Optimization of a Filter-Lysis Protocol to Purify Rat Testicular Homogenates for Automated Spermatid Counting
The research was funded by Superfund Research Program (NIH/NIEHS) grant P42ES013660 and T32ES007272–17 for “Training in Environmental Pathology” (S.P.).
Abstract
Abstract: Quantifying testicular homogenization-resistant spermatid heads (HRSH) is a powerful indicator of spermatogenesis. These counts have traditionally been performed manually using a hemocytometer, but this method can be time consuming and biased. We aimed to develop a protocol to reduce debris for the application of automated counting, which would allow for efficient and unbiased quantification of rat HRSH. We developed a filter-lysis protocol that effectively removes debris from rat testicular homogenates. After filtering and lysing the homogenates, we found no statistical differences between manual (classic and filter-lysis) and automated (filter-lysis) counts using 1-way analysis of variance with Bonferroni's multiple comparison test. In addition, Pearson's correlation coefficients were calculated to compare the counting methods, and there was a strong correlation between the classic manual counts and the filter-lysis manual (r = 0.85, P = .002) and the filter-lysis automated (r = 0.89, P = .0005) counts. We also tested the utility of the automated method in a low-dose exposure model known to decrease HRSH. Adult Fischer 344 rats exposed to 0.33% 2,5-hexanedione in the drinking water for 12 weeks demonstrated decreased body (P = .02) and testes (P = .002) weights. In addition, there was a significant reduction in the number of HRSH per testis (P = .002) when compared to controls. A filterlysis protocol was optimized to purify rat testicular homogenates for automated HRSH counts. Automated counting systems yield unbiased data and can be applied to detect changes in the testis after low-dose toxicant exposure.
Quantification of testicular homogenization-resistant spermatid heads (HRSH) can be used to estimate daily sperm production rates and is a commonly used method in studies of toxicant-induced testicular injury or dysfunction (Blazak et al, 1993; Ashby et al, 1997; Omura et al, 2000; Wade et al, 2006; Assinder et al, 2007). The use of a hemocytometer in this method necessitates that multiple counts be recorded as a result of the high levels of variation and error inherent in the technique. It has been previously reported (Freund and Carol, 1964; Zrimsek, 2011) that mean differences of greater than 20% are common in manual sperm counts, even when the counts are performed by the same individual, highlighting the need for a reliable automated protocol.
Previous work describes the use of the Coulter Counter for automated semen analysis, but cellular contaminants within the semen tend to inflate the counts (Evenson et al, 1993). In addition, the computer-assisted sperm analyzer (CASA) technology has been applied to enumerate rodent testicular spermatids; however, the CellSoft system also overestimates spermatid number by misidentifying testicular debris as spermatid heads (Working and Hurtt, 1987). Through the addition of filtration and somatic cell lysis steps and the use of an automated counter that can identify trypan blue-stained cells, the classic protocol can be modified for automatic quantification of testicular spermatid heads.
Here we describe a novel update to the classic protocol for counting testicular HRSH to eliminate cellular debris and purify spermatid heads. Using the pure lysates in an automated counting system produces efficient, reliable, and unbiased results that can be applied to detect low-dose toxicant-induced testicular injury.
Materials and Methods
Chemicals
The 2,5-hexanedione (HD; CAS 110-13-4) used in the application study was purchased from Sigma Aldrich (St Louis, Missouri).
Animals
Adult male Fischer 344 rats weighing 175–225 g (Charles River Laboratories, Wilmington, Massachusetts) were maintained in a temperature- and humidity-controlled vivarium with a 12-hour alternating light-dark cycle. All rats were housed in community cages with free access to water and Purina Rodent Chow 5001 (Farmer's Exchange, Framingham, Massachusetts). The Brown University Institutional Animal Care and Use Committee approved all experimental animal protocols in compliance with National Institutes of Health guidelines.
Preparation of Testes and Homogenization Procedures
Body weights were recorded at the time of necropsy and the testes were removed and weighed. The right testis was detunicated, and one-third of the parenchyma was weighed, flash-frozen, and stored at 280°C for later evaluation. At the time of processing, each sample was thawed on ice and homogenized using a Brinkmann Kinematica Homogenizer Polytron PT 10/35 (Brinkmann Instruments, Westbury, New York) in saline-merthiolate-triton (SMT) buffer, following a previously published protocol (Blazak et al, 1993). Briefly, testis samples were homogenized in 25 mL SMT at maximum speed (27 000 rpm) for 2 minutes and used immediately for counting.
Additional Filter and Lysis Protocol
After the homogenization procedure, the testis homogenates were filtered through 10-μm nylon mesh (Dynamic Aqua-Supply Ltd, Surrey, Canada). The filtered homogenates were then combined in a 1:1 ratio with an optimized somatic cell lysis buffer (0.3% sodium dodecyl sulfate and 1% Triton-X 100) derived from a protocol used for lysing somatic cell contamination in human semen (Goodrich et al, 2007). Each sample containing the homogenate and lysis buffer mixture was incubated on wet ice for 5 minutes prior to counting. The lysis of debris was confirmed using phase contrast microscopy, and photographs of trypan blue (Invitrogen, Eugene, Oregon)-stained homogenates and lysates were taken using the Nikon Diaphot microscope (×40; Melville, New York) and a Nikon D40 digital camera.
Manual Testicular Spermatid Head Counts
Testis homogenates or lysates were combined with 0.2% trypan blue in a 1:1 ratio and 10 μL was loaded into both chambers of 2 hemocytometers, resulting in 4 counts per sample that were averaged together to obtain 1 value. The hemocytometers were placed in a humidified chamber for 5 minutes prior to counting, and the samples were counted according to previously published methods (Blazak et al, 1993).
Automated Testicular Spermatid Head Counts
Testis homogenates or lysates were combined with 0.2% trypan blue in a 1:1 ratio and 10 μL was loaded onto both sides of a cell-counting chamber slide (Invitrogen), resulting in 2 counts per sample that were averaged together to obtain 1 value. HRSH were counted using the Countess Automated Cell Counter (Invitrogen), following manufacturer guidelines. The gating parameters “sensitivity,” “minimum size,” “maximum size,” and “circularity” were optimized for rat testicular spermatid heads and were determined to be 5, 5 μm, 10 μm, and 30%, respectively.
Optimization Experiment
Control testis samples (n = 10) were homogenized. Each homogenate was divided and one-half was left “as is,” while the other half was subjected to the additional filter-lysis steps described earlier. Both preparations of each sample were counted manually by 2 individuals (S.P. and L.A.) and automatically using the automated counter. Coefficients of variation were calculated for each approach ([standard deviation (SD)/mean] × 100), and the manual counts of S.P. and L.A. were averaged to obtain a single value to reduce variability. This resulted in 4 counts for each sample: 1) classic manual, 2) classic automated, 3) filter-lysis manual, and 4) filter-lysis automated. One-way analysis of variance (ANOVA) with the Bonferroni's multiple comparison test was applied to determine if there was a statistical difference in the average number of HRSH per testis among the 4 counting approaches. Correlation between the groups was determined using Pearson's correlation coefficients. Results from both tests were considered significant if P was less than .05. A scatter diagram of the number of HRSH obtained using the classic manual method vs the filter-lysis automated method was fitted with a Deming regression line. In addition, a Bland-Altman plot was generated to determine the agreement between the classic manual and filter-lysis automated approaches (Zrimsek, 2011).
Application Experiment
To determine if the adapted protocol was capable of detecting changes in the testes after low-dose toxicant exposure, rats were exposed to either water (control, n = 8) or 0.33% HD in the drinking water (n = 10) for 12 weeks. We chose this dose of HD because it induces minimal but detectable testicular injury in Fischer 344 rats (Moffit et al, 2007) and because pilot experiments in our laboratory indicated that HD decreased the number of HRSH in the testis when counted manually (Pacheco and Boekelheide, unpublished). The testes were homogenized and the homogenates were filtered, lysed, and counted automatically as described earlier. The total numbers of HRSH in the testis of the control and HD rats were calculated and compared using a 2-tailed Student's t test, and the results were considered significant if P was less than .05.
Statistical Analyses
All statistical analyses were performed using the Prism 5 software (GraphPad Software, La Jolla, California).
Results
Optimization Experiments
The Additional Filter-Lysis Protocol Removes Testicular Debris—
A comparison of the samples prepared using the classic protocol (Figure 1A and B) and those prepared following an additional filter-lysis (Figure 1C and D) clearly demonstrated the efficacy of the updated method to remove the majority of cellular contaminants that would inflate automated counting methods.

Filter-lysis optimization. Testis homogenates were prepared using each of the 2 methods described (A, B, classic; C, D, filter-lysis) and stained with trypan blue for visualization. White arrowheads indicate rat spermatid heads, while white arrows indicate cellular debris. Photomicrographs were taken at ×40, and the scale bar = 20 μm.
Manual Counting Has High Interobserver Variability Compared to Automated Counts—
Correlation coefficients were calculated among the 4 sets of manual counts and there was poor correlation between the 2 individuals doing the counting (Table 1). The correlation coefficient calculated comparing “Classic Manual #1” vs “Filter-Lysis Manual #2” was low (0.425). Likewise, the correlation coefficient calculated comparing “Filter-Lysis Manual #2” and “Filter-Lysis Manual #1” was also low (0.438). These findings indicate that the variability in the counts was mainly due to the variation between the 2 individuals counting and not to the methods used to prepare the samples. The coefficients of variation (CV) for the mean manual counts (“Classic Manual #1,” “Classic Manual #2,” “Filter-Lysis Manual #1,” and “Filter-Lysis Manual #2”) for all 10 samples were 32.2%, 28.6%, 26.4%, and 33.7%, respectively. However, the “Filter-Lysis Automated” had less variation (CV = 25.5%). To reduce variability we averaged the 2 individual counts for the classic manual and filter-lysis manual approaches, and the CVs were reduced to 28.72% and 25.43%, respectively.
| Classic Manual #1 | Classic Manual #2 | Filter-Lysis Manual #1 | Filter-Lysis Manual #2 | |
|---|---|---|---|---|
| Classic Manual #1 | … | 0.010b | 0.0005b | 0.220b |
| Classic Manual #2 | 0.767a | … | 0.0215b | 0.019b |
| Filter-Lysis Manual #1 | 0.891a | 0.710a | … | 0.206b |
| Filter-Lysis Manual #2 | 0.425a | 0.720a | 0.438a | … |
- a Correlation coefficients.
- b P-values. Data were generated comparing the average number of homogenization-resistant spermatids per testis.
There Is High Correlation Between the Classic Manual and the Filter-Lysis Automated Approaches to Counting Testicular Spermatid Heads—
One-way ANOVA determined that there were no significant differences between the observer-averaged manual (both classic and filter-lysis) and the filter-lysis automated methods. However, there was a significant increase in the total number of HRSH counted using the classic automated method compared to the other 3 protocols (Figure 2A). These results demonstrate that the additional filter-lysis steps did not alter overall testicular HRSH numbers and that the automated cell counter produced accurate HRSH counts. Furthermore, Pearson's correlation coefficients were calculated to compare the similarity among the 4 preparation techniques. The classic manual protocol was highly correlated with the filter-lysis manual (r = 0.85, P = .002) and automated protocols (r = 0.89, P = .0005) but not with the classic automated counts (r = 0.65, P = .04; Table 2). The strongest correlation occurred when the filter-lysis manual and automated counts were compared (r = 0.90, P = .0004; Table 2). Scatter plots with a fitted Deming regression line (Figure 2B) and Bland-Altman plots (Figure 2C) were used to get the best overview of comparative data generated using the classic manual and filter-lysis automated methods. Deming regression was applied to describe the relationship between the 2 methods that were both generated with error, taking into account the analytical imprecision of each method. This regression analysis yielded a best-fit line with a slope and Y-intercept (when X = 0) of 0.84 ± 0.15 (95% confidence interval [CI], 0.50–1.18) and 0.20 ± 0.25 (95% CI, 20.37–0.78), respectively (P = .005). The Bland-Altman plot calculated a bias of 0.06 when comparing the classic manual and the filter-lysis automated approaches (Figure 2C, dashed line) with a SD of 0.21. The SD of the differences between the 2 assay methods was used to calculate the limits of agreement according to the following formula: bias ± 1.96 ×SD. Our 95% limits of agreement are between 20.35 and 0.47 (Figure 2C, solid lines). As expected, the 2 methods give very similar results on average, and the level of agreement among the samples is good, with 90% of the data falling within the limits.
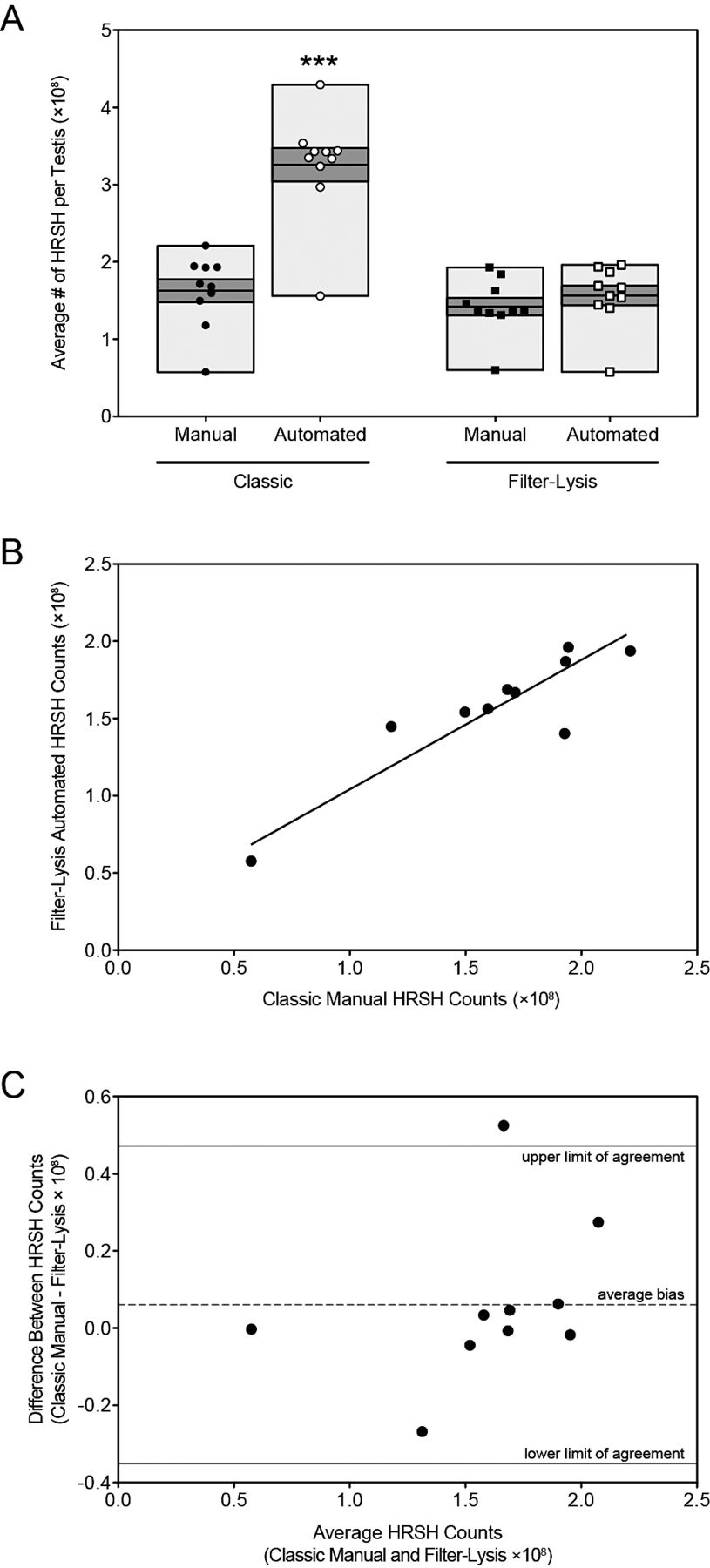
Optimization experiment: Number of homogenization-resistant spermatid heads (HRSH) per testis. (A) The average number of HRSH per testis were calculated for 10 rats using each protocol and values were graphed as follows: classic manual (black circle); classic automated (open circle); filter-lysis manual (black square); and filter-lysis automated (open square). The light gray boxes represent the range of values and the dark gray boxes contain the standard error of the mean, with the mean as the line in the middle of the dark gray box. Statistical differences between the average number of spermatids per testis among groups was determined using 1-way analysis of variance with the Bonferroni's multiple comparisons test; *** P < .001 relative to all other methods. (B) Scatter diagram of the number of HRSH obtained using the classic manual method vs the filter-lysis automated method, with Deming regression line fitted (solid line). (C)Bland-Altman absolute bias plot of the number of HRSH obtained using the classic manual method vs the filter-lysis automated method showing the average bias (dashed line) and limits of agreement (solid lines).
| Classic Manual | Classic Automated | Filter-Lysis Manual | Filter-Lysis Automated | |
|---|---|---|---|---|
| Classic manual | … | 0.044b | 0.002b | 0.0005b |
| Classic automated | 0.645a | … | 0.007b | 0.0066b |
| Filter-lysis manual | 0.846a | 0.790a | … | 0.0004b |
| Filter-lysis automated | 0.894a | 0.789a | 0.897a | … |
- a Correlation coefficients.
- b P-values. Data were generated comparing the average number of homogenization-resistant spermatids per testis.
Application Experiment
Exposure to 0.33% HD Decreases Body and Testes Weights—
Rats were exposed to either water (control) or 0.33% HD in the drinking water for 12 weeks, and all rats were weighed at the time of necropsy (Table 3). Rats exposed to HD displayed significantly decreased body weights when compared to control rats (P = .02). Testis weights were also recorded during the necropsy, and rats exposed to HD had significantly decreased testis weights compared to controls (P = .002; Table 3).
| Treatment | Body Weights, g | Testis Weights, g |
|---|---|---|
| Control (n = 8) | 315.8 ± 6.1 | 1.58 ± 0.02 |
| 2,5-hexanedione (n = 10) | 286.9 ± 8.9* | 1.48 ± 0.02** |
Exposure to HD Decreases the Average Number of Homogenization-Resistant Spermatids in the Testis—
Following optimization of the technique, the utility of the automated cell counter was tested in the context of toxicant exposure that we had previously seen reduce the number of HRSH in the testis when counted manually (Pacheco and Boekelheide, unpublished). Testes from HD and control rats were prepared with the additional filter-lysis steps and counted using the automated cell counter. The total number of HRSH per testis was calculated for each sample, and consistent with the significant decrease in testis weights, HD rats displayed significantly decreased HRSH counts (P = .002) relative to control rats (Figure 3).
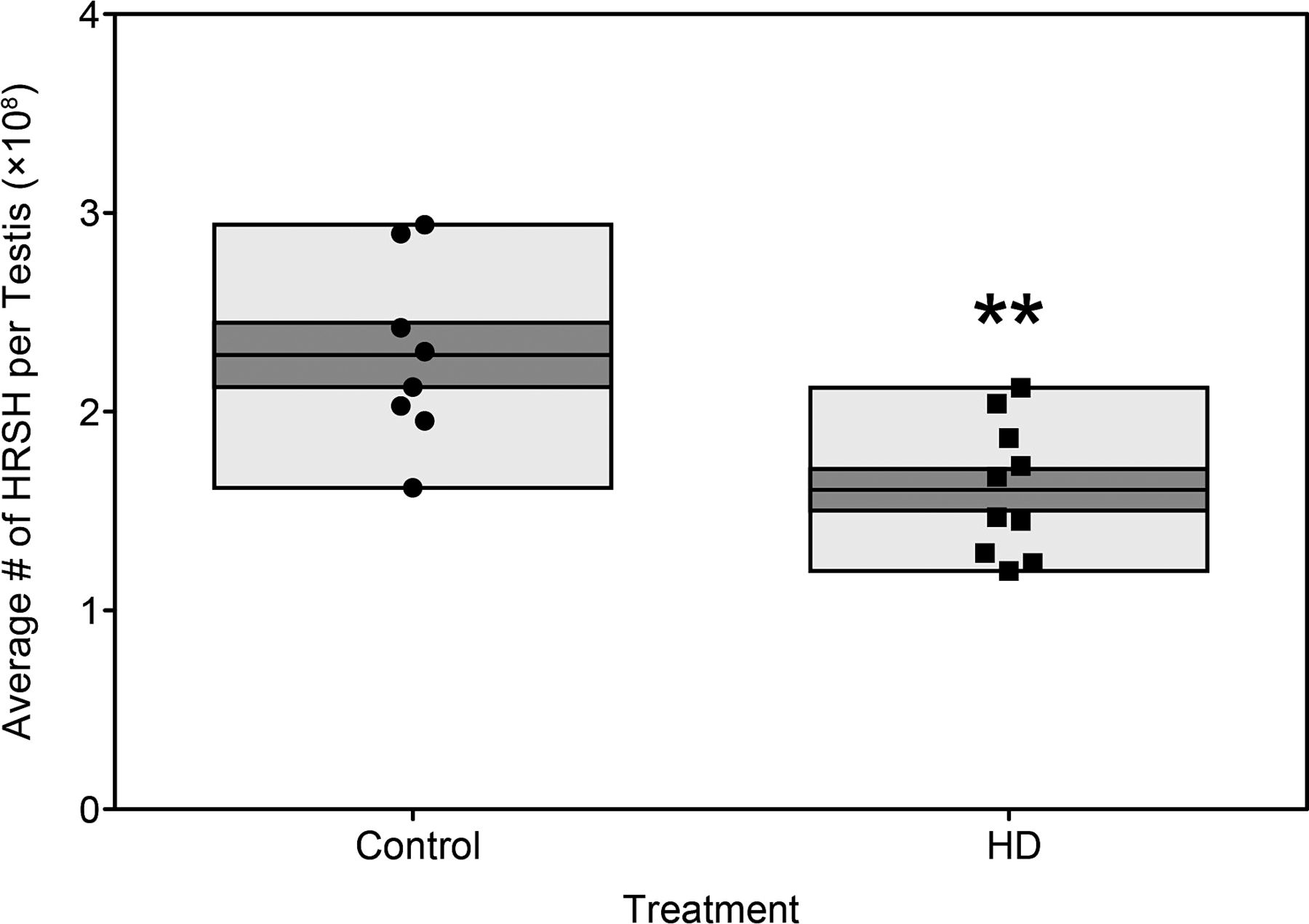
Application experiment: Number of homogenization-resistant spermatid heads (HRSH) per testis after toxicant exposure. Testicular spermatid head counts were obtained from 2,5-hexanedione (HD; n = 10; black square) and control rats (n = 8; black circle) using the filter-lysis automated technique and graphed as the total number of HRSH per testis. The light gray boxes represent the range of values and the dark gray boxes contain the standard error of the mean, with the mean as the line in the middle of the dark gray box. ** P = .002 relative to control.
Discussion
The most recent World Health Organization (2010) laboratory manual for the examination and processing of human semen emphasizes the necessity for automated systems for analyzing sperm because they have the potential for greater objectivity, precision, and reproducibility than do manual systems. The CASA system evolved because of the need to obtain objective and bias-free data when analyzing sperm motility, but this system has been incorporated into other aspects of the semen analysis (Amann and Katz, 2004). Unfortunately, not all laboratories with interests in male fertility have access to a CASA system, and these laboratories have to rely on manual methods to examine sperm parameters. Here we present a novel update to the classic protocol for quantification of rat testicular HRSH. This update utilizes a filtration step followed by somatic cell lysis of the homogenate in order to rid the sample of debris while preserving spermatid heads. These additional steps allow for the use of an automated cell counter, rather than a hemocytometer, to quantify HRSH.
Optimization of the protocol was performed using normal rat testes, and 4 sample preparation/counting methods were compared to demonstrate the effectiveness and reproducibility of the newly modified protocol. We were able to show that automated counts of classically prepared samples produced an artificially high HRSH count due to inclusion of cellular debris, but with the addition of filtration and lysis steps, the automated counts were no different from those obtained using a hemocytometer. In fact, we saw the strongest correlation between the filter-lysis manual and automated protocols. These results indicate that the additional filter-lysis steps are beneficial for both manual and automated counting, and this may be because the lysates are cleaner, which reduces the misidentification of debris as HRSH or prevents debris from masking the HRSH.
Additionally, the automated method can be applied to detect differences in testicular HRSH counts following low-dose exposure to a known testicular toxicant. Previous work in our lab has documented the deleterious effects of HD on the rat testis, including reductions in testis weights and histological alterations to the seminiferous tubules, including germ cell sloughing, Sertoli cell vacuolization, and retained spermatid heads (Moffit et al, 2007). These manifestations of testicular injury can reduce the number of spermatids in the testis, which we have observed in HD-exposed testes after performing manual HRSH counts (Pacheco and Boekelheide, unpublished). This is consistent with the results presented here that show decreased body and testis weights and decreased testicular HRSH with HD exposure, thereby verifying the utility of the automated protocol in animal studies of testicular injury.
This applicability of the pure testicular lysates to an automated basic cell culture counting platform represents a significant improvement to the established manual protocol for counting testicular HRSH, a procedure that provides an important measure of spermatogenic capacity in animal models. Manual counts using the hemocytometer are variable, time consuming, and susceptible to bias. We have developed a newly modified protocol that is effective and sensitive, allowing testicular HRSH counts to be obtained more efficiently and reliably.




