Long-Term Effect of Colestimide on Glycemic Control in Patients with Type 2 Diabetes with Elevated Low-Density Lipoprotein Cholesterol Levels
Abstract
Objective. Bile acid sequestrants (BASs) have been shown to improve both low-density lipoprotein-(LDL-) cholesterol and hemoglobin (Hb) A1c levels in patients with type 2 diabetes with elevated LDL levels. However, the longitudinal effect on glycemic control in these patients has not been fully examined. In the current study, the effect of colestimide (a BAS used in Japan, called also as colestilan) over a mean period of 18 months was investigated in patients with type 2 diabetes with elevated LDL levels. Methods. The study was performed prospectively in 34 patients with type 2 diabetes with elevated LDL levels who started colestimide (3.0 g a day) between April 2007 and March 2008. Results. HbA1c was significantly decreased after 3 months of colestimide therapy and at the end point of treatment (a mean period of 18 months) compared with baseline values (8.1% ± 1.1% at baseline, 7.1% ± 0.7% at 3 months, and 7.2% ± 0.9% at the end point; both P < .0001 versus baseline; P > .05, 3 months versus end point). Colestimide also significantly reduced LDL-C at 3 months and at the end point (both P < .0001 versus baseline; P > .05, 3 months versus end point). However, fasting plasma glucose, triglycerides, systolic and diastolic blood pressure, and body weight did not differ significantly after 3 months and at the end point compared with the respective baseline values. Conclusion. Colestimide significantly decreased HbA1c over a relatively long-term period in patients with type 2 diabetes with elevated LDL levels.
1. Introduction
Bile acid sequestrants (BASs) have been shown to improve both lipid and glucose metabolism [1–7], although the detailed mechanisms of the glucose-lowering effect of BAS is unknown. In 2008, colesevelam was approved by the United States Food and Drug Administration (FDA) as a BAS for treatment of type 2 diabetes concomitantly with other antidiabetic drugs. Colesevelam decreases hemoglobin (Hb) A1c levels by approximately 0.5% over 3 to 6 months [2–5], but its long-term effect is not fully understood. We and others have recently shown that colestimide (called also as colestilan), another BAS used for treatment of hyper-low density lipoprotein-(LDL-) cholesterolemia in Japan, can also reduce HbA1c in patients with type 2 diabetes over 3 to 6 months [6, 7]. However, the long-term effects of colestimide and colesevelam on glycemic control are unclear. Therefore, in the current study we evaluated the effect of colestimide on glycemic control over a mean period of 18 months in patients with type 2 diabetes with elevated LDL levels.
2. Patients and Methods
2.1. Patients
Patients with type 2 diabetes with elevated LDL levels who started colestimide between April 2007 and March 2008 were prospectively enrolled in the study. All patients received colestimide at 3.0 g a day (1.5 g, twice a day). Of the 38 enrolled patients, 34 who were followed up in an outpatient department for at least 12 months were included in the study. Four patients dropped out because of poor compliance with colestimide due to its main side effect of constipation. The mean duration of followup was 18.9 ±4.3 months (range: 12 to 26 months). Patients exhibiting evidence of liver dysfunction or findings of infectious or autoimmune disease were excluded from the study. The clinical features of the patients are shown in Table 1. As a general rule, patients with statins were excluded from this study (only 2 patients who had received pravastatin in long term were included).
| Patients | |
|---|---|
| No. (male/female) | 34 (12/22) |
| Age (year) | 63.1 ±9.1 |
| Duration of diabetes (year) | 13.0 ±6.8 |
| BMI (kg/m2) | 24.5 ±3.7 |
| Diabetic therapy | |
| Insulin | 6 |
| SU | 19 |
| (SU1/SU2/SU3/SU4/ | 1/3/5/3/2/1/4 |
| SU5/SU6/SU7) | |
| Metformin (Met) | 2 |
| α-GI/α-GI, Met | 1/3 |
| Diet alone | 3 |
| Antihypertensive drugs | 17 |
| ARB/Ca/ACE-I/ARB, Ca/ | 3/5/1/5/2/1 |
| ACE, Ca/ARB, ACE-I, Ca | |
| Statins | 2 |
- Data are expressed as mean ± standard deviation (SD). Comparisons in variables between two groups were made by use of an unpaired t test. P: P value, P < .05 are defined as statistical significance. BMI: body mass index; Diabetic therapy: the number of the patients with respective diabetic therapies; Insulin: insulin therapy; SU: sulfonylurea; α-GI: α glucosidase inhibitor; SU1:SU alone; SU2: SU; α-GI, SU3: SU, Met, SU4: SU, pioglitazone, SU5: SU, α-GI, Met, SU6: SU, α-GI, pioglitazone, SU7: SU, Met, pioglitazone and/or α-GI: Antihypertensive drugs: the number of the patients with respective antihypertensive drugs; ARB: angiotensin-II receptor blocker; Ca: calcium channel blocker, ACE-I: angiotensin-I converting enzyme inhibitor.
2.2. Methods
Blood tests and body weight (BW) measurements were evaluated at 0 months (baseline), after 3 months of treatment, and at the end point of the study (a mean treatment period of 18 months). Blood was sampled in the outpatient department from 8:30 to 10:00 AM. after at least 10 hours of overnight fasting. At the time of blood sampling, the patients were weighed in their underwear. Plasma glucose, HbA1c, and serum lipid concentrations were measured as described elsewhere [8].
Ethical Considerations All subjects gave informed consent to treatment of hyper-LDL-cholesterolemia with colestimide. This study was performed according to the guidelines proposed in the Declaration of Helsinki.
Statistical Methods Data are presented as means ± standard deviation (SD). Comparison of two time points for an individual was performed using a paired t-test. P < .05 was considered to indicate statistical significance in all analyses.
3. Results
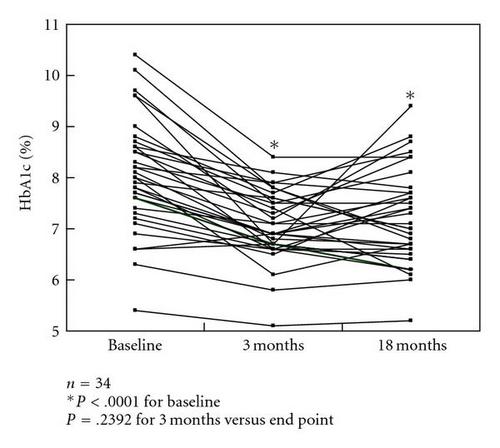
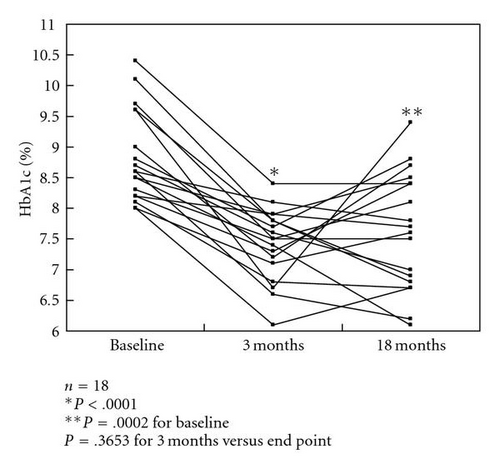
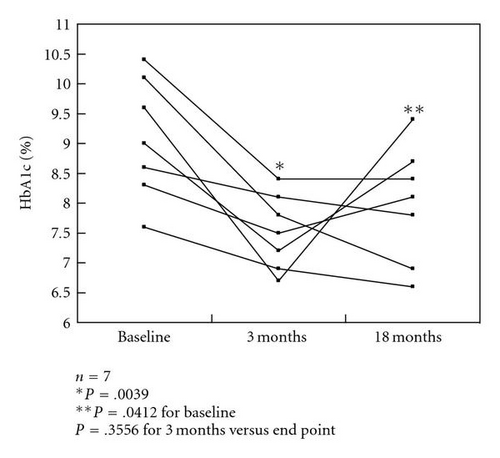
HbA1c levels were significantly decreased after 3 months of colestimide therapy and at the study end point (a mean treatment period of 18 months) compared with baseline: 8.1% ±1.1% at baseline, 7.1% ±0.7% after 3 months, and 7.2% ±0.9% at the end point (both P < .0001 versus baseline; P > .05, 3 months versus end point) (Figure 1(a)). In a subgroup of patients with poorly controlled type 2 diabetes (n = 18; HbA1c >8.0%, defined as poor glycemic control by the Japan Diabetes Society [9]), the HbA1c levels were 8.8% ±0.7% at baseline, 7.4% ±0.6% after 3 months, and 7.6% ±1.0% at the end point (P < .0001, P = .0002 versus baseline, resp.; P > .05, 3 months versus end point). However, about one-third of the patients had reelevation of HbA1c levels at the end point compared with those at 3 months (Figure 1(b)). In patients receiving multiple drug therapy, including glibenclamide at more than 5.0 mg/day, the HbA1c levels were 9.1% ±1.0% at baseline, 7.5% ±0.6% after 3 months, and 8.0% ±1.0% at the end point (P = .0039, P = .0142 versus baseline, resp.; P > .05, 3 months versus end point). However, approximately half of the patients showed reelevation of HbA1c compared with those at 3 months (Figure 1(c)).
| Base line | Final points | P value | |
|---|---|---|---|
| (18 months) | |||
| FPG (mg/dL) | 165.2 ± 48.3 | 144.2 ± 30.0 | .0911 |
| HbA1C (%) | 8.1 ± 1.1 | 7.2 ± 0.9 | <.0001 * |
| TC (mg/dL) | 245.9 ± 44.7 | 204.0 ± 42.5 | <.0001 * |
| TG (mg/dL) | 165.7 ± 112.9 | 169.6 ± 146.7 | .8384 |
| LDL-C (mg/dL) | 149.3 ± 32.4 | 114.4 ± 36.2 | <.0001 * |
| HDL-C (mg/dL) | 55.3 ± 10.7 | 55.6 ± 11.0 | .8444 |
| SBP (mm Hg) | 128.5 ± 12.7 | 129.7 ± 10.7 | .5573 |
| DBP (mm Hg) | 71.0 ± 5.9 | 70.8 ± 4.9 | .7921 |
| BW (kg) | 62.0 ±13.3 | 62.4 ± 12.5 | .2573 |
- All data are expressed as mean ± standard deviation (SD). The two time points for an individual were compared by use of a paired t-test.
- P: P value; P < .05 are defined as statistical significance ( *).
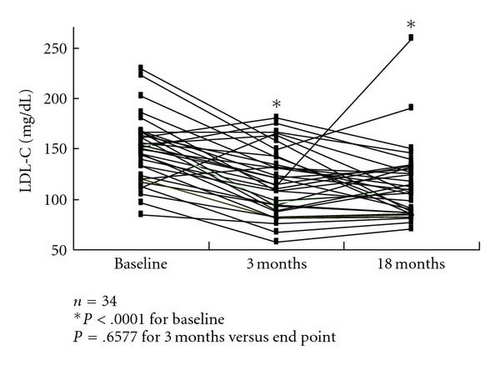
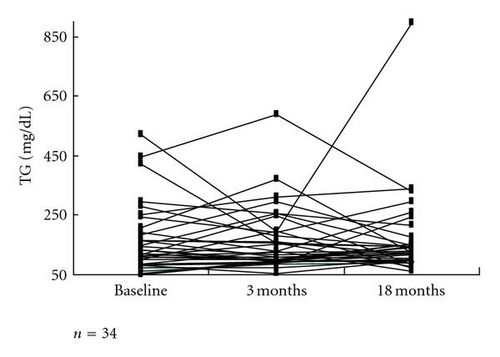
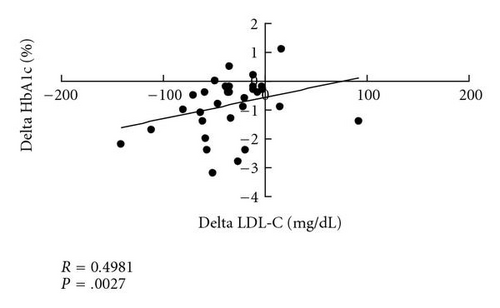
The LDL-C level of 149.3 ±32.4 mg/dL at baseline was significantly decreased to 117.3 ±32.6 mg/dL after 3 months and to 114.3 ±36.1 mg/dL at the end point (both P < .0001 versus baseline; P > .05, 3 months versus end point) (Figure 2(a)). However, fasting plasma glucose (FPG) triglyceride (TG) (Figure 2(b)), high-density lipoprotein cholesterol (HDL-C), systolic blood pressure (SBP), diastolic blood pressure (DBP), and body weight (BW) did not show significant changes after 3 months or at the end point compared with the respective baseline values. These data are summarized in Table 2. There was a significant positive correlation (R = 0.4981, P = .0027) between the changes in HbA1c and LDL-C before and after colestimide therapy (Figure 3).
4. Discussion
Our results show that colestimide decreases HbA1c by about 1.0% in 3 months in patients with type 2 diabetes with elevated LDL cholesterol. Most of the patients had already been treated with other antidiabetic drugs, including insulin or sulfonylureas. This result corresponds to that in a recent study showing a 0.9% reduction of HbA1c levels [6]. Furthermore, we found that the reduction in mean HbA1c was maintained over a longer treatment period of about 18 months. However, the standard deviation in HbA1c tended to be higher at 18 months than at 3 months; in about one-third of the patients, HbA1c had reelevated after 18 months compared with the value at 3 months, while other patients had lower HbA1c levels at the end of the study. This suggests that the percentage of patients who obtain a beneficial effect of colestimide for glycemic control may decrease in long-term administration, compared with that over 3 months, despite the similar mean reduction in HbA1c levels.
Notably, the glucose-lowering effect of colestimide was more apparent in patients with poor glycemic control (HbA1c >8.0%), compared with those with HbA1c <8.0%. This result corresponds to findings with colesevelam treatment [2]. Furthermore, colestimide was effective in patients who were receiving multiple antidiabetic drugs, including high-dose sulfonylureas, although approximately half of patients showed reelevation of HbA1c in long-term observation. Taken together, these results suggest that colestimide may be particularly beneficial in patients with poor glycemic control or those being treated with multiple antidiabetic drugs.
Interestingly, the decrease in HbA1c produced by colestimide was larger than that found for colesevelam (a 0.5% reduction in 3 to 6 months), despite the longer treatment period in the current study. However, differences in ethnicity and body mass index (BMI) between the studies make it difficult to compare the glucose-lowering effects of the two drugs directly, and studies in the same population are required to examine this issue. The detailed mechanisms of glucose-lowering by BASs are not fully understood, although several hypotheses have been proposed [10]. In the current study, we observed a positive correlation between the changes in HbA1c and LDL-C before and after colestimide therapy. Since BASs mainly decrease LDL-C through effects on bile acid metabolism, colestimide may decrease HbA1c, at least in part, via a similar mechanism.
Regarding serum TG levels, colestimide did not apparently affect TG levels. This is in contrast with recent studies with colesevelam in type 2 diabetic patients [4, 5]; however, more detailed analysis in further study with large sample size is needed for the influence of colestimide on TG levels.
In the current study, only 2 patients were receiving statins, because as a general rule the patients who had received these drugs were excluded from the study. However, statins are the cornerstone of lipid management in diabetic patients, as supported also by the results of CARDS study [11]. Therefore, we emphasize that statins should be first choice of treatment for hyperlipidemia in these patients despite the potentially beneficial effect of BAS for glycemic control.
This study has several limitations. The most important concern is associated with the study design; although the study was prospective, it was not randomized and we did not use a placebocontrolled group. Furthermore, the number of patients was relatively small and most had already taken antidiabetic drugs. Thus, it would be of interest to investigate the effect of single-use of colestimide on glycemic control.
In conclusion, we found that colestimide is effective for glycemic control in relatively long-term use (about 18 months) in patients with type 2 diabetes with elevated LDL cholesterol. However, a noninsignificant number of patients showed redeterioration of glycemic control over this period. These patients may require other antidiabetic treatments, including a change to insulin therapy in noninsulin treated patients or a change of insulin dose in insulin-treated patients.
Abbreviations
-
- FPG:
-
- Fasting plasma glucose
-
- HbA1C:
-
- Hemoglobin A1C
-
- TC:
-
- Total cholesterol
-
- TG:
-
- Triglyceride
-
- LDL-C:
-
- Low-density lipoprotein cholesterol
-
- HDL-C:
-
- High-density lipoprotein cholesterol
-
- SBP:
-
- Systolic blood pressure
-
- DBP:
-
- Diastolic blood pressure
-
- BW:
-
- Body weight.




