Leptomeningeal Carcinomatosis Secondary to Gastroesophageal Adenocarcinoma: A Case Report and Literature Review of a Rare Occurrence
Abstract
We present a 68-year-old male with leptomeningeal carcinomatosis (LC) from gastroesophageal junction carcinoma. Three months following epirubicin, cisplatin, 5-flurouracil (ECF) chemotherapy, the patient suffered from gait imbalance, headache, and dysarthria. CT and MRI imaging revealed LC throughout the brain and spine. The patient was prescribed dexamethasone and treated with a course of palliative radiation to the whole brain, 2000cGy/5. Additionally, the regions of symptomatic disease in the spine included the top of L4 vertebrae to the bottom of the S2 vertebrae which was treated with 2000cGy/5, and the top of the C5 vertebrae to the bottom of the T4 vetebrae received 800cGy/1. The radiation treatment did provide short-term symptom control; however, the patient eventually passed away from his illness. While LC remains a devastating complication of malignant disease, it has been rarely discussed in GI tumors, specifically GE junction adenocarcinomas. Therefore treatment options must be considered using first principles based on management of LC in more common disease sites. With early detection, and for patients with good performance status, palliative radiation utilizing hypofractionated regimens to sites of symptomatic involvement may improve quality of life for this group of unfortunate people.
1. Introduction
Leptomeningeal carcinomatosis (LC) results from “diffuse infiltration of the leptomeninges by malignant cells originating from an extrameningeal primary tumor site” [1]. LC caused by metastatic gastroesophageal adenocarcinoma has been rarely reported in the English literature. To date, only two cases have been described, the first was in 2001 and the other in 2003 [2, 3]. LC has mostly been reported with breast cancer, prostate cancer, and melanoma [2–5]. We describe the third reported case of LC with brain involvement secondary to adenocarcinoma of the gastroesophageal (GE) junction (Figure 1). In particular, unlike the first two reported cases where supportive measures were used to palliate symptoms; this is the first reported case where radiotherapy was used to control local symptoms.

2. Case History
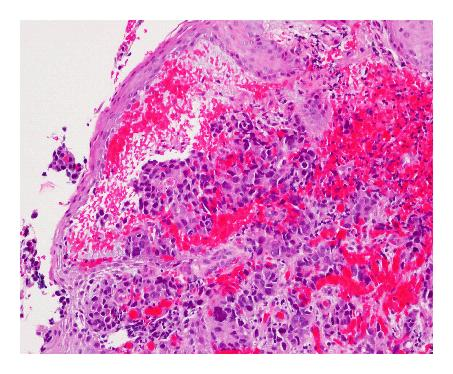
A 68-year-old male presented with a history of progressive dysphagia and weight loss, and was subsequently referred to a gastroenterologist. Outpatient endoscopy revealed, in the lower esophagus, an area of narrowing with significant ulceration, nodularity, and friability consistent with neoplasm. The gastroesophageal junction was at 38 cm from the incisors and the upper limit of the abnormality was at 34 cm from the incisors. The stomach revealed mild diffuse antral erythema, and the duodenum appeared normal. Biopsy of the gastroesophageal junction lesion revealed poorly differentiated adenocarcinoma (Figure 1). As the patient also had a history of prostate cancer in 1997 that was treated with surgical resection and radiation therapy, immunohistochemistry was performed on the GE biopsy, revealing that the tumor cells were positive for CK7 and CDX2, and negative for CK20 and PSA, consistent with a primary gastrointestinal adenocarcinoma. In addition, serum PSA levels had remained stable with no evidence of recurrent prostate cancer. He also had a history of hypertension and peptic ulcer disease, a remote history of smoking, and reported drinking three to four alcoholic drinks per week.
On examination, his weight was 66.9 kg and his height was 163.5 cm. His general physical examination including his abdominal examination was normal. There was no evidence of clubbing, scleral icterus, or peripheral lymphadenopathy.
Computed tomography (CT) revealed circumferential thickening involving eight centimeters of the esophagus/stomach averaging 1.5 centimeters in thickness with associated enlarged gastrohepatic and retroperitoneal lymph nodes as well as a 2.3 centimeter liver mass. There were two soft tissue nodules noted within the left lung, each measuring 6 millimeters, and a less-defined density noted within the right middle lobe. The impression was that of a gastroesophageal junction carcinoma with lymph node metastatic disease. Endoscopic ultrasound revealed a carcinoma, extending between 34 and 43 centimeters from the incisors to the Z-line with a fundus perfectly normal, as well as the rest of the stomach. The tumor penetrated through the muscularis propria at approximately 35 centimeters, invading the surrounding structures.
He was treated with standard epirubicin, cisplatin, 5-fluorouracil (ECF) chemotherapy from September 2007 through March 2008 for a total of six cycles. Following 3 cycles of chemotherapy, CT scans demonstrated an excellent response with decreased esophageal/stomach thickening and decreased lymphadenopathy. The liver lesions and lung nodules had also improved. After completion of six cycles of ECF chemotherapy, further regression of the esophageal/stomach thickening was seen and no liver metastases were identified. After completing his sixth cycle of chemotherapy, the patient developed progressive fatigue and mucositis. Given the excellent response to treatment and the significant toxicities experienced, the patient elected to take a break from chemotherapy following the completion of the sixth cycle of ECF.
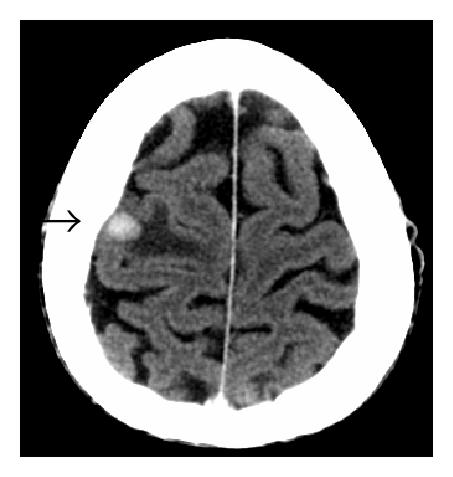
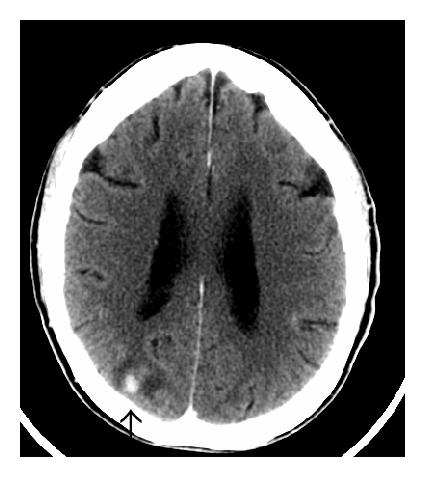
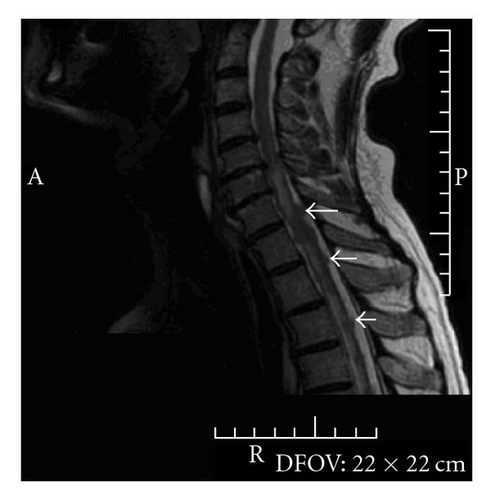
Three months after discontinuing chemotherapy, the patient presented to the emergency department with a two-day history of gait imbalance, headache, and dysarthria. He had no other neurological symptoms. Contrast-enhanced head CT revealed several enhancing lesions at the gray white junction throughout the cerebrum and cerebellum consistent with CNS metastases. There was edema evident around the lesions; the largest lesion measured 11 millimeters in the right frontal lobe (Figure 2). CT scan from the base of the skull to T6 revealed no definite evidence of skeletal metastases. There were equivocal foci of enhancement posteriorly within the upper thoracic spinal canal and/or cord. Concern about meningeal and spinal cord metastases prompted further imaging. Subsequent MRI revealed diffuse drop metastasis around the cervical and thoracic cords, associated with a large area of abnormal signal in the cord, consistent with nodular leptomeningeal disease (Figure 3).
The patient was started on dexamethasone and referred to the Rapid Response Radiotherapy Clinic in Toronto, Canada. This clinic accepts patients on an urgent basis for consideration of palliative radiation therapy. The patient was treated with a course of palliative whole brain radiation treatment, 2000cGy in 5 fractions using 6MV photons. Management of the leptomeningeal carcinomatosis with radiation therapy was deferred because of concerns of toxicity and would be reserved for any area of focal neurological involvement.
After the first treatment of whole brain radiation therapy, the patient developed bowel and bladder dysfunctions and left-sided weakness, requiring admission to hospital. An urgent MRI of the brain and spine revealed the same findings as previously mentioned. As a result of the new neurological deficits, the patient was prescribed radiation to the lumbar-sacral spine, specifically from the top of the L4 vertebrae to the bottom of the S2 vertebrae. A prescription of 2000cGy in 5 fractions was given using 6MV photons, using a parallel opposing technique. Additionally, one fraction of radiotherapy from the top of the C5 vertebrae to the bottom of the T4 vertebrae was also prescribed to treat the localized pain described by the patient. This was treated with a single 800cGy dose using 6MV photons with a direct posterior beam. All three sites were delivered concurrently.
The patient was unable to return home due to persistent symptoms and was transferred to the hospital of his treating oncologist. Given the patient poor performance status and prognosis, there was no further chemotherapy recommended. The patient symptoms which included urinary retention, bowel incontinence, neck pain, and headaches resolved postradiation treatment. He regained control of his bowel function and was able to ambulate with the assistance of a walker. The patient was subsequently transferred to a palliative care facility. Following transfer, the urinary retention returned about 1 week afterwards, requiring permanent catheterization. However, the pain was under fairly good control, and there were no more complaints of headache. However, about 3 weeks after transfer, the patient slowly succumbed to his illness and passed away.
3. Discussion
Leptomeningeal carcinomatosis is a serious metastatic complication of solid tumors. Its related morbidity can be devastating to patients. The median survival despite treatment is between 3–6 months. The occurrence of LC remains to be relatively uncommon; however, there is no precise published estimate of its incidence. It has, generally, been quoted as occurring in approximately 5% of cancer patients [2, 3, 5]. For patients with solid tumors, the incidence of LC can range from 4–15%. Postmortem results of 2375 patients published in 2005 by Memorial Sloan-Kettering Cancer Centre found an incidence of 8%. LC may occur at any stage of a neoplastic disease as either a presenting sign or late complication. LC mainly manifests in primary breast (12–14%), lung (10–26%), and melanoma (17–25%) [1].
While the incidence of LC from a GI primary cancer has been reported to be between 4–14%, it is worth examining this statistic further [1]. A literature review published by Lisenko et al. of the incidence of LC from solid tumors from 1975–2001 found only 7 out of 210 patients to have a stomach cancer primary (3.33%) and 1 out of 210 patients to have LC from a GE junction tumor (0.48%) [4]. Ku at el. have documented an incidence of LC from a colorectal cancer primary to be approximately 1–3% [5], and according to Okumura et al. there have only been 3 reported cases in the literature of LC from esophageal cancer [3, 6]. Since gastroesophageal tumors may be classified as either gastric or esophageal, or else placed in their own separate category, it is useful to review its treatment as a separate category within GI cancers. To our knowledge, there have only been two case reports of the management of LC from a gastroesophageal junction primary cancer [4, 7] (Table 1). All three cases presented with histology of adenocarcinoma, and this was consistent with the most commonly reported histology in the literature [2, 4, 7]. Table 2 compares our case with the most common cerebral, cranial nerve, and spinal signs and symptoms of LC.
| Patient demographics | Case 1 (2001) | Case 2 (2003) | Case 3 (2008) |
|---|---|---|---|
| Age at primary diagnosis (years) | 47 | 50 | 67 |
| Sex | Male | Male | Male |
| Presenting symptoms related to LC | None | Confusion, fluctuating levels of consciousness | Left-sided weakness; urinary incontinence; constipation for 5 days |
| Other sites of metastases | Mediastinal, supraclavicular lymph nodes, lungs, skin, adrenal gland | None | Brain; liver; lung; spinal vertebrae |
| Pathology | Poorly to moderately differentiated adenocarcinoma | Adenocarcinoma | Poorly differentiated adenocarcinoma |
| Headache | None | None | Yes |
| Mental status | Confusion | Confusion | Normal |
| Neurological signs and symptoms | None | None | Left-sided weakness; urinary incontinence; constipation for 5 days |
| CT (head) findings | Normal | Prominent nodular enhancement involving meninges | Head: demonstrate diffuse brain metastases |
| Spine: sclerosis of vertebrae; disc degeneration; and hyperdensity; no comment on LC | |||
| MRI findings | Not ordered | Not ordered | Nodular LC throughout the spinal cord |
| CSF cytology | Positive | Positive | Not ordered |
| Treatment for LC | Palliative care measures | Palliative care measures | Radiation to symptomatic areas; referral for intrathecal chemotherapy after radiotherapy |
| Outcome | No mention of final outcome | Patient level of consciousness continued to deteriorate and passed away 5 months after the initial onset of symptoms | Good symptom control, recurrent urine retention following radiation, passed away 3-4 weeks posttreatment |
| Symptom | Initially (%) | At any time (%) | Sign | Initially (%) | At any time (%) | |
|---|---|---|---|---|---|---|
| Cerebral | *Headache | 38 | 40 | Papilledema | 12 | 12 |
| Mental change | 25 | 30 | Abnormal mental status | 50 | 50 | |
| Nausea/vomiting | 12 | 20 | Seizures | 14 | 15 | |
| Gait difficulty | 46 | 68 | Extensor plantar(s) | 50 | 66 | |
| Cranial nerve | Visual loss | 8 | 12 | Ocular muscle paresis | 30 | 38 |
| Diplopia | 8 | 20 | Trigeminal neuropathy | 12 | 14 | |
| Hearing loss | 6 | 9 | Facial weakness | 25 | 26 | |
| *Dysphagia | 2 | 4 | Hearing loss | 20 | 20 | |
| Spinal | *Pain | 25 | 40 | *Nuchal rigidity | 16 | 17 |
| *Back | 18 | 50 | Reflex absence/decrease | 60 | 76 | |
| Radicular | 12 | 25 | Dermatomal sensory loss | 50 | 50 | |
| Paresthesias | 10 | 42 | *Lower motor neuron weakness | 78 | 78 | |
| *Weakness | 22 | 50 | — | — | — |
- *Indicates signs or symptoms which this case presented with [1].
- **Total may be more than 100% as patients may have more than one sign or symptom.
Our patient presented to our clinic with neurological spinal symptoms and headaches, however, experienced no change in mental status. Since LC from the GE junction is not well documented in the literature, this case was managed with the same rationale as LC from other primary adenocarcinomas. Lumbar puncture, CT, and MRI are used to diagnose leptomeningeal carcinomatosis. CT and MRI of the brain and spine can be used to diagnose leptomeningeal carcinomatosis; however, MRI with T1-weighted gadolinium-enhanced sequencing is the gold standard for delineating changes in the spinal cord. In particular, it can detect abnormal meningeal enhancement that is characteristic of LC. MRI is also 1.5–2 times more specific and sensitive for LC [4]. Particularly, detecting LC from solid tumors on MRI as compared to hematological cancers is easier [1]. Interestingly, in our reported case the CT scan of the spinal cord was reported as normal. However, it was the MRI that detected the nodular LC throughout the spine. The nodular intradural enhancement is documented in the literature as a common finding for LC [1].
Further workup for leptomeningeal carcinomatosis is obtained in cerebral spinal fluid (CSF) from a lumbar puncture test. However, 40–50% of patients will have a false negative result upon initial cytology [1]. The two cases documented in the literature did obtain a positive cytology report, while in our case CSF was not obtained. This is because a diagnosis through cerebrospinal fluid cytologic examination or by gadolinium-enhanced MRI scans of the brain and spine is equally acceptable. As a result, relying on imaging results to make the diagnosis of LC prevented any delay in treatment for this patient.
For LC that arises from solid tumors, the intent of treatment is palliative. Surgery is often reserved for relief of hydrocephalus. Radiotherapy to the whole spine is usually deemed to be very toxic for this patient population. Thus, radiotherapy is often reserved for focal palliative treatment to symptomatic sites or bulky areas of disease as visualized on imaging [1, 8]. Additionally, intrathecal chemotherapy (commonly methotrexate) can also be offered adjuvantly to patients who have low-tumor burden, good performance status, and a relatively good prognosis. However, to date the optimal treatment regime has not been defined and therapeutic outcomes remain poor [5, 9]. Given that the median survival without treatment is 4–6 weeks, a combination of radiation and chemotherapy for such patients has shown an improvement in 50% of cases along with prolonging the median survival [4]. While the two reported cases in the literature were not offered any active treatments, our patient did receive focal radiotherapy due to his relatively good performance status and his eagerness to receive active treatment. Particularly, lumbar-sacral irradiation (top of L4 vertebrae to bottom of sacrum) was prescribed to provide relief of pain and stabilize the cauda equine symptoms the patient was experiencing (left-sided weakness, urinary incontinence, and constipation). Additionally, since the patient was complaining of focal nuchal rigidity and pain, a single 800cGy using 6MV was also prescribed to the neck to help alleviate this particular symptom. This did prove to provide symptom relief for a short duration of time, arguably providing him with an improved quality of life for that time period.
The treatment care plan executed for this particularly rare case of leptomeningeal carcinomatosis from a primary GE junction adenocarcinoma was in keeping with documented standards of care for symptomatic LC from solid tumors. However, to date the optimal treatment regime has not been defined and therapeutic outcomes unfortunately remain poor. A review of LC in gastric carcinomas (which behave similarly to GE junction tumors) suggests that good performance status and use of intrathecal chemotherapy were independent prognostic factors for survival [10]. In this case, the patient was treated with radiotherapy because of good performance status at the time of treatment. However, he was unfit to undergo further chemotherapy, particularly because of ongoing toxicity from previous ECF treatment. When comparing this case to the others in the literature, the ultimate outcomes were very short survivals, as predicted. It is unclear why radiotherapy was not used in these other cases, and it can be postulated that the benefit would have been minimal in the first case [4], given the extensiveness of extraaxial disease. In the second case [7], it seems that the patient poor performance status precluded any form of active treatment. It was also mentioned in that paper that earlier identification of disease may influence the overall poor outcome. It is, therefore, important to utilize the literature on more common sites of LC involvement, including breast and melanoma, in order to individualize patient treatment. If LC is identified early and is localized to the CNS, with good performance status, this may influence the use of radiotherapy and/or chemotherapy as a treatment modality. However, one must determine if the treatment will do more harm than good, especially as time intervals to death remain so short once LC is diagnosed. Therefore, although the goal of treatment for such cases should always be to provide symptom relief if possible, this should also always be balanced against the quality of life that the patient and family wish to accept for the remainder of their illness.
4. Conclusion
Leptomeningeal carcinomatosis remains a devastating complication of malignant disease. It has been rarely discussed in GI tumors, specifically GE junction adenocarcinomas. Treatment is supportive in nature. Because of the scarcity in the literature, one must utilize first principles based on management of LC in more common disease sites. If identified early, and in patients with good performance status, palliative radiation utilizing hypofractionated regimens ranging from 800cGy/1 to 2000 cGy/5, to sites of symptomatic involvement may play a role in improving quality of life for patients, despite the poor life expectancy in this group of unfortunate people.




