Nonsyndromic congenital retinal nonattachment gene maps to human chromosome band 10q21
Abstract
Nonsyndromic congenital retinal nonattachment (NCRNA) comprises congenital insensitivity to light, massive retrolental mass, shallow anterior chamber, microphthalmia, and nystagmus. We searched for the location of the gene responsible for an autosomal recessive form of NCRNA by using DNA samples from 36 individuals from a founding population. To this end we applied homozygosity mapping and a DNA pooling strategy using genomewide screen polymorphic microsatellite markers. We used two DNA pools, one pool contained DNA from 16 individuals affected with NCRNA. The second pool contained DNA from 20 normal carrier individuals, the parents of the patients. The polymorphic microsatellite markers were polymerase chain reaction (PCR)-amplified in each DNA pool; the PCR products were electrophoresed on polyacrylamide gels and visualized by silver staining. The banding patterns from DNA pools of affected and unaffected persons were compared, and linkage was detected between the NCRNA and D10S1225, a marker in 10q21. To confirm linkage of NCRNA to chromosome 10q21 by homozygosity mapping, the patients and their carrier parents were genotyped for a number of other microsatellite polymorphic markers in the 10q21region. Statistically significant linkage was observed with multiple polymorphic markers in the 10q21 region. At θ = 0 with markers D10S1225, D10S1428, D10S1422, and D10S1418 maximum LOD scores of 3.74, 3.58, 3.79, and 3.48 were generated, respectively. TDT P values for markers D10S1225 and D10S1418 were 0.0000021 and 0.000021, respectively. Am. J. Med. Genet. 90:165–168, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Nonsyndromic congenital retinal nonattachment (NCRNA; MIM 221900) comprises congenital insensitivity to light, massive retrolental mass, shallow anterior chamber, microphthalmia, and nystagmus in otherwise normal individuals [Warburg, 1979]. NCRNA is heterogeneous; autosomal dominant [Weve, 1938] and autosomal recessive [Ghiasvand et al., 1998] modes of inheritance are known. However, no gene for this phenotype had been identified or localized.
Identification of the gene responsible for NCRNA was deemed important for understanding the pathophysiology of retinal nonattachment and also for the development of DNA-based diagnostic tests for this disease. To achieve these, the determination of the location of NCRNA gene was essential. Because there was no candidate region for this disorder, a genomewide screen for finding the location of this gene was performed.
Previous success of homozygosity mapping in localizing disease genes in consanguineous populations [Lander and Botstein, 1987] and also the efficiency of DNA pooling strategy in genomewide screen in such populations [Sheffield et al., 1994, 1995a; Nystuen et al., 1996] have been reported before. In this study we used the human subjects from an earlier described founding population segregating NCRNA [Ghiasvand et al., 1998] and applied a DNA pooling strategy and homozygosity mapping. This article reports the mapping of NCRNA gene to chromosome area 10q21.
MATERIALS AND METHODS
Patient Population
The pedigree showing the participants is shown in Figure 1. Informed consent for this study was obtained from all the participating members or their guardians. Blood samples from 36 individuals were collected, comprising 16 affected and 20 normal but carrier individuals. All of these 36 individuals had undergone ophthalmological and general health examinations before linkage studies, and the only abnormal findings were the eye defects in the patients [Ghiasvand et al., 1998]. Research was conducted under the Institutional Review Board approved guidelines.
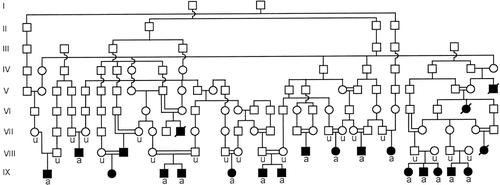
Pedigree of families with autosomal recessive nonsyndromic congenital retinal nonattachment. Squares and circles represent males and females, respectively. Filled symbols represent affected individuals, and double-line marriages indicate consanguineous unions. A line over the symbols (only filled symbols) represent deceased individuals. Individuals marked “u” and “a” are the unaffected and affected individuals whose DNA samples were used to make the “unaffected” and “affected” DNA pools, respectively.
DNA Pools
DNA was prepared from blood samples by using a standard “salting out” protocol [Miller, 1988] and quantitated spectrophotometrically. To ensure equal amplification between samples prepared from different individuals, ∼6 ng of DNA from each individual DNA sample was PCR amplified. Two separate pools of genomic DNA were prepared. One pool, “affected pool,” contained equal amounts of DNA from each of the 16 affected individuals, and the second DNA pool, the “unaffected pool,” was prepared from equal amounts of DNA from 20 obligate carriers (the parents of the patients).
On the basis of homozygosity mapping, in the genome screen, the relative intensity of each allelic band on the polyacrylamide gel approximates the frequency of that allele in the DNA samples included in the pool. Therefore, a short tandem repeat polymorphic (STRP) marker that is linked with the disease phenotype will show a shift in intensity toward a single allele or a reduction in the number of alleles in the affected DNA pool compared with the unaffected DNA pool.
Genome Screening and Genotyping
The DNA pools were used as templates for polymerase chain reaction (PCR) amplification with microsatellite polymorphic markers developed by the Cooperative Human Linkage Center [Sheffield et al., 1995b]. Amplification took place with the following conditions: ∼6 ng pooled DNA, 1.25 μL PCR buffer (100 mM Tris-HCl, 15 mM MgCl2, 500 mM KCl, pH 8.3), 300 μM each of dCTP, dGTP, dTTP, and dATP, ∼2.5 pmol each primer, 0.25 U Taq polymerase (Roche Molecular Biochemicals, Indianapolis, IN) in a total volume of 8.4 μL. Reactions with 1–3 primer pairs were amplified for 35 cycles of 94° for 30 sec, 55° for 30 sec, and 72° for 30 sec after an initial 3 min at 94°. Products of amplification were analyzed by the silver staining method of Bassam et al. [1991] after gel electrophoresis on 6% polyacrylamide containing 7.7 M urea.
After the initial linkage was found, additional microsatellite markers in the region of interest were identified from Marshfield Medical Research Foundation. Genotyping of individuals for these markers was performed in a manner similar to the pooling strategy except ∼6 ng of DNA from each of the 36 individuals was used as the PCR template in each reaction.
Statistical Analysis
The complexity of the known familial relationships made it impossible to perform formal linkage analysis using all of the pedigree information. Four subgroups of more closely related individuals were used for linkage analysis. Analysis was performed by using the LINKAGE and FASTLINK software suites [Lathrop and Lalouel, 1984; Cottingham et al., 1993]. A recessive disease model with 100% penetrance and a disease allele frequency of 10% was used. The subgrouping would be expected to result in conservative estimates of LOD because not all pedigree information is retained.
RESULTS
Linkage Results of Genome Screening and Individual Genotyping
More than 160 STRP markers used in the initial genome screen showed an identical or nearly identical allelic pattern in the affected and the unaffected DNA pools. However, for five markers, which were known to be unlinked to each other, the allelic pattern in the affected DNA pool was less complex compared with that in the unaffected DNA pool. Therefore, we suspected that the disease locus might be linked to one of these markers.
All affected and unaffected individuals were genotyped by using each of the five markers suspected to be linked to the disease. Among these markers only a 10q21 marker, D10S1225, showed a clear association with the disease. To confirm linkage of the NCRNA gene to area 10q21 region, each of the 16 affected individuals and the 20 obligate carriers was also genotyped with additional microsatellite markers located in 10q21. Genotyping data of the 36 individuals showed linkage of the disease locus to markers D10S1428, D10S1422, and D10S1418, three other markers in the 10q21 region.
Statistical Analysis
The complexity of the relationships between the affected individuals in the nine-generation pedigree did not allow using all of the consanguineous loops in the pedigree. However, linkage analysis on four subgroups of the pedigree showed linkage to microsatellite markers on chromosome region 10q21. At θ = 0 with markers D10S1225, D10S1428, D10S1422, and D10S1418 maximum LOD scores of 3.74, 3.58, 3.79, and 3.48 were generated, respectively (Table I). TDT P values for markers D10S1225 and D10S1418 were 0.0000021 and 0.000021, respectively (Table I).
| Marker | Recombination fraction (θ) | θ at Zmax | Zmax | TDT P value | ||||
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.05 | 0.10 | 0.20 | ||||
| D10S1232 | 2.20 | 2.16 | 1.96 | 1.66 | 1.05 | 0.00 | 2.20 | 0.02 |
| D10S1428 | 3.58 | 3.46 | 2.98 | 2.41 | 1.40 | 0.00 | 3.58 | 0.002 |
| D10S1225 | 3.74 | 3.67 | 3.35 | 2.86 | 1.83 | 0.00 | 3.74 | 0.0000021 |
| D10S1418 | 3.48 | 3.38 | 2.97 | 2.47 | 1.54 | 0.00 | 3.48 | 0.0000210 |
| D10S1422 | 3.79 | 3.73 | 3.42 | 2.93 | 1.89 | 0.00 | 3.79 | 0.002 |
| D10S0676 | 1.80 | 1.99 | 2.06 | 1.84 | 1.22 | 0.05 | 2.09 | 0.043 |
DISCUSSION
NCRNA includes a heterogeneous group of severe retinal abnormalities causing blindness. Few reports on the mode of inheritance are published, but there is no information on mapping of a NCRNA locus. To begin a search for understanding the pathophysiology of this severe retinal disorder, we have set up a series of studies to find and study the gene responsible for this disease.
In the absence of a candidate region for NCRNA, the only way to search for the location of the gene of this Mendelian disorder was to make a genomewide screen using STRP markers. To choose an efficient strategy for mapping the disease gene, we used the known biological characteristics of the study population. Because of the genetic isolation of this founding population and also the uniformity of the disease phenotype in affected individuals, for the purpose of this study, we assumed that all the affected individuals have descended from a common ancestor. Hence, all the copies of the NCRNA allele in this population were assumed to be identical by descent. Therefore, homozygosity and linkage-disequilibrium mapping and also pooling strategy were chosen to be used for mapping the NCRNA gene in the study population.
In the genome screen using pooling strategy we came across five unlinked markers, which when compared for the banding patterns for the affected and the unaffected DNA pools, showed a less complex allelic pattern for the affected DNA pool. The simpler allelic pattern for these unlinked markers led us to consider them as candidate markers linked to the disease locus. The simpler allelic pattern for these unlinked markers indicates high homozygosity of the patients for these markers. For one of these markers this could be explained by linkage of the marker to the disease gene. For the other markers it could be explained by the fact that these patients were products of consanguinity in an isolated founding population and could be identical by descent for numerous segments of their genome.
However, genotyping of all the affected individuals and their parents for these five unlinked markers using homozygosity mapping clearly showed that the disease was only associated with marker D10S1225 from 10q21. To rule out a random association between the disease and marker D10S1225, all the affected and unaffected individuals were also genotyped for a number of other microsatellite markers in 10q21. Statistical analysis of the results showed a significant association between NCRNA and four of these markers, making 10q21 a strong candidate region for the location of NCRNA gene. Because of the complexity of the pedigree, we could not use all the pedigree information in our statistical analysis. Hence four subgroups were used, and this subgrouping would be expected to result in conservative estimates of LOD because not all pedigree information is retained. Therefore, the actual LOD for the linkage of NCRNA to the markers in 10q21 must be greater than our estimates.
Narrowing the disease region, using the public databases such as the UniGene database would be a good start to the finding of candidate genes for NCRNA. Positional cloning and identification of the gene responsible for NCRNA would contribute to the understanding of the pathophysiology of this phenotype and eventually to the prevention and perhaps even to the treatment of this disease.
Acknowledgements
We thank the NCRNA families for their support, cooperation, and encouragement. We also thank Drs. Hamid Reza Ghorbanzadeh, Avisa Aledavood, and Henry Kaplan for their support and Ali Reza Aledavood, Mahmood Kalbasi, Mahmood Hasani, Mohammad Ali Sheikhamirloo, Ali Jafarnejad, and Arezoo Dehghanie for their logistic support. This work was in part supported by National Institutes of Health grant 5F33-EY06846-02.




