Electrocatalytic Reduction of Hydrogen Peroxide on Palladium-Gold Codeposits on Glassy Carbon: Applications to the Design of Interference-Free Glucose Biosensor
Abstract
Following our previous studies on the catalytic activity electrochemically codeposited on graphite Pd-Pt electrocatalysts for hydrogen peroxide electroreduction, a series of glassy carbon electrodes were modified with Pd or (Pd+Au) deposits aiming at the development of even more efficient electrocatalysts for the same process. The resulting electrodes were found to be very effective at low applied potentials (−100 and −50 mV versus Ag/AgCl, 1 M KCl). The surface topography of the electrode modified with Pd+Au mixed in proportions 90% : 10%, exhibiting optimal combination of sensitivity and linear dynamic range towards hydrogen peroxide electrochemical reduction, was studied with SEM and AFM. The applicability of the same electrode as transducer in an amperometric biosensor for glucose assay was demonstrated. At an applied potential of −50 mV, the following were determined: detection limit (S/N = 3) of 6 × 10−6 M glucose, electrode sensitivity of 0.15 μA μM−1, and strict linearity up to concentration of 3 × 10−4 M.
1. Introduction
- (i)
the simplicity of operating with electrical signals (current, potential, conductivity, or impedance);
- (ii)
compact and versatile instrumentation capable of supporting several different electrochemical techniques (steady state or dynamic) in a single device;
- (iii)
a possibility to finely tune the electrochemical detection and to customize it so that the tedious sample pretreatment stage could be avoided;
- (iv)
broad opportunities for miniaturization to afford portable devices, needing very small amounts of a sample (e.g., only several microlitres) to be analyzed;
- (v)
a possibility to perform an automated on-line monitoring, that is, a determination to be carried out directly in the system where the process/product of interest occurred.
Moreover, the selectivity of the analysis might be drastically improved through coupling sensor with a biological component for molecular recognition (enzymes, cells, or tissues), thus providing the opportunity to selectively determine the analyte in complex liquid matrices. These are the main prerequisites for their wide practical application in important spheres, such as medicine [1, 2], food industry [3], and ecology [4].
A broad spectrum of oxidative enzymes (e.g., glucose oxidase, xanthine oxidase, etc.) is known to produce hydrogen peroxide as a by-product of the target reaction they catalyse in presence of molecular oxygen. Therefore, a large group of amperometric biosensors functions on the principle of measuring the current variation upon the addition of the enzyme substrate which is due to the electrochemical transformation (oxidation or reduction) of the generated H2O2. The biosensors based on registering the current from the electrochemical oxidation of the formed hydrogen peroxide suffer from poor selectivity, mostly due to the high operational potentials, at which the compounds normally attending biological liquids (organic acids, neurotransmitters, and drugs) are co-oxidised, thus leading to overestimated analyte levels. Alternatively, reducing electrochemically H2O2 at low working potentials (usually around 0 V versus Ag/AgCl) [5], where interfering substances are electrochemically inert, allows for a much more selective assay of the analyte. In respect to studies on the surface modified with micro- and nanostructures carbonaceous electrodes as efficient electrocatalysts of hydrogen peroxide, both oxidation and reduction [6–13] are currently on high demand.
Various types of carbonaceous materials have been investigated most often as modified electrodes for determining H2O2: graphite [6, 14], glassy carbon [7, 8, 10, 15–19], carbon-paste electrodes [20, 21], carbon nanotubes [22–24], and so forth. With these electrodes, the selectivity of the electrochemical response to hydrogen peroxide is improved by using different catalytically active modification components: transition metals and their oxides [15, 21], platinum metals, dispersed on their own or mixed with other platinum metals [7, 25], and with nanostructured composite films [8, 26]. Studies aimed at the electroanalytical detection of H2O2 with micro- and nanostructured carbon films containing metal particles (2–5 nm), such as Pt, Ni, Cu, and Ir [26], have become common in recent years.
In electrochemical sensor technologies, special attention is paid to carbon nanotubes (CNTs) as a promising electrode material. CNTs have demonstrated to possess a unique combination of excellent structural, mechanical, and electrochemical characteristics [22]. CNT-based electrodes, promoted with metal nanoparticles, are distinguished with their high catalytic activity and have been successfully used for detecting H2O2 [23, 24]. The modification was performed through the adsorption of nanoparticles from colloidal solutions or through electrodeposition from a solution of the salt of the respective metal. The second approach is often preferred due to the possibility of strict and accurate control allowing for the high reproducibility of the modification procedure. Contemporary studies proved the electrodeposition as an attractive and cheap method for building-up nanosized metal structures, which does not require expensive equipment [27, 28]. By varying the conditions of modification (applied potential, composition of the electrolyte, deposition time, nature of the carrier, etc.), micro- and nanostructured composite metal deposits with high electrocatalytic activity can be obtained.
Our research has shown that highly porous graphite materials, modified through potentiostatic deposition of Pt, Pd, and mixtures of (Pd+Pt) and (Pd+Au) in varied proportions, prove to be effective electrocatalysts for the reduction of hydrogen peroxide at low working potentials [9, 10]. In addition, modified graphites were characterised with a simple modification protocol, high operational stability over a wide pH-range, and high operational stability with retaining catalytic activity for over 1 year that motivated us to use them as electrochemical transducers in the design of amperometric biosensors based on the electroreduction of hydrogen peroxide. This study continues our research in the above-mentioned direction, with glassy carbon being the solid support to be modified with microquantities of pure Pd and a mixture (Pd+Au) deposited electrochemically on it. The choice of the carbon material, glassy carbon, is motivated by its high mechanical and chemical stability in aggressive media, and above all by its fullerene-related structure [29], that is, properties that distinguish it from graphite. In addition, substituting one of the components of the catalytically active phase, Pt with gold would potentially affect the catalytic activity towards the target reaction (H2O2 electroreduction), and the new data would provide us with a better understanding of the factors affecting the catalytic process as a whole. Based on the above, the present study deals with the development of an efficient and selective electrocatalyst for the reduction of H2O2 through modification of glassy carbon with microdeposits of Pd and a mixture of (Pd+Au). Their applicability as transducers in amperometric biosensors, based on hydrogen peroxide producing oxidoreductases, is also demonstrated.
2. Experimental
2.1. Materials
Glucose oxidase (GOx) (E.C. 1.1.3.4) from Aspergillus niger (Fluka Biochemica), with homogeneous activity of 198 U mg−1 (1 U corresponds to the amount of the enzyme which oxidizes 1 μmoL glucose per min at pH 7.0 and 25°C); D-glucose monohydrate (Valerus, Sofia, Bulgaria). Hydrogen peroxide and chemicals used for preparing buffer solutions, Na2HPO4 × 12H2O, citric acid, KOH, and H3PO4, were purchased from Fluka. All solutions were prepared with double-distilled water. The β-D-glucose solution (5 × 10−3 M in phosphate-citrate buffer, pH 7.0) was allowed to mutarotate for 24 h before use.
2.2. Preparation of the Electrodes
Glassy carbon (GC) lamellae with a geometric surface Sgeom = 1.35 cm2 were used. The catalytically active components were deposited in potentiostatic regime (Edeposit = +0.05 V versus reversible hydrogen electrode) via a brief electrolysis (tdeposit = 10 s) from the following electrolytes: 2% solution of PdCl2 in 0.1 M HCl, alone or mixed with 2% solution of HAuCl4 in 0.1 M HCl in the following volume to volume ratios: 90% : 10%, 70% : 30%, or 50% : 50%. The respective molar proportions of Pd to Au in the electrodeposition baths have been determined to be 17 : 1; 9 : 2; 2 : 1. The surface coverage was estimated to be ca. 18 μg cm−2. The prepared electrodes were allowed to age for 3–5 weeks until the stabilization of the surface occurs. By the end of ageing period, the chronoamperometric data showed a decrease of the active electrode surface with 15%.
- (i)
glassy carbon modified with individual Pd (electrode type A);
- (ii)
glassy carbon modified with Pd+Au mixed in a ratio of 90% : 10% (w/w, electrode type B);
- (iii)
glassy carbon modified with Pd+Au mixed in a ratio of 70% : 30% (w/w, electrode type C);
- (iv)
glassy carbon modified with Pd+Au mixed in a ratio of 50% : 50% (w/w, electrode type D).
2.3. Apparatus and Measurements
All electrochemical measurements were performed in a three-electrode cell with separated compartments (working volume 10–15 mL). An Ag/AgCl, 1 M KCl electrode was used as a reference electrode, and platinum wire as a counter electrode. The electrochemical setup also involved a bipotentiostat, type BiPAD (TACUSSEL, Villeurbanne, France); a generator, type EG-20 (Elpan, Lubawa, Poland); a digital voltmeter, type 1AB105 (ZPU, Pravets, Bulgaria). The solutions were softly bubbled with argon during the measurements, in order to decrease the background current, but not to completely deaerate the solutions. For the experiments with the enzyme electrodes, the access of oxygen to the electrolyte was assured by keeping the cell open. The operational characteristics of the series-modified electrodes were examined by the polarization curves’ method in potentiostatic regime (phosphate-citrate buffer, pH 7.0). For maintaining constant temperature a thermostat UH (VEB MLW Prüfgeräte-Werk, Medingen, Germany) was used. The pH of the buffer solutions was adjusted with a pH-meter OP-208 (Radelkis, Budapest, Hungary).
3. Results and Discussion
3.1. Electrocatalytic Reduction of Hydrogen Peroxide
The steady-state polarization curves on glassy carbon electrodes, modified with deposits of Pd only (type A electrode) and with the mixtures of (Pd+Au) (types B, C, and D electrodes) in presence of hydrogen peroxide recorded over the potential range from −250 to +250 mV (versus Ag/AgCl) at 25°C, are shown in Figure 1.
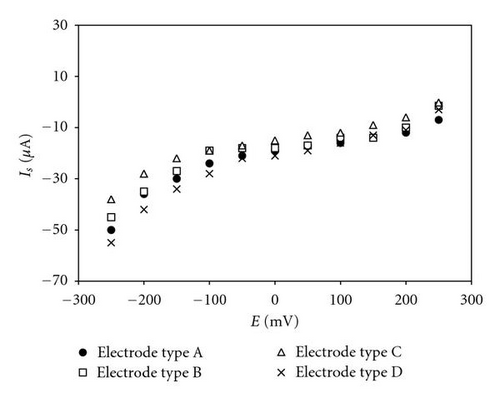
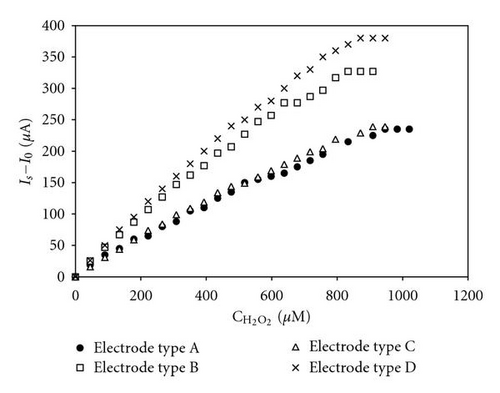
For modified glassy carbon of types B, C, and D, the sensitivity increases as the working potential shifts to the negative direction. As a general rule, the electrode sensitivity at potentials −50 and −100 mV was found to be between 1.2 and twice as high as the sensitivity registered at 0 V (Table 1). For glassy carbon modified with Pd only (type A), there were no differences observed in the values of the electrode signal over the same potential range. The linear dynamic range of the electrode signal (in μM) also differs for all electrodes as dependent on the applied potential and on the type of the modifying component For the type A electrode; however, the linearity of the signal, as well as the sensitivity, increases as the potential shifts negatively. For the other three electrodes—of types B, C, and D, there is no correlation between the linearity of the response and the shifts of the potential in the cathode direction. There is no dependence between the linearity of the signal and the percentage of Pd and Au in the microdeposits of the modifying mixture, either.
| E (mV) | T (°C) | Sensitivity (μA μM−1) | Linearity (μM)* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| electrode type | electrode type | ||||||||
| A | B | C | D | A | B | C | D | ||
| −100 | 25 | 0.27 | 0.42 | 0.28 | 0.47 | 900 | 800 | 870 | 830 |
| 35 | 0.27 | 0.45 | 0.40 | 0.55 | |||||
| −50 | 25 | 0.26 | 0.35 | 0.19 | 0.36 | 800 | 980 | 1020 | 760 |
| 35 | 0.19 | 0.31 | 0.30 | 0.51 | |||||
| 0 | 25 | 0.25 | 0.31 | 0.14 | 0.30 | 680 | 900 | 830 | 800 |
| 35 | 0.12 | 0.23 | 0.21 | 0.35 | |||||
- *Linearity ranges for all 4 types of GC electrodes were determined at 25°C.
The activation energy of the electrochemical reduction of hydrogen peroxide on the C type GC was found to be virtually the same over the studied range of potentials: Ea = 31.6 ± 1.9 kJ moL−1. Similarly, the activation energy of H2O2 electroreduction on the D type electrode has been found to be independent of the working potential over the chosen potential region; however, the temperature effect on the reaction rate was much lesser, and the activation energy was calculated to be 11.2 ± 1.9 kJ moL−1. The low effective activation energy values as well as their independence of the working potential suggest that the electrocatalytic process in both cases is limited by diffusion.
From the basic operational characteristics of glassy carbon electrodes (Table 1), it is evident that the glassy carbon electrodes with the highest sensitivity (dI/dC) are the ones of types B and D at working potentials of −50 and −100 mV. At a potential of −50 mV, the type B electrode is characterized by a longer linear dynamic range of the calibration graph (up to 980 μM) as compared to the type D electrode (up to 760 μM).
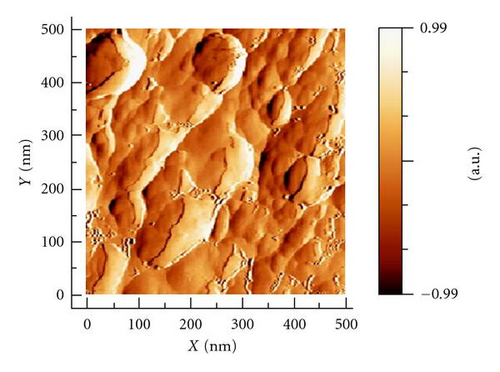
The surface topography of electrode type B was studied by means of scanning electron microscopy (SEM) and atomic force microscopy (AFM). SEM photograph (Figure 3(a)) shows that the catalytically active phase (atomic ratio of Pd to Au 90% : 10%) is deposited on the glassy carbon support in the form of well-defined oval formations with a size of 100 to 500 nm, while the AFM image (Figure 3(b)) gives a much detailed information about the substructure of the deposits; they consist of fine grains with nearly spherical shape and approximate diameter of 10–20 nm.

Ensuring a high selectivity of the analysis is a factor of key importance when designing amperometric biosensors. In this connection, the response of the type B electrode was studied in the presence of electrochemically active substances, which usually attend biological fluids. At potentials of −50 and −100 mV, no electrode response was registered in the presence of uric acid, ascorbic acid, glutathione, and paracetamol, in concentrations of up to 700 μM, which largely exceed the possible physiological levels. In addition, for the type B electrode, the lowest background currents at potentials of −50 and −100 mV were registered as well. The discussed advantages of the modified glassy carbon electrode of type B were the main prerequisites in choosing it as a transducer in designing an amperometric glucose biosensor.
3.2. Glucose Oxidase Enzyme Electrode
The high activity and selectivity, the sufficiently long dynamic range of the response, the low background currents, and the great number of catalytically active centres of glassy carbon modified with microquantities of (Pd to Au 90% : 10%) were the main prerequisites for its use as a basic biosensor transducer. On it, by immobilizing GOx in accordance with a previously reported procedure [10], a new glucose oxidase enzyme electrode was developed. The possibility to determine quantitatively glucose at potentials of −100 and −50 mV in a phosphate-citrate buffer pH 7.0, at temperatures of 25°C and 35°C (Table 2), was examined.
| E (mV) | T (°C) | Sensitivity (μA μM−1) | r2 | Linearity (μM) | (μM) |
|---|---|---|---|---|---|
| −100 | 25 | 0.05 | 0.968 | 800 | — |
| 30 | 0.11 | 0.968 | 380 | 680 | |
| −50 | 25 | 0.10 | 0.982 | 450 | 710 |
| 30 | 0.15 | 0.985 | 300 | 405 | |
The presented data shows that a rise of the temperature with 5°C results in an increased current response which is more extensive at E = −100 mV. As the temperature increases and the potential shifts into positive direction, the linear dynamic range shortens, and the electrode sensitivity (dI/dC) increases (Table 2). At E = −100 mV, the linear part of the calibration graph is shortened more than twice when the temperature is increased by 5°C. Furthermore, higher background currents were registered at this potential. At a temperature of 25°C, the electrode sensitivity at E = −50 mV is 2.2 times as high as that at E = −100 mV, and at 30°C, 1.3 times as high. The detection limits of glucose at E = −50 mV were found to be 9 μM, and at 30°C were found to be 6 μM (estimated at signal/noise ratio of 3 : 1).
Therefore, the working potential of E = −50 mV was selected as optimal for determining glucose quantitatively. The experimental data on the selected working potential and temperatures 25 and 30°C were presented in Eadie-Hofstee coordinates Is versus (Is/C) in accordance with the equation , where the steady-state electrode response (Is) for a certain concentration of the substrate is represented as a function of the electrode sensitivity at this given concentration (Is/C) (the ratio of the current to the respective concentration of the substrate) (Figure 4). From the calibration graph, (Figure 4(a), left side) it is obvious that the linear dynamic range of the glucose oxidase enzyme electrode spans over the concentration range up to ca. 300 μM. From the presentation of these experimental data in Eadie-Hofstee coordinates (Figure 4(a), right side) it can be deduced that at low substrate concentrations (up to ~300 μM), the sensitivity of the electrode remains practically constant, which is typical for diffusion restrictions over the processes in the bioelectrochemical system. The inclined area between 300 μM and 455 μM indicates the presence of enzyme-kinetic control, while the horizontal area of the graph refers to high substrate concentrations exceeding 455 μM and can be assigned to the effect of saturation of the enzyme with substrate molecules. It is due to the fact that it was impossible for the enzyme to react with all the glucose molecules that reach it. The value of the apparent Michaelis constant = 710 μM was determined from the slope of the inclined region. Analogous analysis related to the working mode of the electrode performed at a temperature of 30°C (Figure 4(b)) suggests that the same three working regimes of the electrode, diffusion, kinetic, and substrate limited, are present.

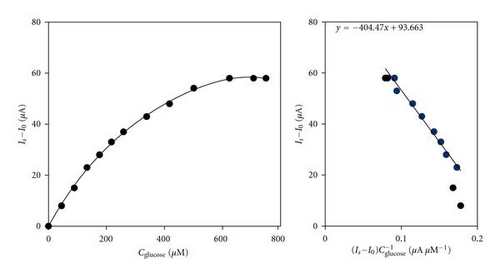
The diffusion area at this higher temperature, however, is considerably shorter (up to 130 μM) compared to that at 25°C, while there is an increase in the section in which the electrochemical process takes place under kinetic control (from 130 to 635 μM). The value of the apparent Michaelis constant = 405 μM was found to be 1.8 times as low as the one determined at 25°C. In Table 3, the operational characteristics of the examined electrode were compared to the ones of the glucose oxidase enzyme electrode, determined through immobilization of GOx on porous graphite (GMZ), modified from a solution containing Pd and Au mixed in a ratio of 70% : 30% [10].
| Electrode type | T (°C) | Sensitivity (μA μM−1) | r2 | Linearity (μM) | (μM) |
|---|---|---|---|---|---|
| type AGOx | 20 | 0.04 | 0.977 | 550 | >1600 |
| 25 | 0.10 | 0.982 | 450 | 710 | |
| type BGOx | 20 | 0.08 | 0.966 | 240 | 470 |
| 25 | 0.13 | 0.974 | 180 | 310 | |
- (i)
the GOx-immobilised electrode based on modified GC (this work)—AGOx type;
- (ii)
the GOx-immobilised electrode based on modified GMZ-graphite [10]—BGOx type.
From the data presented in Table 3, it is evident that at both working temperatures the sensitivity of the type BGOx electrode is as high as 1.7 times (at 20°C) and as high as 1.3 times (at 25°C) compared to the sensitivity of the type AGOx electrode. The lower sensitivity of the type AGOx electrode is accompanied by linear dynamic range of the response that is 2.5 times as long, as well as with higher values of the apparent Michaelis constant . The differences in the operational parameters of the glucose oxidase enzyme electrodes shown above can be due to both the different proportions of Pd and Au microquantities in the modifying mixture, and to the different basic carbon matrix graphite and glassy carbon. These lead to a difference in the structure of the electrodeposited active phase [9] which eventually affects the conformation of GOx during its immobilization (which means it affects its activity).
4. Conclusions
Efficient electrocatalysts for the reduction of hydrogen peroxide at low potentials, around and below 0 V (versus Ag/AgCl), have been obtained by modifying glassy carbon matrices with microquantities of Pd and mixtures (Pd+Au), in different proportions of the catalytically active components. The basic kinetic (Ea) and operation parameters (electrode sensitivity, linear dynamic range, and detection limits) of the electrodes in the target reaction have been resolved. A selective biosensor for amperometric determination of glucose based on electrocatalytic peroxide electrode of the B-type (Pd to Au mixed in atomic ratio 90% : 10% deposits on glassy carbon) has been developed.
- (i)
working potential of −50 mV,
- (ii)
a temperature of 25–30°C.
Under the above conditions, a detection limit of 6 × 10−6 M, electrode sensitivity of 0.15 μA μM−1, and linear dynamic range of the response up to 300 μM have been determined.
Acknowledgments
The authors acknowledge the support from the Bulgarian National Science Fund (Grant no. DVU-02/38).




