Syringocystadenocarcinoma Papilliferum In Situ: Case Report with Immunohistochemical Observations
Abstract
A 23-year-old man was operated for a skin tumor in the right axilla. Histological examination revealed findings consistent with a syringocystadenoma papilliferum. However, most of the papillary structures showed a disorganized proliferation of atypical cells without invasive growth, that is, a syringocystadenocarcinoma papilliferum in situ. Immunohistochemistry disclosed a different staining profile between the basal cells of the papillary structures and those of the columnar and atypical cells which reacted similarly. However, the atypical cells showed a stronger and more extensive expression of S-100 protein and CD56 than the columnar epithelium and a few of them also reacted with CEA. Ki-67 staining highlighted the increased proliferative activity of the atypical cells. The best antibody for depicting the basal cells was p63. In the present case immunohistochemistry was of little help to support the diagnosis of malignant transformation since the differences between the atypical and regular columnar cells were small and of a quantitative character only.
1. Introduction
Syringocystadenoma papilliferum (SP) is a benign skin adnexal tumor of apocrine or eccrine derivation. The tumor is mainly seen in the head and neck region and it may be present at birth [1]. About 40% are associated with nevus sebaceous in the same area, some of them developing a basal cell carcinoma as well [1]. There are only few reports of transformation of an SP into an infiltrating syringocystadenocarcinoma papilliferum (SPCA) [2–10] and even fewer cases showing an SPCA in situ (SPCIS) [4, 5, 11–13]. The latter tumors are characterized by a disorganized proliferation of atypical cells in the papillary structures of an SP but without tumor infiltration of the surrounding dermis. Some of the reported cases with SPCA have revealed findings consistent with SPCIS in the noninfiltrating cystic component indicating that this change probably precedes infiltrative tumor growth [2–5, 7, 14]. There are only few reports of immunohistochemical findings in SP and its malignant forms. In this paper we report a case of SPCIS with application of a broad panel of antibodies relevant for classification of epithelial tumors. In particular, we focus on the different staining profiles between the two cell layers of the ordinary papillary structures and those of the proliferating atypical cells.
2. Case Report and Histopathological Findings
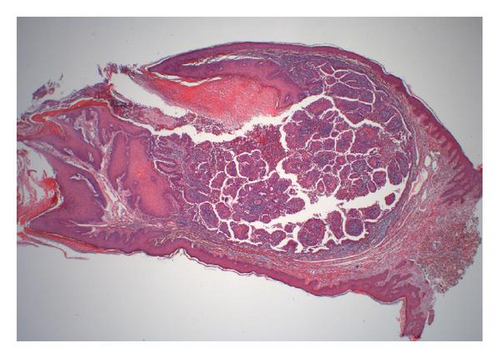
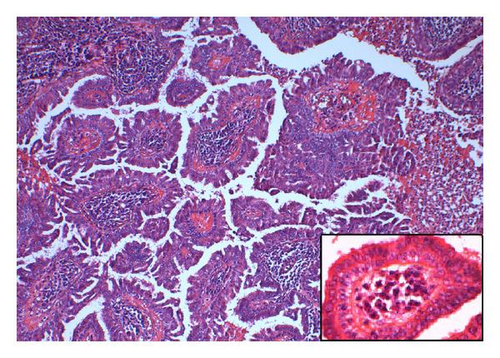
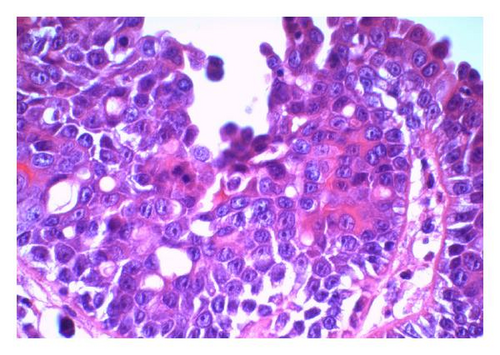
The reported patient is a man born in 1984 with a negative family history regarding inheritable and skin diseases. In 1994 a granulation polyp was removed from his right external ear. Since childhood he has had a skin tumor in the right axilla which increased somewhat in size and became irritating during puberty. The lesion was removed in October 2008. The specimen was described as a pale skin nodule measuring 11 × 8 mm with a central pore. Microscopical examination revealed a well-demarcated cystic tumor opening to the skin surface (Figure 1(a)). In the superficial part the cyst was lined by a hyperkeratotic squamous epithelium, in the deeper part by one layer of cuboidal or columnar cells with multiple papillary projections into the cyst lumen. Some of these papillae showed the typical picture of a benign syringocystadenoma papilliferum with a two-layered epithelium resting upon a connective tissue core heavily infiltrated with plasma cells. The inner basal cell layer was cuboidal, the outer columnar (Figure 1(b), inset). However, most of the papillae displayed a disorganized proliferation of cells with eosinophilic cytoplasm and enlarged nuclei and nucleoli as well as scattered mitoses (Figures 1(b) and 1(c)). The surrounding dermis showed neither tumor infiltration nor changes of a nevus sebaceous. The resection margins were free.
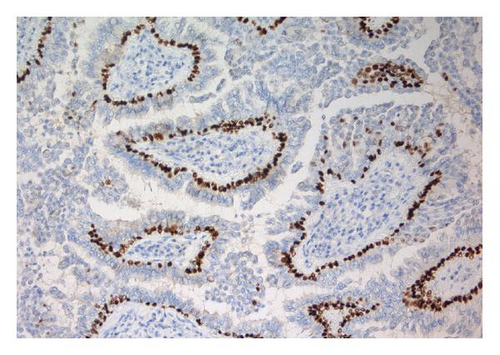
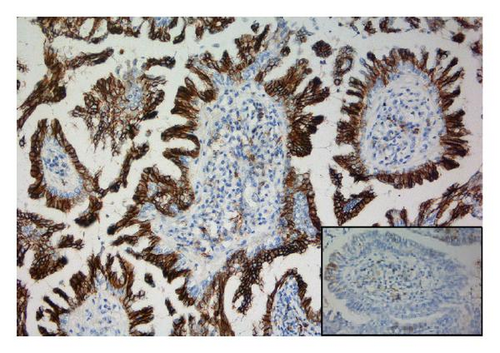
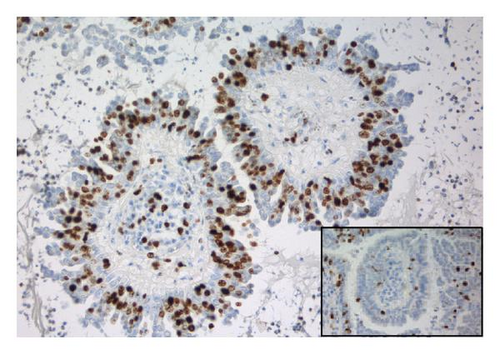
The tumor was immunohistochemically stained with a panel of antibodies to demonstrate differences in staining properties between the ordinary two-layered papillary epithelium and the atypical cells. The antibodies applied and the staining characteristics of the various cell types are displayed in Table 1. As can be seen, the basal cells had a staining profile different from the columnar and atypical cells which, on the other hand, showed a similar reaction. Thus, the basal cells stained positively for smooth muscle actin and p63 (Figure 1(d)); negatively for Ber-Ep4, CK8, EMA, and CD56 in contrast to the columnar and atypical cells. Furthermore, the basal cells gave a stronger staining reaction for CK5/6 than the other two cell types. Generally, the atypical cells revealed a stronger and more extensive staining for CD56 (Figure 1(e)) and S-100 protein than the columnar cells and a few of them also expressed CEA. Ki-67 staining confirmed the high proliferation activity of the atypical cells (≈50%) in contrast to the much lower activity in the basal and columnar cells (Figure 1(f)).
| Antibody | Source | Typical two-layered epithelium | Atypical cells | |
|---|---|---|---|---|
| Basal cells | Columnar cells | |||
| AE1/AE3 | Dako | +++ | +++ | +++ |
| BCCK | Dako | +++ | +++ | +++ |
| Ber-Ep4 | Dako | − | +/++ | +/++ |
| CK5/6 | Dako | +++ | ++ | ++ |
| CK7 | Dako | + | ++ | ++ |
| CK8 | Zymed | − | +++ | +++ |
| EMA | Dako | − | ++ | ++ |
| P63 | Dako | +++ | − | − |
| CEA | Dako | − | − | + |
| CD56 | Nordic biosite | − | + | +++ |
| Chr.A | Dako | − | − | − |
| SYN | Novocastra | − | − | − |
| S-100 | Dako | − | −/+ | +/++ |
| TTF-1 | Novocastra | − | − | − |
| SMA | Dako | ++ | − | − |
| VIM | Dako | ++ | + | + |
| ER | Dako | − | − | − |
| PGR | Dako | − | − | − |
| Ki-67 | Dako | ≈10% | ≈3% | ≈50% |
- +++ = strong staining reaction, ++ = moderate staining reaction, + = weak or focal staining reaction, BCCK = basal cell cytokeratin, EMA = epithelial membrane antigen, CEA = carcinoembryonic antigen, Chr.A = chromogranin A, SYN = synaptophysin, S-100 = S-100 protein, TTF-1 = thyroid transcription factor-1, SMA = smooth muscle actin, VIM = vimentin, ER = estrogen receptor, PGR = progesterone receptor.
3. Discussion
The reported tumor fulfils all criteria for a syringocystadenoma papilliferum (SP). However, it also revealed an intracystic proliferation of atypical cells without infiltration of the dermis in conformity with lesions previously reported as “syringocystadenocarcinoma papilliferum in situ” (SPCIS) [5, 12, 13]. There is probably a continuum from a benign SP via various grades of atypia to an infiltrating syringocystadenocarcinoma papilliferum (SPCA), but criteria for grading of these rare tumors have to our knowledge not been determined. Whereas an SPCA may metastasize to regional lymph nodes [2, 14], this is not the case for SPCIS which is cured by total surgical resection.
The tumor of our patient was located at the axilla which apparently is a rare location for SPs. In a survey of 145 cases with benign SPs, Mammino and Vidmar [1] found no tumor at this site whereas one previously reported case of SPCIS had an axillary location like ours [4]. However, the number of such lesions is too small to suggest that the axilla represents a predilection site for malignant transformation of SPs.
There are only few reports on immunohistochemical findings in SPs and their malignant forms, and little attention has been paid to demonstrate staining differences between the various cell types. Benign SPs have been reported to be positive for CEA [11, 12, 15, 16], CK AE1 [12], and EMA [11], but variable for S-100 protein [11, 12]. Ansai et al. [8] employed a large panel of antibodies in a case of SPCA comparing the staining properties of the upper noninfiltrating and the deeper infiltrating parts of the tumor. They found that both components reacted positively for CEA, S-100 protein and several cytokeratins including CK8. However, only the infiltrating part stained positively for EMA. Thus, their findings are in discrepancy to ours. In cases of SPCIS, Arai et al. [12] found positive staining for CK AE1, variable for CEA, and negative for S-100 protein whereas Ishida-Yamamoto et al. case [11] was positive for EMA, partly positive for S-100 protein and negative for CEA in accordance with our findings. However, classification of the latter tumor as an SPCIS must be questioned since the authors state that “double-layered cuboidal and columnar epithelia typical of syringocystadenoma papilliferum were not seen in this tumor.” Furthermore, the somewhat divergent immunohistochemical findings between the various studies may partly be explained by the use of different staining procedures and different antibody clones.
In conclusion, the immunohistochemical staining panel applied in the present study was of little help to support the diagnosis of malignant transformation in our case of SP since the differences in staining properties between the ordinary columnar and the atypical cells were of a quantitative character only. Further studies are necessary before this matter can be definitely settled. A Ki-67 stain will, however, highlight the increased proliferation activity of the atypical cells. Furthermore, p63 proved to be the best antibody for visualizing the basal cells. In case of an SPCIS, we will recommend to examine sections cut at several levels of the blocks to rule out an infiltrating tumor component.




