Ammon's horn as well (Fig. 2B–D). These two experiments showed very similar anterograde labeling in the hypothalamus, so the results of case 1028 are discussed. Many labeled fibers descended through the precommissural and postcommissural portions of the fornix but not through the mcht. Among these fibers, only those through the postcommissural fornix were distributed in the hypothalamus. The preoptic region contained few labeled fibers (Fig. 12A). In the anterior hypothalamic region, many labeled fibers with terminal boutons were seen to form a plexus surrounding the fornix, some of which ran ventrolaterally to innervate the lateral hypothalamic area (Fig. 12B). In the tuberal region, a less dense terminal field of labeled axons was situated in an area just below the subincertal nucleus (Fig. 12C). Some labeled fibers emerging from this terminal field ran dorsally or dorsomedially to innervate the subincertal nucleus and the reuniens thalamic nucleus. In the mammillary region, dense terminal labeling was detected bilaterally in the dorsal part of the MM throughout its rostrocaudal extent (Fig. 12D).
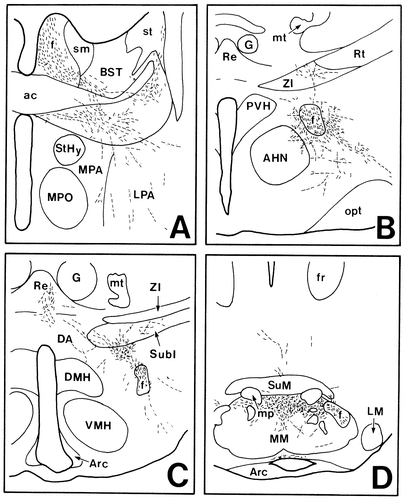
Projection drawings arranged rostrocaudally (A–D) showing the distribution of labeled fibers (fine lines) and terminals (fine dots) in the hypothalamus after PHA-L injection into the Sd (case 1028; Fig. 2).
In case 1156, the injection site involved the medial part of the Sd (Fig. 2B,C). Labeled fibers coursed exclusively through the postcommissural fornix. Few labeled axons were detected in the preoptic, anterior, and tuberal hypothalamic regions. In the mammillary region, moderate terminal labeling was found in the MM: Labeled fibers were distributed ipsilaterally in the dorsolateral part of the rostral MM, whereas they were distributed bilaterally with an ipsilateral predominance in the dorsal part of the caudal MM.
Retrograde tracing with CTb
The most prominent finding of the anterograde experiments was that the temporal-to-septal axis of origin in the subiculum determined a ventromedial-to-dorsolateral axis of termination in the anterior and tuberal regions of the medial hypothalamus. This topography was further confirmed by the retrograde labeling experiments, in which CTb injections were made representatively into the anterior hypothalamic region. In 4 of 7 operated rats, the injection site was successfully placed within the areas where the dense terminal field of labeled axons had been found after PHA-L injection into the subiculum (Figs. 13, 14). The results obtained in these 4 rats are representatively described below.
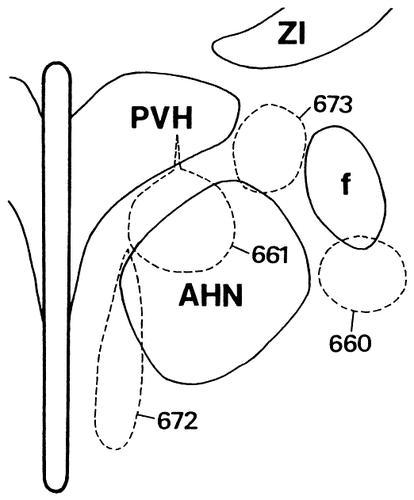
Line drawing illustrating CTb injection sites of the four representative cases in the anterior hypothalamic region. Each area encircled by broken lines indicates the injection site. Each numeral indicates the case number.
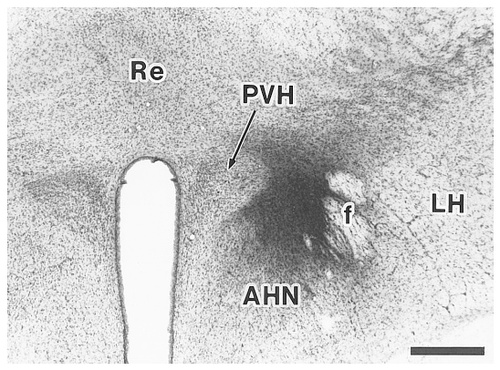
A site of CTb injection into an area between the paraventricular hypothalamic nucleus and the fornix (case 673). Scale bar = 500 μm.
In case 672, CTb was injected into an area involving the ventral half of the SPVZ and the medial tip of the AHN (Fig. 13). In the rostral part of the Sv, many labeled neurons were distributed throughout the entire superficial-deep and mediolateral extent of the pyramidal cell layer (Fig. 15A). These neurons were more abundant in the medial half than in the lateral half. Some labeled neurons were also present in the amygdalohippocampal transition area. When followed caudally, labeled neurons in the Sv significantly decreased in number (Fig. 15B,C). In the Si, some labeled neurons were scattered throughout its entire dorsomedial extent (Fig. 15D,E). The Sd contained no labeled neurons (Fig. 15A–C).
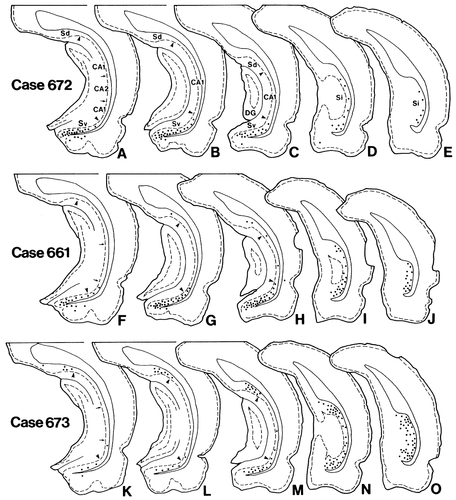
Line drawings showing the distribution of retrogradely labeled neurons in the hippocampal formation after CTb injections into the anterior hypothalamic region (Fig. 13). In each case, the drawings are arranged rostrocaudally. Each dot indicates about 10 labeled neurons. Arrows and arrowheads indicate the border between the CA1 and the CA2 and the border between the CA1 and the subiculum, respectively.
In case 661, the injection site was centered in an area involving the dorsal one-third of the SPVZ and the dorsomedial part of the AHN (Fig. 13). Numerous labeled neurons were distributed throughout the rostrocaudal, mediolateral, and superficial-deep portions of the pyramidal cell layer in the Sv and were concentrated in the more caudomedial parts of the Sv (Fig. 15F–H). The entire area of the Si also contained many labeled neurons, with a ventral predominance (Fig. 15I,J). Some labeled neurons were also detected in the amygdalohippocampal transition area and a few in the Sd and the field CA1 of Ammon's horn.
In case 673, the injection site was centered in an area between the PVH and the fornix (Figs. 13, 14). In case 660, the injection site was placed just beneath the fornix. The results obtained in case 673 are representatively described, because these two experiments showed very similar retrograde labeling in the subiculum. The rostral part of the Sd as well as that of the Sv contained a small number of labeled neurons (Fig. 15K). Proceeding caudally, labeled neurons in the Sv and those in the Sd gradually increased in number, with a concentration mainly in the lateral two-thirds of the subiculum (Fig. 15L,M). In the Si, many labeled neurons were seen throughout its entire extent, although the distribution was relatively less dense in the middorsoventral part (Fig. 15N,O). In addition, only a few labeled neurons were detected in the field CA1 of Ammon's horn. In all cases, the presubiculum and parasubiculum contained no labeled neurons.
DISCUSSION
In the present study, we reexamined the projections from the subiculum to the hypothalamus using the anterograde PHA-L and retrograde CTb methods in the rat and have extended considerably the understanding of the organization of this pathway.
Subicular projections to the hypothalamus
The efferent fibers of the subiculum passing through the fimbria–fornix system separate into three components at the caudal end of the septal region: the precommissural fornix, the mcht, and the postcommissural fornix. As was shown in the present and previous studies (Canteras and Swanson, 1992), the preoptic area receives subicular fibers that run through the mcht or through the precommissural fornix. Our results are also nearly identical to those of previous studies (Swanson and Cowan, 1975a, 1977; Meibach and Siegel, 1977; Canteras and Swanson, 1992), in that the rostral part of the Sv sends almost all of the efferent fibers to the medial hypothalamus through the mcht and only a few through the postcommissural fornix. We further observed that the progressively more caudal parts of the Sv send more fibers into the postcommissural fornix than into the mcht and that the Si sends projection fibers predominantly into the postcommissural fornix. It is clear that the efferent fibers of the Sd to the hypothalamus run exclusively through the postcommissural fornix (Swanson and Cowan, 1975a, 1977; Witter et al., 1990; present study). In addition to the projections through the fimbria–fornix system, a ventral pathway to the hypothalamus via the amygdala has been suggested by Köhler (1990). The present and previous PHA-L studies (Canteras and Swanson, 1992) showed very few fibers in this pathway.
The present findings indicate that the Sv sends projection fibers to the medial preoptic area, as did the findings of previous PHA-L studies (Canteras and Swanson, 1992; Cullinan et al., 1993). Moreover, we demonstrated that the Sv projections to the medial preoptic area are, to some extent, topographically organized: Fibers from the rostral part of the Sv are distributed in the medial half rather than the lateral half, whereas those from the caudal part of the Sv tend to be distributed in the lateral half. The fibers from the ventral part of the Si are also present in the lateral half, although those from the dorsal part of the Si as well as from the Sd are a few in number in the medial preoptic area. Groenewegen et al. (1987) noted that most of the PHA-L-labeled fibers following Sv injections were found in the lateral preoptic area, although in their figure they indicated more prominent labeling in the medial preoptic area than in the lateral preoptic area. In the present study, we found some labeled fibers, most of which probably represent passing fibers, in the lateral preoptic area after every PHA-L injection into the subiculum.
In agreement with findings of previous PHA-L studies (Köhler, 1990; Witter and Groenewegen, 1990; Canteras and Swanson, 1992; Cullinan et al., 1993), we observed that the Sv fibers give rise to a dense plexus of labeled fibers and terminals in the SPVZ and its caudal continuum around the VMH. Furthermore, the present study indicates for the first time that the Si sends massive projection fibers to the region just medial to the fornix at the anterior and tuberal levels of the hypothalamus. In an earlier autoradiographic study of the subicular efferents, Meibach and Siegel (1977) failed to detect labeled Si fibers in these perifornical regions; this may be due to the relatively less sensitive autoradiographic techniques used in those early experiments. The present findings also indicate that the Sd sends projection fibers to the perifornical region and that they originate from the proximal part but not from the distal part. Witter et al. (1990) also demonstrated an accumulation of labeled fibers in the perifornical region after PHA-L injection into the proximal part of the Sd.
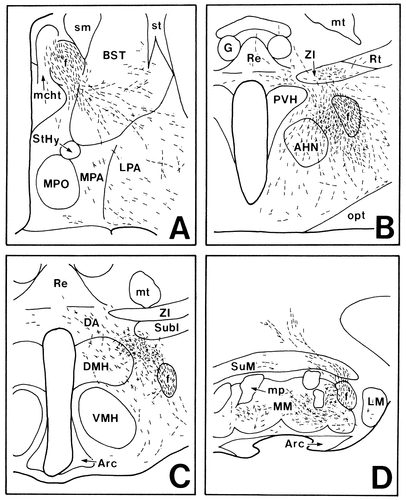
Here we clearly demonstrate by use of PHA-L that the temporal-to-septal axis of origin in the subiculum determines a ventromedial-to-dorsolateral axis of termination in the medial hypothalamus: Fibers from the temporal part of the subiculum give rise to dense plexuses of terminals in the SPVZ and in the medial and dorsal part of the “shell” of the VMH, and those from progressively more septal parts of the subiculum provide dense terminal fields in progressively more dorsolateral parts of the medial hypothalamus (as diagrammed in Fig. 16). This topographical organization of the projections is further confirmed by the present CTb experiments: following a shift of the CTb injection site from the ventromedial part to the dorsolateral part of the anterior hypothalamic region, the distribution area of labeled neurons shifted from the temporal part to the septal part of the subiculum. It has been suggested that the transverse organization of subicular efferents exists. According to Witter and Groenewegen (1990), this organization is target-specific, and the ventromedial hypothalamic area receives projection fibers predominantly from the distal (medial) part but not from the proximal (lateral) part of the Sv. However, Namura et al. (1994) observed that neurons labeled with a fluorescent dye (diamidino yellow) injected into the ventromedial hypothalamic area were distributed throughout the proximal-distal extent of the Sv. Our data also show that not only the distal part but also the proximal part of the Sv sends projection fibers to the ventromedial hypothalamic area, although the more distal parts generate more projection fibers and that there are no significant differences between the distribution patterns of projection fibers from these two parts. The results of our retrograde experiments further indicate that the perifornical region receives projection fibers from the proximal part rather than the distal part of the Sv.
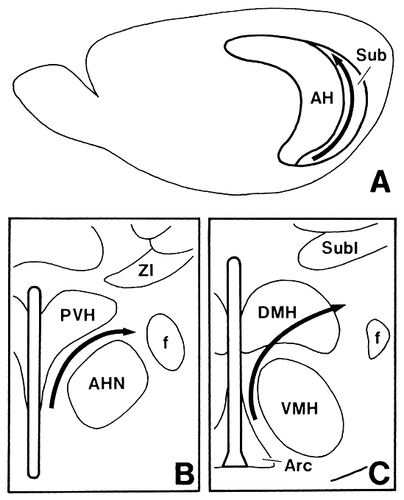
Schematic drawings showing that the rostrocaudal axis of origin in the subiculum (A) determines a ventromedial-to-dorsolateral axis of termination in the anterior (B) and tuberal (C) hypothalamic regions.
In the present study, we did not detect heavy anterograde labeling in the lateral hypothalamus after any of the PHA-L injections into the Sv, which is in accordance with the results of other PHA-L studies (Canteras and Swanson, 1992; Cullinan et al., 1993). On the other hand, Köhler (1990) stated that the innervation of the lateral hypothalamic area was quite prominent after deep ventral PHA-L injections into the Sv. The reason why the findings of Köhler are discrepant from ours is not clear, because he did not indicate the injection site either by photomicrographs or by illustrations.
The results of the present study confirm previously published observations that the subiculomammillary projections arise not only from the Sd (Swanson and Cowan, 1975a, 1977; Meibach and Siegel, 1977; Donovan and Wyss, 1983; Shibata, 1989; Allen and Hopkins, 1989; Köhler, 1990; Witter and Groenewegen, 1990; Witter et al., 1990) but also from the Si and Sv (Meibach and Siegel, 1977; Shibata, 1989; Allen and Hopkins, 1989). Furthermore, the present finding that the septal part of the subiculum projects to the dorsal part of the MM and progressively more temporal parts of the subiculum project to more ventral parts of the MM is consistent with that in the previous reports (Meibach and Siegel, 1977; Allen and Hopkins, 1989; Shibata, 1989). Additionally, we present another topography in which the temporal-to-septal axis of origin in the septal two-thirds of the subiculum corresponds to a ventrolateral-to-dorsomedial axis of termination in the rostral part of the MM.
Functional considerations
As was mentioned above, the full rostrocaudal extent of the Sv sends projection fibers to the SPVZ. This zone also receives dense inputs from the suprachiasmatic nucleus (Swanson and Cowan, 1975b; Berk and Finkelstein, 1981; Watts and Swanson, 1987; Watts et al., 1987; Watts, 1991; Vrang et al., 1995), where a circadian rhythm generator exists (Moore, 1983; Card and Moore, 1991; Reuss, 1996), and has been considered to integrate circadian and metabolic information for regulating the hypothalamopituitary axis (Elmquist et al., 1998) and to regulate behavioral rhythms (Watts, 1991). Based on these data, we raise the possibility that the Sv may participate in the control of behavioral rhythms and associated endocrine responses. The present study further indicates that the caudal part of the Sv projects to the AHN. According to Fuchs et al. (1985a,b) and Siegel and Pott (1988), the anteromedial hypothalamus including the AHN may play a central role in the expression of aggressive–defensive behavior by relaying information in part from the AHN to the central gray. Therefore, the caudal part of the Sv may also be involved in such expression.
The present study indicates that the Si projects heavily to the region just medial to the fornix at the anterior and tuberal levels of the hypothalamus. The tuberal component of this region is a part of the so-called perifornical area (PFA), which has been considered to have effects on cardiovascular responses (Gelsema et al., 1989; Smith et al., 1990) as well as on those associated with behavioral responses (LeDoux et al., 1988; Diamant et al., 1992). The PFA may influence cardiovascular activity through its projections to autonomic centers in the brainstem (Allen and Cechetto, 1992) and/or in the spinal cord (Saper et al., 1976; Cechetto and Saper, 1988; Allen and Cechetto, 1992). It therefore appears likely that information flowing from the Si to the PFA might regulate these cardiovascular responses.
The hippocampal formation has been known to exert inhibitory influences on the hypothalamic–pituitary–adrenocortical (HPA) axis (Saphier and Feldman, 1987; Herman et al., 1992). However, hippocampal outputs are generally considered to be excitatory (Walaas and Fonnum, 1980; Finch et al., 1986). Previous PHA-L studies reported that the PVH rarely received direct inputs from the Sv (Groenewegen et al., 1987; Köhler, 1990; Canteras and Swanson, 1992; Cullinan et al., 1993). Furthermore, the present study clarifies that neither the Sv nor the Si and Sd send projection fibers to the PVH. Accordingly, there may be inhibitory relay neurons that transmit subicular outputs to the PVH, such as GABAergic neurons in the bed nucleus of the stria terminalis, which receives Sv efferents (Cullinan et al., 1993; Herman et al., 1997). In addition, PVH-projecting GABAergic neurons exist in the perifornical region at the anterior and tuberal levels of the hypothalamus (Roland and Sawchenko, 1993; Boudaba et al., 1996), where the terminal fields of labeled axons were found after PHA-L injections into the Si and Sd in the present study. The Si– and Sd–PVH projections may be relayed by GABAergic perifornical neurons.
As was mentioned above, the medial zone of the hypothalamus is divided into functionally distinct regions, which receive projection fibers from distinct parts of the entire longitudinal extent of the subiculum. Therefore, it is likely that different populations of neurons existing along the longitudinal axis of the subiculum exert their influences on the execution of different hypothalamic functions. This view has also been suggested by Risold and Swanson (1996, 1997), on the basis of their finding that the indirect hippocampohypothalamic pathway via the lateral septal nucleus is topographically organized along the longitudinal axis of the hippocampal formation.
Acknowledgements
The authors are grateful for photographic help of Mr. Makoto Oshita and Mr. Tsunao Yoneyama.




