Evaluation of Sodium Carbonate Peroxyhydrate as a Potential Catfish Egg Disinfectant
Abstract
Two experiments were conducted to evaluate the efficacy of sodium carbonate peroxyhydrate (SCP) in improving the hatching success of channel catfish Ictalurus punctatus when used as a prophylactic chemotherapeutant during egg incubation. In the first experiment, the efficacy of SCP was evaluated in 379-L aluminum incubation troughs similar to those used in commercial hatcheries. Egg masses treated daily with 254 mg of SCP/L of water had significantly higher mean hatching success than untreated controls, and a pathogen-inhibiting effect was also evident (i.e., no gross infection was observed on the treated egg masses). In the second experiment, the hatching success of egg masses treated daily with 254 mg/L was compared with that of egg masses treated daily with hydrogen peroxide (70 mg/L). The effects of both treatments on the pH, dissolved oxygen, and hydrogen peroxide concentrations in the trough were also examined. Both SCP and hydrogen peroxide significantly improved hatching success. Unlike in the treatment with hydrogen peroxide, water pH increased during the treatment with SCP; however, no negative effects on hatching success were observed. The results of this research suggest that SCP acts similarly to hydrogen peroxide in improving channel catfish hatching success and warrants further research to determine whether it could be a practical and effective alternative for managing catfish egg infections in commercial hatcheries.
Because of adhesive and collective properties of egg masses of channel catfish Ictalurus punctatus, fungal and bacterial egg infections can be a significant problem in commercial hatcheries (Brunson 1992). Dead eggs and other organic matter in hatchery culture systems provide excellent substrates for these pathogens. Without chemotherapeutic treatment, pathogens quickly infect moribund eggs and spread to live eggs, overtaking the egg mass. If left untreated, embryo survival often falls to zero. Historically, potential infections were controlled by prophylactic immersion treatments of chemotherapeutants such as malachite green (now banned in several countries because of toxicological considerations; Alderman 1985), povidone iodine, or formalin (Brunson 1992; Walser and Phelps 1993). More recently, hydrogen peroxide has been shown to be an efficacious therapeutant for managing channel catfish egg diseases (Rach et al. 1998; Small and Wolters 2003; Rach et al. 2004; Small 2004).
The U.S. Food and Drug Administration (FDA), which regulates the approval and use of aquaculture drugs in the USA, has designated povidone iodine as a low regulatory priority aquaculture drug when used as an egg surface disinfectant. Formalin and hydrogen peroxide are the only FDA approved therapeutants for the control of fungi on finfish eggs. Although formalin is an effective therapeutant, concerns of safety exist due to its suspected carcinogenicity and odoriferous nature. On the contrary, hydrogen peroxide has little odor and is not considered carcinogenic. Hydrogen peroxide has the added benefit of being considered environmentally friendly because it decomposes into water and oxygen. However, even with these positive attributes, hydrogen peroxide has not been well received or adopted by hatchery staff at commercial catfish operations because, as a strong oxidant, liquid hydrogen peroxide (35%) irritates and burns the skin.
Therefore, there has been an interest in finding an egg disinfectant alternative to formalin and hydrogen peroxide that would provide strong oxidizing potential but no odor or carcinogenicity, and yet that would be easy and safe to use in a hatchery setting. Sodium carbonate peroxyhydrate (SCP), commonly referred to as sodium percarbonate, appeared to meet the desired attributes and was thus identified as a possible alternative worth evaluating. Sodium carbonate peroxyhydrate products are marketed in the USA as fast-acting algaecides or algaestats. Several brands of SCP are on the market and have been registered by the U.S. Environmental Protection Agency as algaecides for use in ponds, lakes, reservoirs, and drinking water. A free-flowing granular substance, SCP is made by combining two molecules of sodium carbonate with three molecules of hydrogen peroxide. This granular product provides a stable source of alkaline hydrogen peroxide, which when added to water, breaks down into hydrogen peroxide and sodium carbonate. In algaecides such as PAK27 (Solvay Chemicals, Inc., La Porte, Texas), the SCP concentration is 85%, which corresponds to 27.6% hydrogen peroxide. In two experiments using SCP as a prophylactic chemotherapeutant during channel catfish egg incubation, we evaluated its efficacy for improving hatching success.
Methods
Channel catfish eggs used in both experiments were collected from U.S. Department of Agriculture (USDA), Agricultural Research Service, Catfish Genetics Research Unit ponds in Stoneville, Mississippi, during the months of May and June. The eggs were from USDA103-strain channel catfish. Spawning containers were checked in the morning once every 2 d for egg masses, and eggs were microscopically staged to determine embryo development (Silverstein and Small 2004). Only fertilized egg masses of similar ontogenic stage (<24 h old) were used in these studies. No visible infections (characterized as white or gray patches on the egg mass) were observed by gross observation on any of the egg masses before starting each experiment. The number of eggs per mass was calculated after determining the number of eggs per gram on a sample of approximately 2 g.
In both experiments, a paired experimental design was used in which sample size is the number of paired samples, untreated and treated. Pairing was used to control for extraneous sources of variability and to increase statistical power. By matching the untreated and treated effects on the same egg mass, confounders such as pond environment, genetics, parental health, nutrition, and so forth affecting individual egg mass hatching success were controlled (i.e., not affect the difference between untreated and treated). By analyzing only the differences, therefore, a paired test corrects for those sources of variation in dependent samples (Louis et al. 1984).
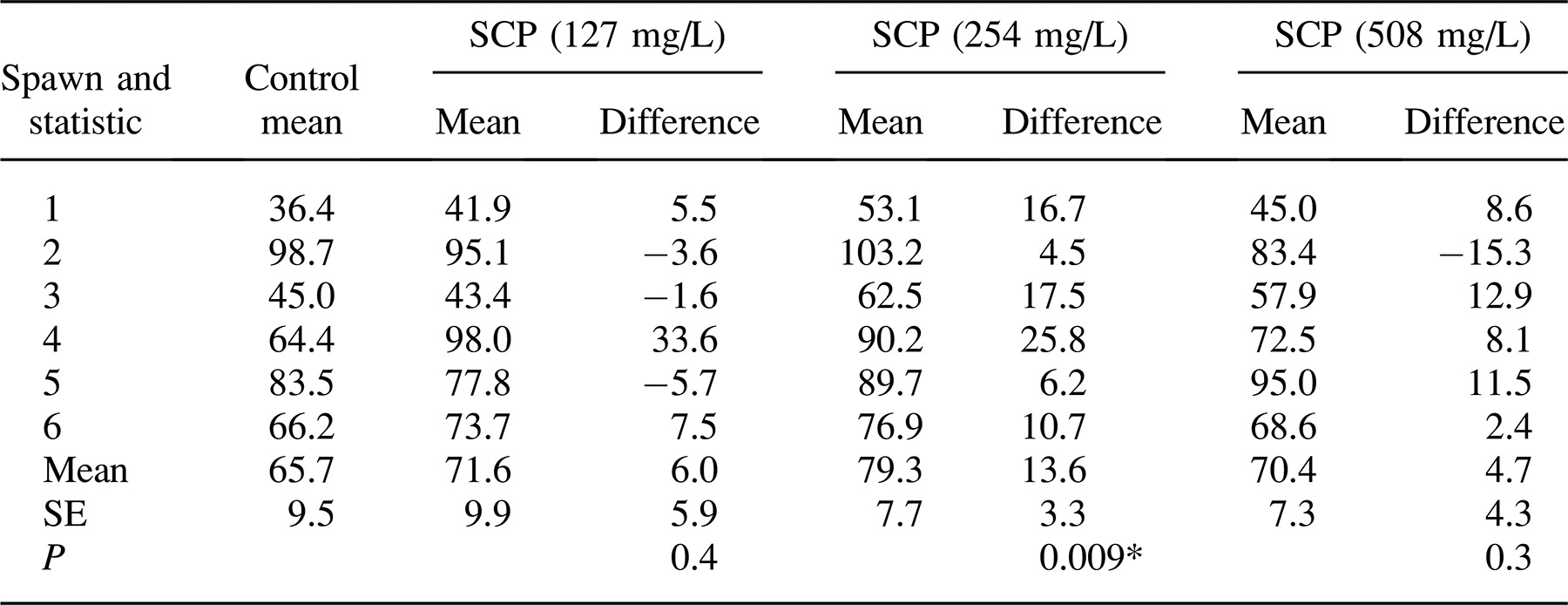
In the first experiment, catfish eggs from six spawns of approximately 200–400 g each were subdivided into equal masses of approximately 50 g, placed in individual hatching baskets, and assigned to four 379-L spawning troughs. Each trough contained a subsample of each spawn placed in similar positions relative to the trough. Hatching baskets were lined with standard window screen to prevent escape of eggs and hatched fry. Each spawning trough received flowing well water at a rate of 7.6 L/min. Four large air stones were placed in each trough to maintain DO and gently agitate the egg masses. Initial water quality variables measured in the troughs were temperature (26.5°C), dissolved oxygen (7.1 mg/L), pH (8.9), total hardness (120 mg/L), and alkalinity (410 mg/L).
A 500-μL homogenate from a grossly infected egg mass collected 4-d prior was pipetted onto each experimental egg mass 20 min before SCP treatment; the water flow was off for 5 min in an attempt to encourage pathogen growth. Flow of water was then returned, and SCP treatments were begun 15 min later. The SCP (PAK27, Solvay Chemicals) treatments (0, 127, 254, and 508 mg/L) were randomly assigned to the four troughs. Treatments were administered every morning until embryonic eye pigmentation became apparent without magnification; this minimized accidental treatment of newly hatched fry in the trough. The daily treatments consisted of adding the appropriate amount of SCP to 6-L of hatchery water in a bucket, mixing for 1 min to dissolve the SCP, and adding the solution at once to the trough at the water inlet. Continuous flow of well water was maintained throughout the treatment. After 3 d, egg masses were photographed to record observable areas of infection. Egg masses were allowed to hatch to completion within the hatching baskets. When hatching was complete, the fry were siphoned into a graduated cylinder, and the volume of live fry recorded. The total number of live fry was calculated after determining the number of live fry in 1 mL then multiplying times the total volume of fry collected. Hatching success was calculated as the percentage of eggs hatched. Dead fry were not included in the calculation of hatching success.
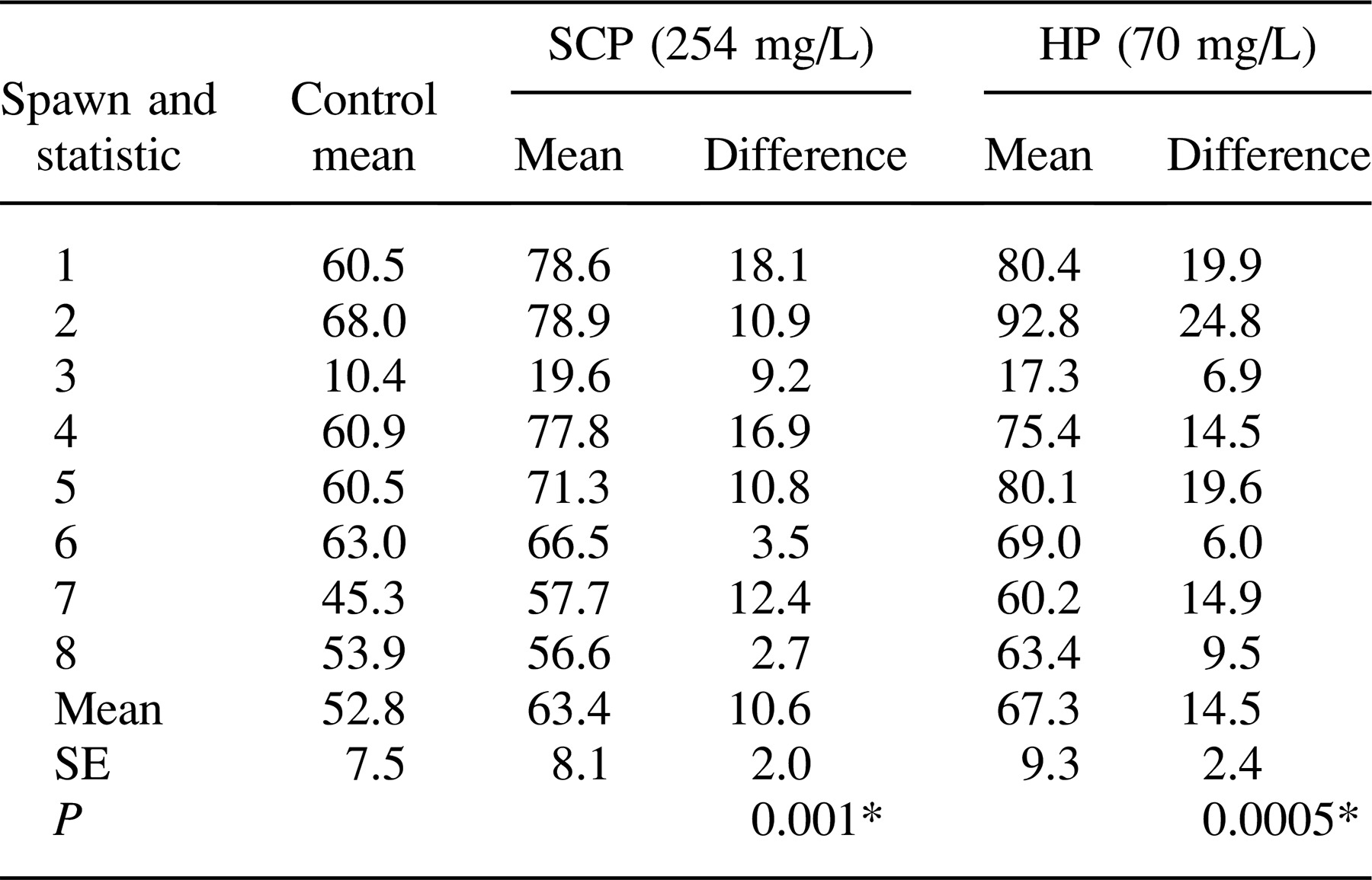
In the second experiment, a comparison was made between the optimal daily treatment with SCP established in the first experiment (254 mg/L) and daily treatment with hydrogen peroxide (Harcos Chemical, Kansas City, Kansas) at the optimal concentration of 70 mg/L (Small and Wolters 2003) and an untreated control. Catfish eggs from eight spawns (200–400 g) were subdivided into equal masses of approximately 60 g and assigned to three 379-L spawning troughs as described above. Environmental conditions and the treatment protocol for SCP were similar to the first experiment. Hydrogen peroxide was administered directly to the inlet end of the hatching through (Small and Wolters 2003). Dissolved oxygen (DO), pH, temperature, and hydrogen peroxide concentrations were measured at the inlet, middle, and outlet of the trough immediately prior (0 min) to addition of SCP and hydrogen peroxide and at 5, 10, 20, 40, 60, 80, 120, and 240 min following addition. We used a model 58 DO meter (YSI, Yellow Springs, Ohio) to measure DO and temperature, a pHTestr 30 m (Oakton Instruments, Vernon Hills, Illinois) for pH, and a semiquantitative Merchoquant and Em Quant peroxide test strips (EMD Chemicals, Gibbstown, New Jersey) for hydrogen peroxide. Hatching success was calculated as previously described.
Statistical comparisons for each trial were conducted using the SAS software system version 9.2 (SAS Institute 2008). Assumptions of normality of the data were tested by the Shapiro–Wilk W test for normality. Data from the first experiment were found to be normally distributed and differences in mean hatching success between the untreated controls and treated eggs masses were analyzed using a paired t-test. Hatching success in the second experiment was not normally distributed, and as such, the sign test was performed (as a nonparametric equivalent to the paired t-test) to compare the differences in median hatching success between untreated controls versus treated egg masses and between SCP versus hydrogen peroxide treated egg masses. Differences were considered significant at α = 0.05.
Results
In the first experiment, visual observation (Figure 1) indicated decreased areas of infection on the egg masses as SCP concentration increased; no areas of infection were apparent on eggs treated with 254 mg of SCP/L of water (input concentration) or higher. Untreated eggs masses were most heavily infected (light gray patches covering the majority of the egg mass (Figure 1A), whereas small to moderate sites of infection were observed on egg masses treated at 127 mg/L (smaller distinct white to light gray patches on the egg mass; Figure 1B). No improvement in hatching success was observed at 127 mg/L (Table 1). A significant improvement in hatching success (13.6 ± 3.3%) was observed when eggs were treated at 254 mg/L, and no sites of infection were observed at this concentration (Figure 1C). When the concentration of SCP was increased to 508 mg/L, there was no difference in hatching success compared with the controls. Dead eggs were observed at 508 mg/L.
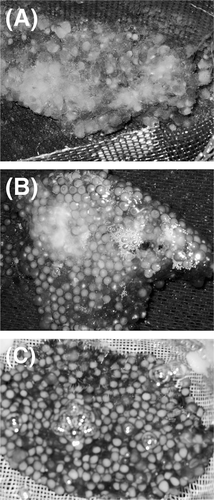
Efficacy of sodium carbonate peroxyhydrate (SCP) in reducing infection on channel catfish egg masses in standard 379-L hatching troughs. Panel (A) shows egg masses that received no therapeutic treatment, which were generally heavily infected; panel (B) shows egg masses that were treated with 127 mg of SCP/L of water, which had distinct areas of infection; and panel (C) shows egg masses that were treated with 254 mg/L, which had no observable infection.
In the second experiment, the most effective SCP treatment from the first experiment (254 mg/L) was compared with the concentration of hydrogen peroxide (70 mg/L of water) recommended for a flush treatment in a 379-L trough with 7.6 L/min of well water influx (Small and Wolters 2003). Both treatments appeared to be effective inhibitors of pathogen growth; no areas of infection were observed, and hatching success improved significantly relative to untreated controls (Table 2).
Comparisons of pH, DO, and hydrogen peroxide profiles in the hatching troughs were made between the SCP and hydrogen peroxide treatments in the second experiment. No change was observed in pH or temperature following addition of hydrogen peroxide to the trough. Temperature also remained constant in the trough following SCP addition; however, pH changed abruptly (Figure 2). Addition of SCP to the trough resulted in an increase from 8.9 to 9.7 at the inlet within the first minute and was up to 9.6 throughout the trough within 10 min, reducing to 9.0 after 4 h. Dissolved oxygen concentrations increased 0.8 mg/L throughout the trough during the 10 min following addition of the hydrogen peroxide, then decreased, returning to the initial concentration by 80 min posttreatment (data not shown). A similar effect on DO was observed following SCP addition (Figure 3); DO peaked between 5 and 20 min posttreatment, increasing by 0.7 mg/L and returning to the initial concentration by 60 min posttreatment. The semiquantitative profile of hydrogen peroxide concentration in the trough was similar between the hydrogen peroxide and SCP treatments; however, the hydrogen peroxide concentration appeared to be reduced more quickly in the SCP treatment (Figure 4).
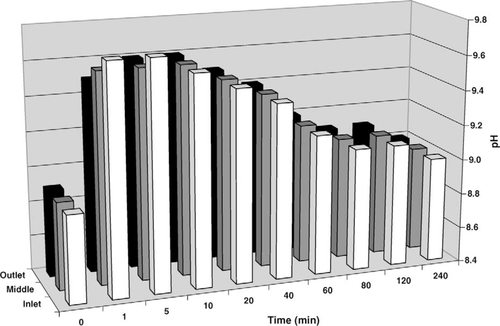
Profile of pH at three points in a 379-L hatching trough with a continuous inflow of well water at 7.6 L/min after the addition of 254 mg of sodium carbonate peroxyhydrate per liter at the inlet.
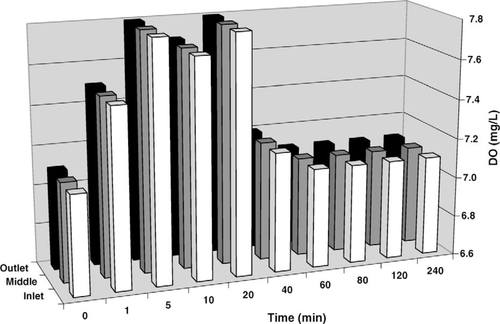
Profile of dissolved oxygen (DO) at three points in a 379-L hatching trough with a continuous inflow of well water at 7.6 L/min after the addition of 254 mg of sodium carbonate peroxyhydrate per liter at the inlet.
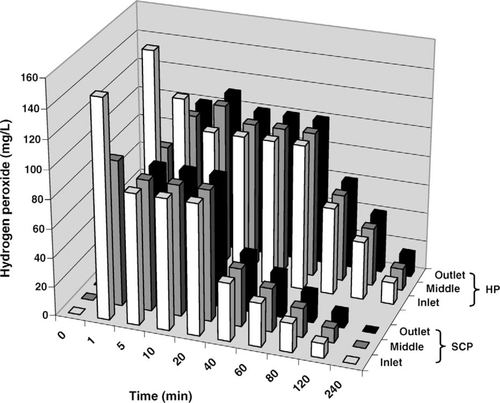
Profile of hydrogen peroxide at three points in a 379-L hatching trough with a continuous inflow of well water at 7.6-L/min after the addition of 254 mg of sodium carbonate peroxyhydrate (SCP) per liter and 70 mg of hydrogen peroxide (HP) per liter at the inlet.
Discussion
Our research was conducted to assess whether SCP has potential as a prophylactic treatment for improving channel catfish hatching success. Hydrogen peroxide is the active component of SCP and is itself an effective chemotherpeutant for treating infections of catfish eggs (Rach et al. 1998; Small and Wolters 2003; Rach et al. 2004; Small 2004). Buchmann and Kristensson (2003) and Buchmann et al. (2003) also sought to capitalize on the hydrogen peroxide releasing activity of SCP for treatment of fish diseases. Buchmann and Kristensson (2003) suggested SCP is an alternative to formaldehyde for reducing Gyrodactylus derjavini populations on rainbow trout Oncorhynchus mykiss, and Buchmann et al. (2003) effectively used SCP to treat the theronts (but not the tomocysts) of Ichthyophthirius multifiliis, the causative agent of white spot disease, in vitro. Buchmann et al. (2003) supported the use of SCP over liquid hydrogen peroxide, arguing that SCP is easier to transport, handle, and apply. Although liquid hydrogen peroxide when diluted to treatment concentrations is safe if precautions are followed, acceptance as a therapeutant has been poor among commercial catfish hatcheries in the USA. In Denmark, SCP at 50–100 mg/L of water is used to treat rainbow trout ponds (Buchman et al. 2003). The granular nature of SCP allows for easier handling than liquid hydrogen peroxide and provides a safer work environment because SCP is less likely to have an immediate adverse effect if spilled on the skin.
In our study, prophylactic treatment of channel catfish eggs with SCP was effective for reducing pathogen growth and increasing hatching success. An SCP concentration of 254 mg/L (input concentration) of water yielded the greatest hatching success increase. The SCP we used, PAK27, has a hydrogen peroxide equivalent of 27.6%. Thus, treatment with SCP at 254 mg/L should yield results similar to a hydrogen peroxide treatment of 70 mg/L of water. Small and Wolters (2003) observed the highest hatching success among channel catfish eggs in troughs under similar conditions and treated with hydrogen peroxide at 70 mg/L. The efficacy of the 254-mg/L SCP treatment was confirmed by the results of the second experiment. Both the SCP (254 mg/L) and hydrogen peroxide (70 mg/L) treatments significantly increased hatching success and were not significantly different from each other in the magnitude of improvement. This level of hydrogen peroxide (70 mg/L) is lower than FDA recommended concentrations of 500-1000 mg/L; however, water and environmental conditions, egg densities, and treatment methodologies of the studies supporting higher concentrations were dissimilar to those used in our study and those of Small and Wolters (2003). The present research and that of Small and Wolters (2003) was conducted in aluminum troughs as a flush treatment using groundwater supplied from a deep aquifer in the Mississippi Delta. Higher concentrations have been recommended for eggs treated for 15 min in McDonald hatching jars (Rach et al. 2004) or aquaria (Rach et al. 1998). Differences in stocking densities may also affect the oxidation potential and, thus, efficacy of these chemicals. Stocking densities (mass) of eggs in our study were less than 1 g/L, whereas egg densities in formulating the FDA label recommendations were more than 100 g/L (Rach et al. 1998; 2004). Industry stocking densities are typically about 18 g/L (Avery and Steeby 2004). Each of these factors should be considered when determining treatment rates for individual hatchery systems.
In our study, increasing the SCP treatment concentration to 508 mg/L decreased hatching success relative to the 254 mg/L concentration. An apparent increase in the number of dead eggs before hatch was also observed in egg masses treated with 508 mg/L. A similar decrease in improvement to hatching success was observed by Small and Wolters (2003) when hydrogen peroxide concentrations of 200 and 300 mg/L were used to treat channel catfish eggs, possibly because of toxicity to the embryos and oxidative decomposition of the eggs; they also observed premature hatching at both 200 and 300 mg/L, which probably contributing to the lower hatching success. No premature hatching was observed in our study with SCP.
Compared with the hydrogen peroxide treatment of 70 mg/L in the second experiment, the SCP treatment of 254 mg/L yielded a similar hydrogen peroxide profile in the trough following treatment; however, the hydrogen peroxide from SCP appeared to flush through the system at a faster rate (Figure 4). This is contrary to the suggestion of Buchman et al. (2003; used SCP from BioCare SPC, BioMar, Denmark) that SCP should release its hydrogen peroxide over a longer period. Although they did not provide evidence to support this claim, differences in formulation could account for differences in hydrogen peroxide release rate. Further examination of hydrogen peroxide release and turnover in commercial hatching troughs is needed. Greater accuracy may be obtained using a potassium permanganate titrimetic method (Jeffery et al. 1989) rather than the test strips we used, which provide only a semiquantitative estimation.
Both SCP and hydrogen peroxide increased dissolved oxygen in the hatching troughs. This is a beneficial effect because DO concentrations above 7.4 mg/L at 26°C have been shown to improve channel catfish hatching success (Torrans and Steeby 2008). The greatest effect of SCP on water quality appeared to be pH. Although pH was unaffected by hydrogen peroxide, it was substantially increased following treatment with SCP. The higher pH coupled with a faster turnover of hydrogen peroxide may have contributed to the somewhat lower (nearing statistical significance) hatching success of eggs treated with SCP compared with hydrogen peroxide.
The SCP we used contained 27.6% hydrogen peroxide and had similar efficacy to an equivalent concentration of hydrogen peroxide in the improvement of channel catfish hatching success. As a free-flowing powder, SCP proved easy to handle and dissolved quickly, yielding a dilute hydrogen peroxide solution. This could be advantageous to commercial hatcheries because hatchery workers would be less likely to suffer skin irritation and burns than with more concentrated liquid hydrogen peroxide. As a relatively stable solid, SCP also has fewer transportation or storage issues than does liquid hydrogen peroxide. Currently, treating with SCP would cost about 5 times the cost of hydrogen peroxide treatments (about $0.50/d to treat one 380-L trough at an SCP concentration of 254 mg/L). Even so, handling and storage attributes and the observed efficacy as a chemotherapeutant could make SCP an attractive alternative to both formalin and hydrogen peroxide for use in fish hatcheries. Further testing is needed to validate efficacy results (including determinations of specific fungicidal and bactericidal activities) and to stimulate interest in SCP as a potential therapeutant for use in catfish hatcheries. Currently, SCP is not approved by the FDA for use in treating diseases of finfish eggs in the United States.
Acknowledgments
I wish to acknowledge the technical assistance of Jimmie Warren of the USDA Agricultural Research Service, Catfish Genetics Research Unit; Tom Goodrich and George Kohan of Aquatic Health Resources, Minnetonka, Minnesota, for their consultation regarding the use of SCP in aquatic systems; and Jef Morgan of Peroxygen Solutions, Jamestown, North Carolina for discussing PAK27 pricing. Mention of trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable.




