Identification of Vibrio harveyi Isolated from Diseased Asian Seabass Lates calcarifer by Use of 16S Ribosomal DNA Sequencing
Abstract
The grow out of Asian seabass Lates calcarifer in marine net-cages is a popular aquaculture activity in Malaysia. Production of this species is greatly affected by the occurrence of vibriosis, which causes heavy mortality. Generally, young fish are more susceptible; they exhibit anorexia and skin darkening, followed by heavy mortality. The acutely affected older fish may also exhibit bloody lesions around the anus and the base of the fins. Twenty-one bacterial isolates obtained from internal organs (kidney, heart, spleen and liver) of the affected specimens were subjected to phenotypic characterization, testing for antibiotic susceptibility, and 16S ribosomal DNA sequencing. The sequencing result showed that all of the bacterial isolates belonged to Vibrio harveyi. The phenotypic characterization, however, identified 4 of the bacterial isolates as V. harveyi, 16 as V. parahaemolyticus, and 1 as V. alginolyticus. These findings suggest that biochemical features alone cannot be reliably used to identify bacterial pathogens, including V. harveyi, in aquaculture. Antibiotic susceptibility assays showed that some antibiotics, including oxytetracycline, nitrofurantoin, furazolidone, streptomycin, sulfamethoxazole, chloramphenicol, nalidixic acid, and oxolinic acid were effective against V. harveyi. Considering the side effects of these antibiotics, however, their use is not recommended in the aquaculture of Asian seabass.
The Asian seabass Lates calcarifer is one of the most important and valuable fish species cultured in open net-cages in Malaysia. However, aquaculture of the fish is hampered by frequent outbreaks of vibriosis. In Asian seabass, the disease is characterized by skin darkening, lethargy, anorexia, reddish ulcerations on the body, and the presence of abdominal fluid. Although many species of Vibrio are reported to result in vibriosis in marine fish (Musa et al. 2003), Tendencia (2002) found that Vibrio harveyi is the most common cause of vibriosis in Asian seabass cultured in open net-cages in the Philippines. In Malaysia, vibriosis was reported to have caused a total loss of US$7.4 million in 1990 (Bondad-Reantaso et al. 2005). Despite disease management programs, the disease continues to affect the net-cage-cultured Asian seabass in the country. The affected fish are characterized by deep skin lesions, hemorrhagic areas on the fin base and anus, tail and fin rot, lack of appetite, swollen intestine, and opaque eyes. The disease spreads rapidly among fish stocked in the same cage and causes high mortality, especially in weak and small-sized fish stocked at high density in poorly managed net-cages. Vibrio harveyi is a major bacterial pathogen capable of infecting a wide range of aquatic animals, including penaeid shrimps (Robertson et al. 1998), bivalves (Pass et al. 1987), cephalopods (Ramesh and Venugopalan 1989), teleosts (Tendencia 2002), and elasmobranchs (Grimes et al. 1985). Accordingly, V. harveyi will doubtless be isolated from many species of marine fish and shellfish. The great diversity of phenotypic features of V. harveyi, however, may cause difficulty in the identification of the pathogen (West et al. 1986). In the present study, bacterial isolates from diseased Asian seabass were subjected to phenotypic characterization, tests for antibiotic susceptibility, and molecular identification by 16S ribosomal RNA (rRNA) gene sequencing.
Methods
Bacterial isolation and preservation
Bacteria were isolated from internal organs (liver, spleen, and kidney) of diseased Asian seabass. Isolation was performed on freshly collected moribund fish. Briefly, the diseased fish were aseptically dissected by using sterile surgical tools to expose internal organs, including spleen, kidney, heart, and liver. A sterile cotton swab was aseptically touched on each organ, streaked on thiosulfate–citrate–bile-salts–sucrose agar (Difco, Becton Dickinson, Sparks, Maryland) plates, and incubated at 28°C for 48 h. Subsequently, the bacteria were serially subcultured on tryptic soy agar (TSA; Difco) plates supplemented with 1.5% NaCl (weight/volume [w/v]) to obtain single, pure colonies. Finally, 21 bacterial isolates were inoculated into tryptic soy broth (TSB; Difco) supplemented with 1.5% NaCl and preserved in 25% glycerol according to the method described by Floodgate and Hayes (1961).
Phenotypic characterization
The 21 bacterial isolates were examined for their biochemical properties according to the method described by Alsina and Blanch (1994). Tests included Gram staining, motility test, oxidative-fermentative test, catalase (enzyme number 1.11.1.6; IUBMB 1992) test, oxidase (1.9.3.1) test, acid production from sugars, citrate utilization, urease (3.5.1.5) test, methyl red reaction, Voges–Proskauer, indole production, phenylalanine test, β-galactosidase (3.2.1.23) test, lysine decarboxylase (4.1.1.18), and arginine dihydrolase (3.5.3.6). The bacterial isolates were also grown at three different temperatures (25, 28, and 30°C) in three concentrations of NaCl: 0, 3, and 5% (w/v).
Antibiotic susceptibility test
Of the 21 bacterial isolates obtained, only 18 were selected for antibiotic susceptibility testing. Three bacterial isolates—VHJR9, VHJR12, and VHJR16—were excluded because of contamination. The 18 isolates tested were first grown on TSA plates for 24 h at 28°C and then were suspended in sterile phosphate-buffered saline (PBS; pH 7.2) and diluted to a turbidity equivalent to a MacFarland Number-0.5 standard solution. After 0.1 mL of bacterial suspension was spread onto Mueller–Hinton agar (Difco) plates, antibiotic discs were added according to the method described by Dalsgaard et al. (1999). The antibiotic disks (Oxoid, Hampshire, UK) used in this assay included ampicillin (10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), sulfamethoxazole (100 μg), furazolidone (100 μg), kanamycin (30 μg), nalidixic acid (3 μg), neomycin (10 μg), nitrofurantoin (300 μg), novobiocin (5 μg), oxolinic acid (2 μg), oxytetracycline (30 μg), penicillin G (10 units), streptomycin (25 μg), sulfonamide (300 μg), tetracycline (10 μg), and vancomycin (30 μg). The plates were incubated at 28°C for 24 h, after which inhibition zones of the bacteria by the antibiotics were scored according to the method described by Barry et al. (1979).
Isolation of DNA
Genomic DNA from the bacterial isolates was obtained by using proteinase K (3.4.21.64) extraction as described by Farto et al. (2003). Bacteria were inoculated into 5 mL of sterile TSB and incubated at 28°C overnight as described by Kim and Jeong (2001). Subsequently, 1.0 mL of the bacterial suspension was transferred into a microcentrifuge tube and centrifuged at 9,000 revolutions/min for 10 min at 4°C. After centrifugation, the supernatant was discarded and the bacterial pellet was resuspended in 600 μL of 1× Tris–EDTA buffer, 30 μL of 10% sodium dodecyl sulfate (w/v), and 3 μL of 20-mg/mL proteinase K (Sigma, St. Louis, Missouri). The mixture was incubated at 37°C for 1 h before adding 100 μL of cetyl trimethyl ammonium bromide–NaCl solution and was further incubated at 65°C for 10 min. Subsequently, protein was extracted by adding an equal volume of chloroform : isoamyl alcohol (24:1) and was precipitated by centrifugation at 9,000 revolutions/min for 10 min at 4°C. A 600-μL volume of the aqueous phase was carefully transferred to a fresh microcentrifuge tube with an equal volume of phenol : chloroform : isoamyl alcohol (25:24:1) and centrifuged at 8,000 revolutions/min for 10 min at 4°C. Thereafter, a 500-μL volume of aqueous phase was transferred to a new microcentrifuge tube, mixed with an equal volume of chilled isopropanol, and centrifuged at 13,000 revolutions/min for 15 min at 4°C. The DNA pellet was washed with 1.0 mL of chilled 70% ethanol. The DNA pellet was briefly air-dried, dissolved into 50 μL of 1 × Tris–EDTA buffer, and stored at −20°C until use. The DNA concentration was determined by using a GeneQuant RNA/DNA Calculator (GE Healthcare).
Polymerase chain reaction amplification, cloning, and sequencing
The 16S rRNA gene underwent polymerase chain reaction (PCR) amplification against the total genomic DNA extracted from all 21 bacterial isolates and American Type Culture Collection (ATCC) reference bacteria (V. harveyi ATCC 35084, V. anguillarum ATCC 19264, and Aeromonas salmonicida subsp. salmonicida ATCC 33658); the primers used were Ecoli9 (5′-gagtttgatcctggctcag-3′) and Loop27rc (5′-gactaccagggtatctaatc-3′) as described by Sfanos et al. (2005). The PCR amplification was conducted in a 25-μL total reaction volume (12.5 μL of PCR Master Mix [Promega, Madison, Wisconsin]; 1.0 μL of each 10-μM primer; 1.0 μL of DNA template [0.307 μg/μL]; and 9.5 μL of nuclease-free water). The amplification was carried out with 1 cycle at 95°C for 3 min; 30 cycles at 95°C for 1 min, 58°C for 1 min, and 72°C for 1 min; and 1 cycle at 72°C for 5 min. The PCR products were purified with an AccuPrep PCR purification kit (Bioneer Corp., Seoul, Korea) according to the manufacturer's instructions. A 2-μL volume of PCR product from the 16S ribosomal DNA (rDNA) fragment was cloned by using pGEM-T Easy cloning vector (Promega) as prescribed by the manufacturer's manual. The plasmid was purified by using the PureLink Quick Plasmid Miniprep Kit (Invitrogen, Carlsbad, California) following manufacturer's instructions. Finally, 20 μL of the purified, plasmid-harboring cloned fragment of 16S rDNA were sequenced with M13 forward and reverse primers (Macrogen, Seoul, Korea). Bacterial isolates were identified based on the results of BLAST (Altschul et al. 1990) analysis of the partial 16S rDNA sequences. The 16S rDNA sequences derived from the bacterial isolates (except ATCC reference bacteria) were then deposited in GenBank (http://www.ncbi.nih.gov).
Results
Phenotypic Characterization
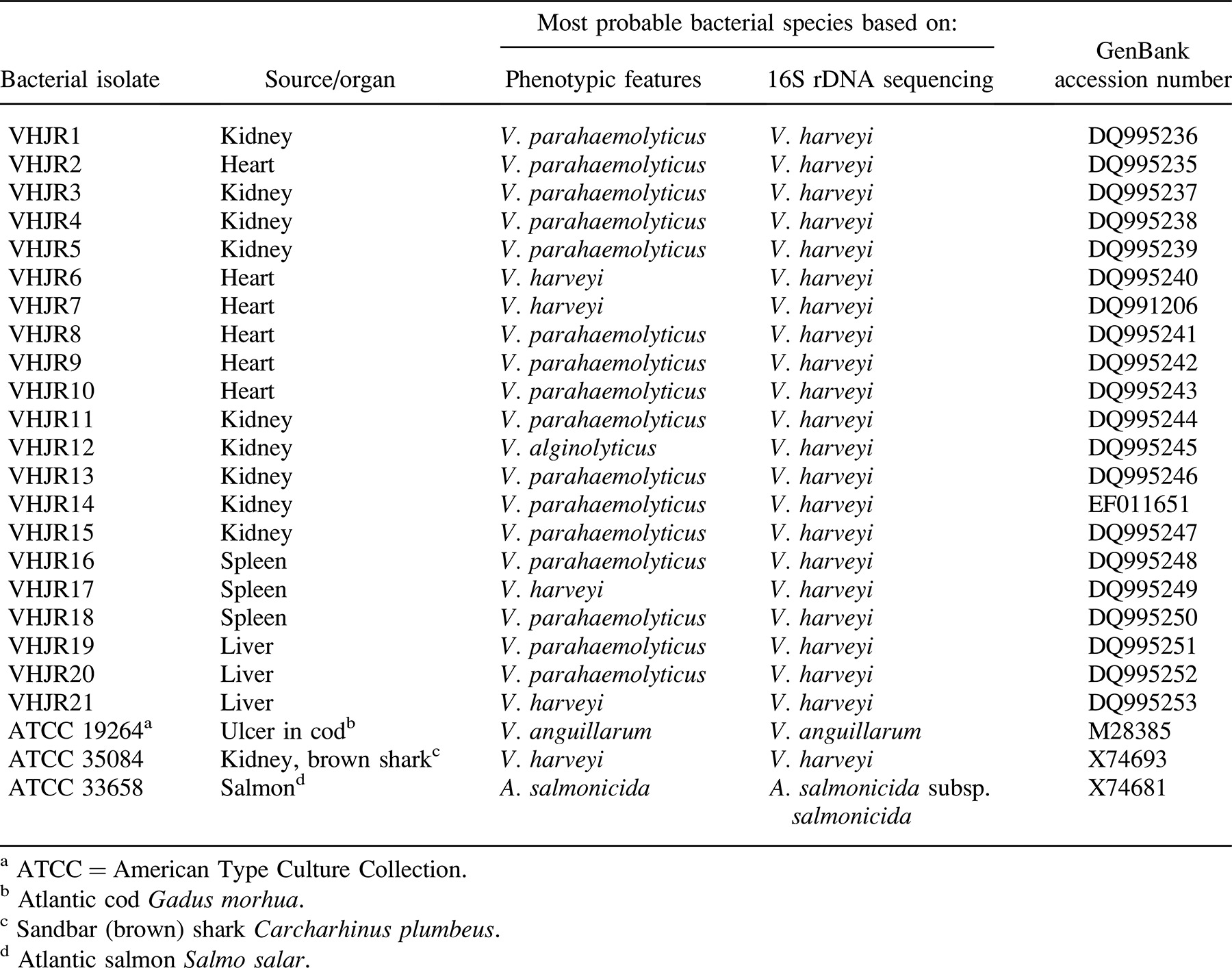
All 21 bacterial isolates were Gram-negative, nonmotile, positive for oxidase and catalase, negative for Voges–Proskauer reaction, and fermentative for glucose but did not produce gas; moreover, all isolates grew at all tested temperatures (25, 28, and 30°C). There were some variations in indole production and citrate utilization. All but one isolate (VHJR21) grew at 0% NaCl, all isolates grew at 3% NaCl, and all but VHJR7, VHJR10, VHJR18, and VHJR19 grew at 5% NaCl. Every isolate was negative for arginine dihydrolase, phenylalanine agar, and β-galactosidase but positive for lysine decarboxylase and methyl red tests. Some isolates (i.e., VHJR1, VHJR6, VHJR7, VHJR17, and VHJR21) were urease-positive. All isolates were able to ferment glucose, fructose, l-arabinose, dextrose, and maltose. The isolates were unable to ferment salicin, lactose, and rhamnose, but they varied in fermentation of other sugars. Based on the biochemical properties, 4 isolates were identified as V. harveyi, 16 as V. parahaemolyticus, and 1 as V. alginolyticus (Table 1).
Molecular Identification
The primers developed by Sfanos et al. (2005) were able to amplify the partial fragment of 16S rDNA from all of the bacterial isolates, including the V. harveyi reference isolate (ATCC 35084) used in this study. The BLAST analysis of the sequences derived from the 21 bacterial isolates examined in this study demonstrated that all isolates had high homology (98–100%) to the 16S rDNA sequences of V. harveyi. On this basis, the bacterial isolates were tentatively identified as V. harveyi. The accession numbers of the 16S rDNA sequences of the 21 bacterial isolates deposited in GenBank are shown in Table 1.
Antibiotic Susceptibility Assay
The 18 isolates were susceptible to 8 of the 17 antibiotics tested: nitrofurantoin, oxytetracycline, furazolidone, chloramphenicol, nalidixic acid, oxolinic acid, streptomycin, and sulfamethoxazole. All isolates were resistant to ampicillin, penicillin, and vancomycin. However, the isolates varied in their sensitivity to novobiocin, kanamycin, neomycin, trimethoprim, ciprofloxacin, and tetracycline.
Discussion
In the present study, Asian seabass with vibriosis were characterized by deep skin and fin ulceration, dark pigmentation, lack of appetite, presence of ascites in the body cavity, enlarged liver and spleen, and swimming at the surface. Generally, young fish are more susceptible to vibriosis and exhibit anorexia, skin darkening, and heavy mortality. Susceptibility of aquaculture animals to vibriosis is enhanced when the animals encounter stressful conditions, such as handling and transport, overcrowding, low dissolved oxygen (Austin and Austin 1993; Inglis et al. 1993), high stocking densities, fluctuating salinity, and high organic loads (Akayh and Timur 2002). This seems to be true in the present study as well, in which Asian seabass were stocked at a high density in net-cages.
Traditional methods of bacteriology can help detect common and easily cultured pathogens but can be time-consuming (Balcázar et al. 2007), less sensitive (Gauger and Gomez-Chiarri 2002), and subject to misinterpretation (Thompson et al. 2002) in comparison with molecular methods. In the present study, the two methods used (biochemical characterization and 16S rDNA sequencing) gave conflicting identifications of the bacterial isolates. According to biochemical tests of the 21 bacterial isolates, 4 were identified as V. harveyi, 16 were characterized as V. parahaemolyticus, and 1 was identified as V. alginolyticus. When 16S rDNA sequences were analyzed, however, all bacterial isolates showed high (98–100%) homology to V. harveyi.
The different results in identification of V. harveyi achieved from biochemical and molecular tools are not surprising and have been reported in the past. Vibrio harveyi has been shown to be phenotypically heterogeneous (Grimes et al. 1993; Alsina and Blanch 1994; Vandenberghe et al. 2003). Moreover, V. harveyi has also been reported to contain mobile genetic elements, such as bacteriophages (Oakey and Owens 2000), which contribute to new phenotypic characteristics of the bacterium (Munro et al. 2003). These situations make it extremely difficult for V. harveyi to be identified with conventional bacteriological tests. Although more complete biochemical characterizations have been proposed to differentiate closely related Vibrio spp. (Bryant et al. 1986; Grimes et al. 1993; Alsina and Blanch 1994), these may be unreliable given variability in biochemical traits and the possibility of errors in interpreting the test results. The weakness of using biochemical profiles as the sole means in bacterial identification is evident from the works of Gauger and Gomez-Chiarri (2002), who found that 8 of 15 putative V. harveyi isolates reported by Pedersen et al. (1998) had 16S rDNA sequences that matched more closely those of other Vibrio species. Similar findings were also reported by Thompson et al. (2002) when they identified V. trachuri, a pathogen of Japanese horse mackerel Trachurus japonicus, on the basis of biochemical tests. However, when the pathogen's 16S rDNA sequence (AJ312382, GenBank) was compared with other 16S rDNA sequences, it exhibited 100% similarity to the 16S rDNA sequence of the V. harveyi type strain (AF426825, GenBank).
Molecular tools, such as 16S rDNA sequencing, have proven to be useful for classification and identification of bacterial species (Kolbert and Persing 1999). One advantage of 16S rDNA sequencing is that it is accessible to any laboratory with technical skills in PCR and sequencing. Computer applications such as BLAST can be used to search databases (e.g., GenBank and the European Molecular Biology Laboratory's Nucleotide Sequence Database), allowing preliminary identifications without needing to include large numbers of reference strains in the study. However, the usefulness of 16S rDNA sequences in bacterial classification and identification may be limited when DNA used in PCR is contaminated or when erroneous sequences are deposited in the databases (Macián et al. 2000). Moreover, 16S rDNA sequences may not be sufficient for a definite identification, particularly for those bacteria that have extremely high rates of horizontal gene transfer and recombination between related species, such as happens in the genus Vibrio (Thompson et al. 2005). For this purpose, Thompson et al. (2005) proposed analysis of other genes, such as recA*, pyrH*, rpoA*, atpA*, and obg*, for greater confidence in Vibrio species identification.
The antibiotic susceptibility results of this study are comparable with those of Vaseeharan et al. (2005), who recorded that 100% of V. harveyi isolates were resistant to ampicillin, 52% were resistant to ciprofloxacin, and 57% were resistant to streptomycin. The results are also in accordance with the findings of Musa et al. (2008), who reported that most V. harveyi isolates were resistant to ampicillin (90%) but demonstrated high susceptibility (96.7%) to chloramphenicol, tetracycline, and furazolidone. Generally, all strains of V. harveyi isolated from shrimp-farming facilities and from unfarmed waters in Asia were resistant to ampicillin (Teo et al. 2000) and several other antibiotics (Abraham et al. 1997). As this study makes clear, some antibiotics could be used as a prophylactic treatment for vibriosis caused by V. harveyi. All isolates were susceptible to nitrofurantoin, oxytetracycline, furazolidone, streptomycin, sulfamethoxazole, chloramphenicol, nalidixic acid, and oxolinic acid. However, most of these antibiotics are either not licensed for aquaculture use or banned completely in some countries (Serrano 2005). Although oxytetracycline has been extensively used in aquaculture in Asia, including Malaysia, the use of this antibiotic in brackish water or seawater is very limited: it binds to calcium and magnesium in seawater to form a complex that is unable to penetrate the fish gill lining (Lunestad and Goksøyr 1990). Moreover, prolonged usage of antibiotics in aquaculture allows bacterial pathogens to develop resistance (Miranda and Zemelman 2002; Sahul and Balasubramanian 2000). It is also possible that antibiotic traces can accumulate in the food chain (Roque et al. 2001; Vaseeharan et al. 2004). Hence, considering the side effects of the antibiotics in aquaculture, other means of controlling vibriosis in Asian seabass aquaculture must be developed. Although the development of vaccine against V. harveyi is still at a preliminary stage, experiments at the laboratory level are already showing some interesting results (Zhu et al. 2006). Perhaps such a development can contribute a great deal to sustainable management of Asian seabass aquaculture in Malaysia.