Infrared Spectroscopic Characterization of Tellurite Glasses Containing Heavy Metal Oxides
Abstract
The infrared (IR) spectra of (100-x)TeO2–xWO3 glasses reveal that the glass network consists of [TeO3]/[TeO3+1], [TeO4], [WO4], and [WO6] groups as basic structural units. Addition of WO3 oxide to the binary TeO2–WO3 glasses increases the amount of lower coordination of [TeO3]/[TeO3+1] units and decreases the higher coordination of [TeO4] units and also the formation of Te–O–W linkages at the expense of Te–O–Te linkages. The IR spectra of 60TeO2–(40-x)WO3–xPbO glasses reveal that the glass network consists of [TeO3], [TeO4], [WO4], [WO6], and [PbO4] units. Changes in the coordination state of tellurium and tungsten ions occur when the PbO and WO3 concentrations are varied. The dual role of the lead ions is confirmed in 60TeO2–(40-x)WO3–xPbO glass system. The W ion coordination state changes from 4 to 6 when WO3 concentration increases beyond 30 mol% in both (100-x)TeO2–xWO3 and 60TeO2–(40-x)WO3–xPbO glass systems. The IR spectra of 60B2O3–10TeO2–(30-x)ZnO–xPbO glasses reveal that the glass network consists of [TeO3], [BO3], and [BO4] groups.
1. Introduction
Glasses with heavy metal oxides (TeO2, GeO2, Bi2O3, WO3, PbO, Ag2O, etc.) are promising materials for IR technologies, nonlinear optics, and design of laser devices [1]. Tellurite-based glasses are the subject of intense current research because of the interesting electrical and optical properties. Main features include extended Infrared transmittance [2], high nonlinear optical indices [3], low fusion temperature, and they constitute an excellent matrix for active element doping, justifying a continuous technological interest [4]. The synthesis of glasses with high refractive index values is of great importance in the glass science and the optical industries. Among tellurite glasses, the glasses based on WO3, PbO, and other heavy-metal oxides (HMO) are known to have high linear refractive indices (n > 2.1) [5–7]. The high linear refractive index of Te+4 containing glasses is attributed to the nonbonding lone electron pair 5s2 of tellurium [8].
The PbO is unique in its influence on the glass structure and is widely used in glasses because it enhances the resistance against devitrification, improves the chemical durability, and lowers the melting temperature [9, 10]. PbO could act both as glass network former and as modifier depending on its concentration in the glasses [11, 12]. B2O3 is one of the best and well-known glass former. Addition of small amount of TeO2 into the borate glass network enhances the glass quality with an improvement in transparency, refractive index. Addition of ZnO into the boro-tellurite glass network produces low rates of crystallization and increases the glass-forming ability [13]. Hence it is of interest to study the structural changes brought about by PbO in tungsto-tellurite and boro-tellurite glasses, which may help in predicting the physico-chemical properties.
The aim of the present work is to study the short range structure and structural changes with compositions of (100-x)TeO2–xWO3, 60TeO2–(40-x)WO3–xPbO, and 60B2O3–10TeO2–(30-x)ZnO–xPbO glass systems by Infrared spectroscopy.
2. Experimental
The tellurium-based glasses (100-x)TeO2–xWO3, 60TeO2–(40-x)WO3–xPbO, and 60B2O3–10TeO2–(30-x)ZnO–xPbO were prepared from 99.9% purity-grade oxides (Aldrich). Powders of TeO2, WO3, B2O3, ZnO, and PbO were weighted to get the required composition and ground in a mortar with a pestle for 1 hour to obtain homogeneous mixtures. Each batch was then transferred to a platinum crucible and melted at about 800–950°C in an electric furnace. This melt was held at this temperature for 30 minutes until a bubble-free liquid was formed. The melts were stirred to achieve desirable homogeneity. The homogeneous melt was quenched by pouring it on to a preheated stainless steel mould to avoid excess thermal shocks. The glasses were annealed for 8 hours at 100°C to relieve the mechanical strains. The compositions of the glass samples employed in the present study are given in Table 1.
| Samples | Oxides (mol%) | ||||
|---|---|---|---|---|---|
| TeO2 | WO3 | PbO | B2O3 | ZnO | |
| TW1 | 90 | 10 | — | — | — |
| TW2 | 80 | 20 | — | — | — |
| TW3 | 70 | 30 | — | — | — |
| TW4 | 60 | 40 | — | — | — |
| TWP1 | 60 | 40 | 0 | — | — |
| TWP2 | 60 | 30 | 10 | — | — |
| TWP3 | 60 | 20 | 20 | — | — |
| TWP4 | 60 | 10 | 30 | — | — |
| TWP5 | 60 | 0 | 40 | — | — |
| BTZP1 | 10 | — | 0 | 60 | 30 |
| BTZP2 | 10 | — | 10 | 60 | 20 |
| BTZP3 | 10 | — | 20 | 60 | 10 |
| BTZP4 | 10 | — | 30 | 60 | 0 |
X-ray diffractograms of powdered glass samples were recorded using a copper target (λ(kα) = 1.54 A°) on a Philips PW (1140) diffractometer at room temperature. The IR spectra of the glass samples were recorded at room temperature using a Perkin-Elmer FT-IS spectrometer model 1605 using KBr disc technique. The investigated samples were ground to fine particles and then mixed with KBr in the ratio (0.002: 0.2 g) glass to KBr, respectively. The weighted mixture was then subjected to a pressure of 5 tons/cm2. The transmission spectra were measured immediately after preparing the desired disks.
3. Results and Discussion
3.1. XRD and IR Spectra of (100-x)TeO2–xWO3 Glasses
The X-ray diffraction spectra show no peaks, indicating that the samples are amorphous.
The IR spectra of (100-x)TeO2–xWO3 glass system recorded in the wave number region 1150–440 cm-1 are shown in Figure 1. The IR spectra of these glasses are characterized by IR absorption bands in the wave number regions 925–945 cm-1, 850–865 cm-1, 730–790 cm-1, 600–680 cm-1, and 460–490 cm-1. The IR band positions are summarized in Table 2. The IR absorption in the range 600–680 cm-1 in the tellurium containing glasses is due to stretching vibrations of the Te–O bond in the TeO4 tbp (trigonal bipyramids) and TeO3 tp (trigonal pyramids) units [14]. The band observed at 640 cm-1 in 90TeO2–10WO3 (TW1) glass is assigned to TeO4 tbp units. The band at around 925 cm-1 in TW1 in the high wave number region of the spectrum is characteristic of the presence of the tungsten ions in [WO4] units or [WO6] units in the structure of the glass [15–17]. The band at around 730–790 cm-1 is due to Te–Oeq bond vibrations of distorted TeO4 units [17, 18]. The shoulder at 865 cm-1 is assigned to vibration of W–O–W linkages [19, 20]. The IR band in the region 460–490 cm-1 is assigned to Te–O–W linkages, which would increase the glass network connectivity and this assignment is made in agreement with the theoretical model for vibrations of mixed bridge bonds containing heavy metal and glass former atoms [21]. The formation of Te–O–W linkages is expected because both W and Te atoms have comparable electro negativity and can therefore substitute for each other in bonding with O atoms [21]. That is, there is a fraction of W cations that have partial covalent bonding and are incorporated in the binary TeO2–WO3 glass network [17].
| Sample ID | IR bands (cm−1) | |||||
|---|---|---|---|---|---|---|
| TW1 | 460–490 | 640 | — | 730 | 865 | 925 |
| TW2 | 460–490 | 645 | — | 735 | 860 | 934 |
| TW3 | 460–490 | — | 665 | 745 | 855 | 940 |
| TW4 | 460–490 | — | 675 | 790 | 850 | 945 |
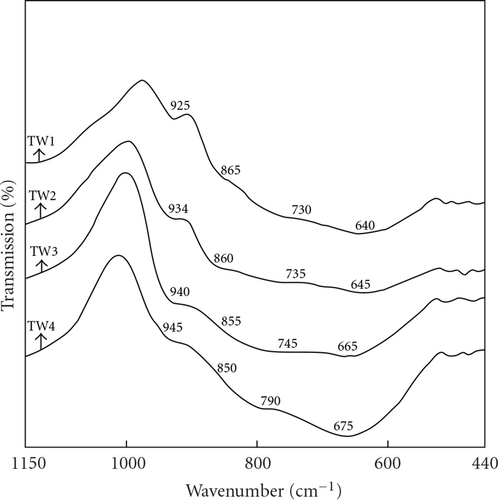
Equimolecular substitution of WO3 for TeO2 causes changes in the structure of the TeO2–WO3 glasses, which is apparent in the IR spectra of glasses (Figure 1). As the WO3 content increases from 10 mol% to 40 mol%, the band at 640 cm-1 in 90TeO2–10WO3 (TW1) shifts towards higher wave numbers: from to 640 to 645, 665, and then to 675 cm-1, while the band at 730 cm-1 shifts to 790 cm-1. This observed shift may be due to higher field intensity of mixed Te–O–W and W–O–W linkages, in which the oxygen is highly polarized, compared with Te–O–Te linkages, since W6+-ions possess higher field intensity than Te4+-ions [18], and this observed shift may be related to the apparition of TeO3 units concomitant to a reduction in the number of TeO4 units [22]. Thus the effect of addition of WO3 content to the TeO2 glass matrix is to transform the TeO4 basic structural units forming the TeO2 glass to TeO3 units. This is supported by the appearance of IR band in the region 665-680 cm-1 of 70TeO2–30WO3 (TW3) and 60TeO2–40WO3 (TW4) glass systems corresponding to TeO3 units.
The shoulder at about 850–865 cm-1 relative to the existence of vibration of W–O–W linkages is present for all the glass samples. As the WO3 content increases from 10 to 40 mol%, this causes a shift in the absorption band at 925 cm-1 due to WO4 units toward higher wave numbers: from 925 to 934, 940, and then to 945 cm-1. The observed shift in the 925 cm-1 band toward higher wave number in the composition range from 10 to 40 mol% WO3 is an indication of the transformation of WO4 units to WO6 units. However, the substitution of TeO2 by WO3 causes an increase of networking oxygens, according to the proportion 3/2 and these oxygens form new Te–O–W and W–O–W linkages. Thus the glass network is highly distorted and this suggests the formation of WO6 units at high WO3 content.
3.2. IR Spectra of 60TeO2–(40-x)WO3–xPbO Glasses
The IR spectra of the ternary TeO2–WO3–PbO (TWP) glass system recorded in the wave number region 1400–440 cm-1 are shown in Figure 2. The IR spectra of the glasses are characterized by absorption bands in the wave number regions 890–945 cm-1, 810–850 cm-1, 724–790 cm-1, 600–680 cm-1, and 460–490 cm-1. The IR band positions are summarized in Table 3. The band in the region 670–680 cm-1 in 60TeO2–40WO3 (TWP1) glass is assigned to TeO3 trigonal pyramids, while the band at around 724–790 cm-1 is due to Te–Oeq bond vibrations of distorted TeO4 units. The shoulder at 945 cm-1 in TWP1 in the high wave number region of the spectrum is a characteristic of the presence of tungsten ions in [WO6] units in the structure of the glass [17]. The shoulder at around 810–850 cm-1 is due to vibration of W–O–W linkages and the band in the region 460–490 cm-1 is assigned to Te–O–W linkages [17]. Equimolecular substitution of PbO for WO3 causes changes in the structure of the TWP glasses, which is apparent in the IR spectra of glasses (Figure 2).
| Sample ID | IR bands (cm−1) | |||||
|---|---|---|---|---|---|---|
| TWP1 | 460–490 | — | 675 | 790 | 850 | 945 |
| TWP2 | 460–490 | 645 | 660 | 761 | 843 | 929 |
| TWP3 | 460–490 | 635 | 670 | 742 | 820 | 911 |
| TWP4 | 460–490 | 630 | 667 | 724 | 810 | 894 |
| TWP5 | 460–490 | 638 | 678 | 731 | — | — |
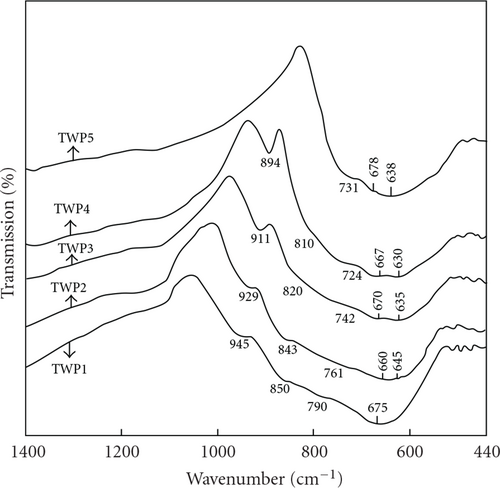
As the PbO content increases from 10 to 30 mol%, this causes a shift in the IR band due to W–O–W linkages towards lower wave numbers: from 850 to 843, 820, and then to 810 cm-1 and also causes a shift in the band 945 cm-1 due to WO6 octahedra toward lower wave numbers: from 945 to 929, 911 and then to 894 cm-1. The observed shift in the 945 cm-1 band and in the band 850 cm-1 toward lower wave number in the composition range from 10 to 30 mol% PbO may be an indication of the transformation of WO6 units to WO4 units. This receives support from the appearance of band at 894 cm-1 in 60TeO2–30PbO–10WO3 (TWP4) and is due to WO4 units [23]. When WO3 is substituted mol by mol by PbO the number of oxygens in the glass network diminishes according to the ratio 3/1. Thus the glass network becomes less distorted and this also suggests that the formation of WO4 units at high PbO content. The shoulder at about 850 cm-1 is absent in the IR spectrum of 60TeO2–40PbO glass.
When the PbO content increases from 10 to 20 mol%, a new band at around 635 cm-1 is observed in addition to that of ~670 cm-1, which indicates the formation of Te–O–Pb bonds in the glass network and this suggests formation of TeO4 units at the expense of TeO3 units. Thus it is concluded that addition of PbO causes a change in the coordination state of the tellurium ions and their partial conversion from 3-coordination to 4-coordination. This is supported by the appearance of band in the 600–645 cm-1 region corresponding to [TeO4] units. At low proportion of PbO (up to 20 mol%), it enters the glass network by breaking up the Te–O–Te, Te–O–W bonds and introduces coordination defects known as dangling bond (Te–O−⋯Pb2+⋯ −O-Te) which in turn decreases the TeO3 units by forming TeO4 units [24]. When the PbO content is increased from 20 to 40 mol%, a considerable proportion may be act as double bridges between adjacent TeO4 and WO4 units such as = Te–O–Pb–O–W = which can be formed beside the formation of PbO4 units [25]. The lead in this case acts as a glass forming agent and is incorporated in the glass structure in the form of [PbO4] units.
Therefore, for PbO up to 20-mol%, it plays a network modifier role, and at x ≥ 30, it plays a network former role in the present system. The study performed clarified the structural species of lead-tungsten tellurite glasses and confirmed the dual structural role of lead ions.
3.3. IR Spectra of 60B2O3–10TeO2–(30-x)ZnO–xPbO Glasses
The IR spectra of the quaternary 60B2O3–10TeO2–(30-x)ZnO–xPbO (BTZP) glass system recorded in the wave number region 4000–440 cm-1 are shown in Figure 3. The IR spectra of these glasses are characterized by absorption bands in the wave number regions 3436–3450 cm-1, 1333–1390 cm-1, 950–1085 cm-1, 665–687 cm-1, and 458 cm-1. The IR band positions of the present glass system are summarized in Table 4.
| Sample ID | IR bands (cm-1) | ||||
|---|---|---|---|---|---|
| BTZP1 | — | 668 | 1022 | 1333 | 3448 |
| BTZP2 | — | 668 | 1081 | 1344 | 3448 |
| BTZP3 | 458 | 687 | 1085 | 1342 | 3448 |
| BTZP4 | 458 | 667 | 1022 | 1342 | 3450 |
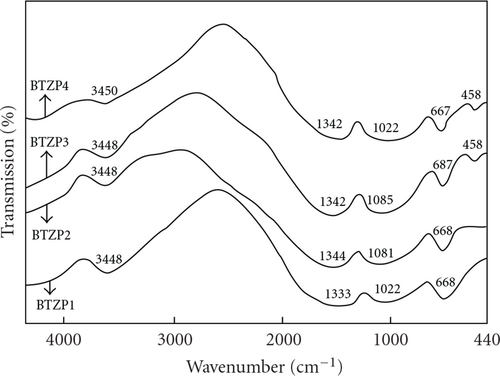
The IR band absorption in the high wave number region of the spectrum with maximum in the 3436–3450 cm-1 region belongs to O–H stretching vibrations [26]. In the present glass system (BTZP), the disappearance of the absorption band at 806 cm-1 indicates the absence of boroxol ring formation and thus clearly indicating the presence of triborate [BO3] and tetra borate [BO4] units. The IR absorption band in the 950–1085 cm-1 in all the glasses is due to stretching vibrations of B–O bond of [BO4] units [27, 28] and the absorption band in the 1333–1390 cm-1 region can be attributed to stretching vibrations of B–O bond of [BO3] units [29].
The absorption band at around 668 cm-1 indicates the presence of tellurium-oxygen groups. The structure pattern of tellurium-containing glasses is determined by trigonal pyramids [TeO3] and trigonal bipyramids [TeO4]. The absorption in the range of 600–700 cm-1 in such glasses is determined by the stretching vibrations of Te–O bonds in [TeO3] and [TeO4] units. The absorption of [TeO3] units has a high-wave number position than [TeO4] units. In general case, the absorption band range of [TeO3] units correlates with 650–700 cm-1 and that of [TeO4] units correlates with 600–650 cm-1 [14, 30]. Analyzing the obtained results and comparing them to the published data [12], it is clear that trigonal pyramids [TeO3] are present in the structure of all the glass systems (BTZP) because the absorption band appears at 667–685 cm-1 region. For lower concentration of PbO (20 mol%), PbO substituted for ZnO in BTZP series causes changes in the structure of the glasses, which is apparent in a shift of the absorption band at 668 cm-1 to higher wave number side up to 687 cm-1. Usually a shift of absorption bands to higher wave numbers occurs as a result of an increase in the degree of polymerization of the structural network [TeO3] units of the glass system. Absorption band at 687 cm-1 shifted to the lower wave number side, as the mole percentage of PbO increased from 20 to 30. These changes are may be due to formation of Te-O-Pb linkages and dual role of lead ions in the glass network.
In Figure 3, the band around 1022 cm-1 in BTZP1 shifts up to 1085 cm-1 as the content of PbO increases up to 20 mol% and the band around 1085 cm-1 shifts up to 1022 cm-1 as the content of PbO increases from 20 to 30 mol%. In lead borate glasses [31], at low concentrations (15–20 mol%) PbO acts as a modifier of the structural network in the form of [PbO6] groups and promotes conversion of [BO3] units to [BO4] tetrahedra. Above 15–20 mol% PbO, some lead ions enter the structure as network former in the form of [PbO4] units. The absorption band at 458 cm-1 in BTZP3 and BTZP4 probably belongs to the Pb-O vibration of [PbO4] units [32]. Thus the fraction of [BO4] units increases as the content of PbO increases from 20 to 30 mol%. It is clear from Figure 3 that there is no significant change in position but there is clear evidence of a decrease in the intensity of the absorption band around 1342 cm-1, and a corresponding increase in the intensity of the band at 1022 cm-1 is observed in the spectra of all the glasses in BTZP series. This observation suggests that the number of [BO3] units decreases and the fraction of [BO4] units increases as the PbO content increases. The study performed clarified the structural species of B2O3–TeO2–ZnO–PbO glasses and confirmed the dual structural role of lead ions.
4. Conclusions
Transparent and stable glasses were obtained in the TeO2–WO3, TeO2–WO3–PbO, and B2O3–TeO2–ZnO–PbO glass systems. The IR results show the progressive transformation of the TeO4 units to more distorted TeO3+1 units for TeO2–WO3 glasses with increasing WO3 content. Addition of PbO and WO3 increases the population of lower coordination units [TeO3]/[TeO3+1] in the TeO2–WO3–PbO glass network at the expense of higher coordination units [TeO4]. Addition of WO3 to the glass systems of TeO2–WO3 and TeO2–WO3–PbO results in the formation of more W–O–W and W–O–Te linkages, while that of the Te–O–Te linkages decreases as WO3 increases. In the case of TeO2–WO3–PbO glasses, the TeO3/TeO3+1 units are transformed to TeO4 units with increasing PbO content. Increasing the PbO content in B2O3–TeO2–ZnO–PbO glasses causes partial conversion of the boron ions from trigonal coordination [BO3] to [BO4] tetrahedral coordination. [TeO3] units are present in all the glass samples of B2O3–TeO2–ZnO–PbO glass system and the dual role of the lead ions is confirmed in this glass system.
Acknowledgment
G. Upender is grateful to UGC (University Grants Commission), New Delhi, for providing financial assistance under the scheme of RFSMS (Research Fellowships in Science for Meritorious Students).




