Critical Swimming Speed and Behavior of Juvenile Shovelnose Sturgeon and Pallid Sturgeon
Present address: Department of Zoology, Fisheries and Illinois Aquaculture Center, Southern Illinois University, Carbondale, Illinois 62901-6501, USA.
Abstract
The swimming performance of hatchery-reared, juvenile shovelnose sturgeon Scaphirhynchus platorynchus and pallid sturgeon S. albus was studied in a laboratory swim tunnel at 20°C and 10°C. The mean 30-min critical swimming speed was not significantly different between species at either temperature (36.9 cm/s for shovelnose sturgeon and 35.9 cm/s for pallid sturgeon at 20°C, 19.4 cm/s for shovelnose sturgeon and 15.0 cm/s for pallid sturgeon at 10°C). Free swimming (swimming without contact with the substrate) was observed less than 18% of the time at speeds greater than 15 cm/s. As speed increased, pallid sturgeon swam significantly less in the water column at 20°C; however, speed had no effect on percent free swimming among shovelnose sturgeon at 20°C. The results of this study indicate that, over the temperature and size range tested, shovelnose sturgeon and pallid sturgeon probably do not segregate in rivers due to different swimming or station-holding abilities
Introduction
Shovelnose sturgeon Scaphirhynchus platorynchus and pallid sturgeon S. albus are sympatric in the main-stem Mississippi and Missouri rivers and are generally associated with flowing water habitats in or near the main channel (Bailey and Cross 1954). Adults of both species are known to use relatively low-velocity areas associated with dike fields, sand bars, and islands (Carlson et al. 1985; Hurley et al. 1987, 1999; Bramblett 1996; Curtis et al. 1997). Collections of young river sturgeons (Scaphirhynchus spp.) in the wild are infrequent, and young pallid sturgeon have only recently been sampled. Juvenile (5.0–21.0 cm) shovelnose sturgeon and pallid sturgeon have been collected by bottom trawling on the sand flats of the Mississippi River, primarily in main channel border areas where velocities 20 cm above the bottom ranged from 20 to 80 cm/s; sand troughs were usually present in general capture locations (M. Petersen and D. Herzog, Missouri Department of Conservation–U.S. Geological Survey Long-Term Resource Monitoring Program, personal communication). The extent of overlap in habitat use by river sturgeons remains relatively unknown (particularly for the early life stages), but a potential consequence of sharing habitat would be an interspecific competition for resources.
Fish occupying similar habitats partition resources along a trophic gradient or through differences in the temporal or spatial use of the habitat (Ross 1986). Closely related, sympatric species may segregate spatially in lotic environments through limits in their ability to occupy increased velocities (e.g., Peake et al. 1997). While most available information suggests that river sturgeons at a given life stage occupy the same general habitats, in some field studies adult pallid sturgeon were found to occupy faster currents and to be more abundant in swift, channel habitats than shovelnose sturgeon (Forbes and Richardson 1905; Carlson et al. 1985). However, the interpretation of adult and juvenile capture data is hindered by uncertainty in identification, the difficulties associated with measuring the focal point velocity of fish in large, turbid rivers, and the differences in fish body size. Also, river sturgeons can maintain station against current by actively swimming or by station-holding (Adams et al. 1997, 1999). The relative abilities of fish to withstand and negotiate currents can be examined in laboratory flumes where fish are subjected to known velocities and behaviors readily observed.
The performance and behavior of river sturgeons have been examined in laboratory swim tunnels. Fifteen-minute critical swimming speeds were determined for a limited number of wild-caught, adult shovelnose sturgeon that ranged in size from 57 to 69 cm fork length (Adams et al. 1997). Swimming endurance curves have been constructed for hatchery-reared juvenile pallid sturgeon (13.0–20.5 cm fork length) at 20°C (Adams et al. 1999). Sturgeons in both studies, if allowed, could maintain station without actively swimming by using the pectoral fins to generate negative lift in a manner similar to that of other benthic fishes (Matthews 1985; Facey and Grossman 1990; Arnold et al. 1991; Webb et al. 1996). In addition to station-holding, two swimming methods were observed in the laboratory flumes: (1) substrate skimming, where the ventral body surface is in contact with the tunnel bottom, but propulsion continues to be generated by body and caudal fin undulation, and (2) free swimming, where swimming occurs within the water column but without contact with the substrate. In previous studies, river sturgeons were found to swim mostly by substrate skimming, but the percent of time free swimming tended to increase at low and extremely high flows (speeds). Whereas free swimming at low speeds was typically steady in the middle of the water column, free swimming at high speeds was characterized by frantic activity and an apparent searching for velocity refugia (Adams et al. 1999).
Previous laboratory studies provide measures of active swimming ability and swimming behaviors of shovelnose and pallid sturgeon, but direct comparisons between the species are hampered by differences in body size, ontogeny, and source (river versus hatchery). Given the ecological and economic importance of river sturgeons and the many alterations to their native habitat (Keenlyne 1997), it is imperative to understand the habitat use patterns of the two species, particularly during their early life stages. Here, we studied relative swimming performance in the laboratory to provide information on one factor potentially influencing spatial habitat use by young river sturgeons. Critical swimming speed and the swimming behaviors of hatchery-reared juvenile shovelnose sturgeon and pallid sturgeon of a similar size were compared at two temperatures (20°C and 10°C). These water temperatures were within the range experienced by juvenile river sturgeon, representing a moderately high and low value (D. Herzog, personal communication).
Methods
Fish were propagated at Gavins Point National Fish Hatchery (Yankton, South Dakota) on approximately 1 July 1997. Parental shovelnose sturgeon and pallid sturgeon broodstock were from the Yellowstone River and the confluence of the Yellowstone and Missouri rivers, respectively. Morphometric and genetic analyses indicated the broodstock did not contain hybrid individuals (Herb Bollig, Gavins Point National Fish Hatchery, personal communication). Approximately 70 juvenile pallid sturgeon arrived at the U.S. Army Corps of Engineers Waterways Experiment Station (Vicksburg, Mississippi) on 2 December 1997, and swimming endurance over a range of velocities was studied (Adams et al. 1999); however, 30 d elapsed prior to the present study. Six juvenile shovelnose sturgeon arrived on 7 January 1998 and were allowed 10 d to recover from transport and handling prior to acclimation to 20°C. From the original 70 pallid sturgeon, we chose 12 individuals equal in size to the six shovelnose sturgeon for use in the present study.
Six shovelnose sturgeon and 12 pallid sturgeon were held in two separate, 300-L Living Streams model 510 (Frigid Units, Toledo, Ohio). A higher number of pallid sturgeon were acclimated because extra fish were available to ensure a minimum sample size of six. The fish were acclimated 28 d to 20°C and tested over a period of 2 weeks. After lowering the water temperature 1.5°C/d to 10°C, the fish were acclimated 35 d before testing. The water temperature was maintained within 1°C, and fish were held on a 12 h light: 12 h dark cycle throughout the study. Dissolved oxygen (8.16 ± 1.31 mg/L [mean ± SD]), pH (7.1–7.8), and conductivity (243.0 ± 34.0 μmhos/cm) were monitored regularly and remained similar for both holding tanks. Fish readily accepted Number 4 Silver Cup Salmon Crumbles (Nelson and Sons, Inc., Murray, Utah), and were fed once daily.
A 100-L, Blazka-type swim tunnel (Beamish 1978) was used to measure sturgeon swimming ability. The tunnel was constructed of Plexiglas and had a working section 39 cm long and 15 cm in diameter. Two flow filters within the inner tube reduced turbulence and promoted rectilinear flow. Water velocities were calibrated with a Marsh-McBirney flowmeter and ranged from 5 to 70 cm/s. During trials, black plastic sheets placed over the working section prevented disturbance of the fish. Temperature was controlled with a Remcor liquid circulator (Glendale Heights, Illinois).
Prior to testing, individual fish were fasted 36 h to achieve a postabsorptive state, thereby limiting the energy allocated to digestion and food absorption. The sturgeon were transported approximately 6 m to the swim tunnel in a water-filled cylinder and allowed a 1-h habituation period, during which tunnel velocity was increased from 5 to 10 cm/s after 30 min. Following tunnel habituation, the fish were subjected to a stepwise, increasing velocity test (Brett 1964) to determine 30-min critical swimming speeds. Beginning at 15 cm/s, if a fish swam for 30 min and successfully completed the bout, the speed was increased to 20 cm/s. A stepwise increase in speed of 5 cm/s every 30 min continued until the fish fatigued. Fatigued fish could no longer maintain position against water current without bracing against the downstream retaining screen and would not respond to mechanical stimulation (gentle prodding of the caudal fin with a small, wooden paddle). Because fish fatigued before the completion of a bout, the following formula was used to calculate critical swimming speed (Brett 1964):

where u1 is the highest velocity at which the fish swam the prescribed time period, u2 is the velocity increment (5 cm/s), t1 is the time the fish swam at the fatigue velocity, and t2 is the prescribed time period (30 min). Since sturgeon occupied less than 10% of the cross-sectional area of the swim tunnel, speeds were not corrected for a solid blocking effect (Webb 1975). Fork length, standard length, total length, caudal filament length, and mass were recorded at the completion of a test. Weisel (1978) reported that the caudal filament of shovelnose sturgeon contained lateral line structures, and he hypothesized that the filament helped the fish orient to current. Therefore, caudal filament lengths may have indirectly affected (perhaps by influencing habituation or orientation in the swim tunnel) critical swimming speeds.
In addition to measuring swimming speeds, we noted the behavior of both species in response to increased velocity. During previous observations in laboratory flumes (Adams et al. 1997, 1999), adult shovelnose sturgeon and juvenile pallid sturgeon exhibited an affinity for the tunnel bottom and could maintain station against water velocity by station-holding. Because our goal was to compare active swimming ability and not station-holding ability, fish that held station without body or caudal fin undulation at speeds greater than 15 cm/s were encouraged to swim (either skimming or free swimming) by a gentle prodding of the caudal fin. The total time spent swimming free of any contact with the tunnel bottom (free swimming) was recorded during each velocity increment with a stopwatch.
The critical swimming speeds and swimming behaviors of shovelnose sturgeon and pallid sturgeon were measured at 20°C and 10°C. Some individuals of both species would not swim in the tunnel (nonperformers) and were not included in the analyses. Given the limited availability of shovelnose sturgeon (six fish), individuals of each species were tested at both 20°C and 10°C. Because fish were retested, we determined if physical conditioning or habituation to the tunnel affected our results. Three pallid sturgeon (not used in the experiments) were tested on three occasions: day 1, day 14, and day 21 at 20°C. These data were analyzed using repeated-measures analysis of variance (ANOVA). Fixed-effects ANOVA and analysis of covariance (ANCOVA) were used to test for differences in critical swimming speed between species at each water temperature. The amount of time fish swam free of the tunnel bottom was expressed as the percent of total time swam per speed. Arcsine-transformed values of percent free swimming (Zar 1984) were used in repeated-measures ANOVA to identify species and speed effects, speed being the within-subjects factor. All data met the assumptions of normality (chi-square test) and homogeneity of variances (Levene's test). Data were analyzed with Statistica 4.2 (Statistica 1994), and significance of differences determined (P < 0.05).
Results
No evidence of training or habituation was observed in individual pallid sturgeon that were tested repeatedly. Mean 30-min critical swimming speeds (34.1 ± 2.56 cm/s; 36.2 ± 1.13 cm/s; 30.0 ± 10.71 cm/s) were not significantly different over time (one-way, repeated-measures ANOVA; F2,4 = 0.794; P = 0.513). These data suggest that previous exposure to the swim tunnel was not a confounding variable in pallid sturgeon.
The fork length and mass of shovelnose sturgeon and pallid sturgeon did not differ significantly (Student's t-test; all P > 0.20) at either water temperature (Table 1). After accounting for body length, no relationship between filament length and critical swimming speed was detected. The caudal filament was partially severed on two fish during transport to and positioning in the swim tunnel. These fish exhibited erratic behavior in the tunnel that resulted in reduced relative swimming performance. These fish were considered nonperformers and were not included in the analyses.

The critical swimming speeds of six shovelnose sturgeon and eight pallid sturgeon at 20°C were compared using ANCOVA with mass as the significant covariate (F1,11 = 9.04; P = 0.011). The assumption of parallelism was met, as the interaction of mass and species was not significant (F1,10 = 0.11; P = 0.751). Shovelnose sturgeon mass-adjusted, mean critical swimming speed (36.98 ± 3.49 cm/s) was not significantly different from that of pallid sturgeon (35.93 ± 3.29 cm/s) at 20°C (one-way ANCOVA; F1,11 = 0.49; P = 0.496; Table 1). At 10°C, the critical swimming speeds of four shovelnose sturgeon and six pallid sturgeon were analyzed using ANOVA, since neither length nor mass were significant covariates of swim speed. No statistical difference was detected (one-way ANOVA; F1,8 = 3.64; P = 0.093) between the mean critical swimming speed of shovelnose sturgeon (19.48 ± 4.35 cm/s) and pallid sturgeon (15.05 ± 3.06 cm/s) at 10°C (Table 1).
Sturgeons spent the majority of bouts skimming (swimming with some degree of contact with the tunnel bottom), but free swimming was also observed in both species. Considering only speeds of 15–30 cm/s (to avoid missing cells in the analysis), we did not see an overall species or speed effect on the percent free swimming at 20°C; however, the interaction of species and speed was significant (repeated-measures ANOVA; F3,36 = 4.69; P = 0.007), warranting an investigation of simple main effects. Though swim speed had no effect on percent free swimming in shovelnose sturgeon at 20°C (repeated-measures ANOVA; F3,15 = 1.47; P = 0.263), the percent free swimming in pallid sturgeon changed significantly with increasing swim speed at 20°C (one-way, repeated-measures ANOVA; F3,21 = 4.17; P = 0.018). Pallid sturgeon swam in the water column significantly more at 15 cm/s than at 25 and 30 cm/s (Student–Newman–Keuls test; Figure 1). At 10°C, there was no significant effect of species or speed on percent free swimming (Figure 1).
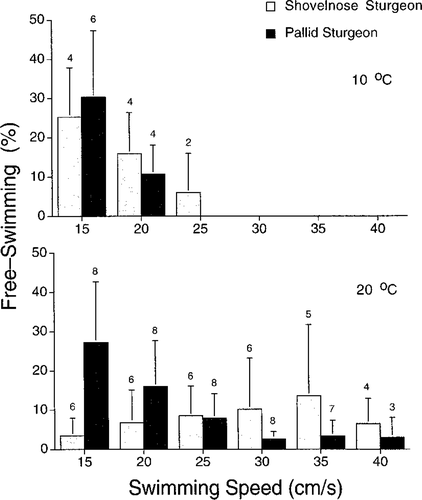
Mean (+2 SEs) percent free swimming (untransformed) versus swimming speed for shovelnose sturgeon and pallid sturgeon at 10°C and 20°C. Sample sizes are given above error bars
Discussion
In a comparison of similar-sized fish, we found no evidence that shovelnose sturgeon and pallid sturgeon have different 30-min critical swimming speeds. The critical swimming speeds for both sturgeon species in the present study corresponded well with the speed at which pallid sturgeon are predicted to endure for 30 min at 20°C (Adams et al. 1999). The two species may not differ in prolonged swimming ability, but the sustained and burst swimming speeds of shovelnose sturgeon have not been determined. The experimental design was limited in its ability to discern the strict effects of temperature on critical swimming speed because temperature treatments could not be randomized. However, critical swimming speed decreased approximately 51–58% in both species when acclimated from 20–10°C, indicating an overall similar physiological response (within this temperature range) as related to performance.
Both species demonstrated an affinity for the Plexiglas bottom during laboratory tests. They generally swam free of the tunnel bottom less than 18% of the time at speeds greater than 15 cm/s (Figure 1). The remaining portion of bouts were spent swimming with the body in contact with the tunnel bottom (skimming). This type of swimming behavior may allow the exploitation of slower currents present at the boundary layer in rivers, enhancing mobility in a physically challenging environment. At 20°C, pallid sturgeon swam in the water column significantly more at low speeds than at faster speeds, but shovelnose sturgeon demonstrated no apparent trend with a change in velocity. This difference in percent free swimming between species at 20°C is not easily explained. At 10°C, both species were active at low speeds, and generally swam more in the water column during tunnel habituation (5–10 cm/s) than at 20°C. All sturgeon could maintain station by remaining motionless at speeds equal to or less than 15 cm/s and were not stimulated to swim; therefore, swimming activity at these speeds was volitional. Free swimming at these low swim speeds was characterized by steady swimming in the middle of the water column, with occasional searches for lower velocity areas. Our observation that shovelnose sturgeon behaved differently at 20°C could be due to many factors (e.g., differences in thermal optima or an artifact of acclimation) and requires further study.
The critical swimming speeds determined for shovelnose and pallid sturgeon are low relative to most speeds reported for teleosts. During active swimming, the heterocercal caudal fin of sturgeons may not generate as much thrust as that of other species, and the presence of bony scutes increases drag (Webb 1986). Poorer relative performance also may be due to the lower metabolic capacity of sturgeons (Singer et al. 1990). Fish morphology (a wide, flat rostrum; large pectoral fins; a flat ventral body surface; and bony scutes) and observations in the laboratory flumes (Adams et al. 1997) suggest that river sturgeons can compensate for low swimming performance by station-holding and skimming. The degree to which river sturgeons, particularly juveniles, use station-holding and skimming in the wild is unknown. Though additional studies are needed to describe habitat use and the extent of overlap, shovelnose sturgeon and pallid sturgeon probably do not segregate in rivers based on different swimming or station-holding abilities within the temperature and size range tested.
Acknowledgments
Sturgeon were spawned and reared by H. Bollig at Gavins Point National Fish Hatchery, Yankton, South Dakota, and provided to us by L. Lee at the Upper Mississippi Science Center, Lacrosse, Wisconsin. Without their generosity, this study would not have been possible. Sturgeon were maintained under the authority of U.S. Fish and Wildlife Permit SA 96-32. We extend our appreciation to J. Killgore, J. Hoover, and P. Kirk at the Waterways Experiment Station, Vicksburg, Mississippi, for providing laboratory space and commenting on the manuscript. The authors benefited from discussions with D. Herzog, J. Hoover, and R. Sheehan. This study was funded by the Environmental Management and Restoration Research Program, U.S. Army Corps of Engineers. Permission to publish this information was granted by the Chief of Engineers.




