Photosensitizing Potential of Ciprofloxacin at Ambient Level of UV Radiation
Abstract
Ciprofloxacin is a widely used fluoroquinolone drug with broad spectrum antibacterial activities. Clinical experience has shown incidences of adverse effects related to skin, hepatic, central nervous system, gastrointestinal and phototoxicity. India is a tropical country and sunlight is abundant throughout the day. In this scenario exposure to ambient levels of ultraviolet radiation (UV-R) in sunlight may lead to harmful effects in ciprofloxacin users. Phototoxicity assessment of ciprofloxacin was studied by two mouse fibroblast cell lines L-929 and NIH-3T3. Generation of reactive oxygen species (ROS) like singlet oxygen (1O2), superoxide anion radical (O2ḃ−) and hydroxyl radical (ḃOH) was studied under the exposure of ambient intensities of UV-A (1.14, 1.6 and 2.2 mW cm−2), UV-B (0.6, 0.9 and 1.2 mW cm−2) and sunlight (60 min). The drug was generating 1O2, O2ḃ− and ḃOH in a concentration and dose-dependent manner. Sodium azide (NaN3) and 1,4-diazabicyclo 2-2-2-octane (DABCO) inhibited the generation of 1O2. Superoxide dismutase (SOD) inhibited 90–95% O2ḃ− generation. The drug (5–40 μg mL−1) was responsible for linoleic acid peroxidation. Quenching study of linoleic acid peroxidation with SOD (25 and 50 U mL−1) confirms the involvement of ROS in drug-induced lipid peroxidation. The generation of ḃOH radical was further confirmed by using specific quenchers of ḃOH such as mannitol (0.5 m) and sodium benzoate (0.5 m). 2′-deoxyguanosine (2′-dGuO) assay and linoleic acid peroxidation showed that ROS were mainly responsible for ciprofloxacin-sensitized photo-degradation of guanine base. L-929 cell line showed 29%, 34% and 54% reduced cell viability at higher drug concentration (300 μg mL−1) under UV-A, UV-B and sunlight, respectively. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay in NIH-3T3 cell line at higher drug concentration (300 μg mL−1) showed a decrease in cell viability by 54%, 56% and 59% under UV-A, UV-B and sunlight, respectively. Results of neutral red uptake assay (NRU) in L-929 cell line were in accordance with MTT assay. The NIH-3T3 cell line showed a higher photosensitizing potential than L-929. The phototoxicity end point shows a time- and concentration-dependent statistically significant (P < 0.001) damage. Ciprofloxacin produced ROS by Type I and Type II photodynamic reactions, interacted with nucleic acid moiety and inhibited cell viability. Further, UV-induced photo-peroxidation of linoleic acid accorded the involvement of ROS in the manifestation of drug phototoxicity. Appearance of ciprofloxacin-induced phototoxicity at the ambient level of sunlight is a real risk for the people of India and for those of other tropical countries. We suggest that sunlight exposure should be avoided (especially peak hours) during ciprofloxacin treatment.
Introduction
Ciprofloxacin is an important antimicrobial agent of the fluoroquinolone group. It is used for a wide range of infectious diseases and is considered relatively safe (1). However, fluoroquinolones produce different adverse effects including phototoxicity and photogenotoxicity (2,3). Some of the fluoroquinolones used in chemotherapy have caused DNA photodamage and phototumorigenesis (4,5). Ciprofloxacin has shown incidence of undesirable adverse effects like gastrointestinal, skin, hepatic and central nervous system function including phototoxicity (6). Gemifloxacin and ciprofloxacin caused phototoxicity in healthy volunteers (7). India is a tropical country and most of the human activities (agricultural, commercial, sports, etc.) take place in bright sunlight, therefore, use of photosensitive drugs may lead to skin disorders and immune suppression. Furthermore, human exposure to UV-R in sunlight is increasing due to ozone depletion and phototoxic effects of the drugs at the ambient level of UV-R in sunlight are worth studying. Various drugs (antibiotics, antimalarials, anti-inflammatory agents, vitamins, etc.) with UV-R are known to cause skin photosensitization via the formation of reactive oxygen species (ROS) (8,9). Information is needed regarding the phototoxicity and photocarcinogenecity of drugs for the safety of human beings (10).
UV-C and UV-B are absorbed largely within the epidermis whereas around 50% of UV-A penetrates into the dermis (11). Approximately 40% of the UV-B falling on the skin is transmitted through the stratum corneum to viable epidermis (12). Longer wavelengths are more penetrating (13). The equivalent of the entire blood volume of an adult may pass through the skin and is exposed within 20 min (14). Patients are generally unaware that longer UV-R penetrates through clouds, window glass and thin clothing and may produce phototoxic responses (15). The phototoxic effects of UV-R are related to the generation of ROS and induction of pyrimidine dimmers (16,17). ROS have been implicated in many pathological conditions including skin cancer and other adverse effects caused by UV-R (18). UV-R caused dose-dependent phototoxic effects on human erythrocytes (19). Quinolone antibiotics, viz. pefloxacin and ciprofloxacin, caused UVA-induced edema and immune suppression in mice (20).
Phototoxicity testing was recommended by the Organization for Economic Co-operation and Development, according to which in vitro tests should be carried out before considering any animal testing (21). The 3T3 NRU test was validated in 1998 for the phototoxicity assessment of chemicals including fluoroquinolones (22). In the present study, the photosensitizing potential of ciprofloxacin was evaluated at the ambient intensity of UV-R in sunlight by using two mouse fibroblast cell lines L-929 and NIH-3T3 (23,24). The phototoxicity of ciprofloxacin was assessed by using 50 to 300 μg mL−1 concentrations of the drug. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative controls, respectively. The purpose of the study was to evaluate the phototoxicity of ciprofloxacin and its mechanism of action at the ambient intensities of UV-R and sunlight by using photochemical methods and a comparative analysis of MTT and NRU assays by cell lines.
Materials and methods
Chemicals and culture wares. N,N-dimethyl-p-nitrosoaniline (RNO), SOD, nitro-blue tetrazolium (NBT), fetal bovine serum (FBS), Dulbecco’s modified eagle’s medium nutrient mixture F-12 HAM (DMEM F-12 HAM), antibiotic and antimycotic solution, trypsin, l-histidine, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), neutral red (NR), sodium azide (NaN3), DABCO, tricarboxylic acid (TCA), sodium chloride (NaCl), ascorbic acid, carbonate and phosphate buffers and 2′-dGuO were procured from Sigma (St Louis, MO). Linoleic acid and Tween-20 were obtained from M.P. Biomedicals Inc. (Solon, OH) and Hank’s Balanced Salt Solution (HBSS) was purchased from Invitrogen Corporation. Ciprofloxacin was procured from Hi-Media India Ltd. Other reagents and chemicals were procured from Hi-Media, Ranbaxy and Merck India Ltd. Potassium ferricyanide [K3Fe(CN)6], ferrous sulfate, ammonium acetate, acetylacetone and formaldehyde (HCHO) were procured from M/s Merck India and M/s Qualigens, India. Pyrogen free Milli Q double-distilled deionized water was used in the study. All plastic wares including 48-well plates and 25 cm2 (polystyrene coated) culture flasks were purchased from Nunc. Cell lines were procured from National Centre for Cell Sciences (Pune, India) and since then it was maintained in our laboratory.
Radiation source and dosimetry. The UV-irradiation system comprised an array of 1.2 m long UV-R emitting tubes manufactured by Vilber Lourmat (France). The intensity of emitted light was measured by a microprocessor-controlled RMX-3 W radiometer (Vilber Lourmat) equipped with calibrated UV-A, UV-B and UV-C detecting probes. The spectral emission of UV-A source ranged from 320 to 400 nm with a peak at 365 nm, whereas the spectral emission of UV-B source ranged from 290 to 320 nm with a peak at 312 nm. The radiation dose was measured in J cm−2. Intensities selected for irradiation were based on dosimetry carried out at our laboratory’s roof top between 12.00 P.M. and 2.00 P.M. and were parallel to the ambient intensities of UV-A and UV-B reaching in sunlight at Lucknow (26o45′N latitude and 80o50′E longitude at 146 m above the mean sea level).
Radiation exposure. Radiation (UV-A and UV-B) exposure was carried out at 25 ± 2°C in a radiation chamber. The samples were put at a distance of 22.0 cm from the source of radiation. Glass petri dishes (60 × 15 mm) were used for photochemical determination. Cell lines were exposed in 48-well culture plates. Sunlight exposure was carried out during clear sunny days between 12.00 P.M. and 2.00 P.M. at 25 ± 2°C. Petri dishes/culture plates were kept on a platform surrounded by ice packs (Polar Tech Industries, Genoa, IL) to prevent increase in temperature.
Photo-degradation of ciprofloxacin. The drug was dissolved in Milli Q deionized double distilled water and 10 μg mL−1 solution was prepared. Drug solution was exposed to sunlight, aliquots were drawn at intervals of 30 min, from 0 to 120 min. The photo-degradation spectrum of the drug was recorded between 200 and 700 nm (Cintra 40 UV-visible spectrophotometer).
Photochemical assays. Determination of singlet oxygen ( 1 O 2 ). The generation of 1O2 under aerobic conditions was measured in aqueous solution as per the method of Ray et al. (25). RNO solution (0.35–0.4 × 10−5 m) was prepared in 0.01 m potassium phosphate buffer (pH = 7.0) and l-histidine (10−2 m) was added as a selective acceptor of 1O2. A 10 mL assay solution in a petri dish with or without the drug was irradiated under UV-A (4.104–7.92 J cm−2), UV-B (2.16–4.32 J cm−2) and sunlight (60 min). The production of 1O2 was monitored by measuring the decrease in RNO absorbance at 440 nm. The generation of 1O2 was further substantiated by the administration of two specific quenchers NaN3 (1 and 2 mm) and DABCO (2 and 10 mm) (26,27).
Determination of superoxide anion radical (O 2 ḃ− ). The generation of O2ḃ− was monitored by recording the photosensitized reduction of NBT to nitro-blue diformazan (NBF) spectrophotometrically (28). NBT solution (1.67 × 10−4 m) was prepared in 0.01 m sodium carbonate buffer (pH = 10). A 10 mL assay system containing the drug (1–25 μg mL−1) was irradiated with UV-A (1.368–2.64 J cm−2), UV-B (0.72–1.44 J cm−2) and sunlight (20 min). The production of NBF was monitored by measuring the increase in the absorbance at 560 nm. The generation of O2ḃ− was further confirmed by carrying out quenching with SOD (25 U mL−1).
Determination of hydroxyl radical ( ḃ OH). The ḃOH generation was measured by ascorbic acid-iron-EDTA system proposed by Cohen (29). The iron-catalyzed oxidation of ascorbic acid at 37°C was used. The standard reaction mixture contains 100 mm potassium phosphate buffer pH 7.4, 167 μm iron-EDTA (1:2 mixture), 0.1 mm EDTA, 2 mm ascorbic acid and 33 mm dimethyl sulfoxide in a final volume of 3.0 mL and irradiated. Ascorbic acid was replaced by the drug (25–100 μg mL−1). After the completion of irradiation, 1.0 mL of TCA (17.5%, wt/vol) was added. The samples were then assayed for formaldehyde formation by the method proposed by Nash (30). Ammonium acetate acetylacetone reagent was prepared by 2.0 m ammonium acetate, 0.05 m acetic acid and 0.02 m analytical grade redistilled acetylacetone. Equal volumes of aliquot (1.5 mL) and reagent (1.5 mL) were mixed and kept at 37°C for 40 min. The production of formaldehyde was monitored at 412 nm. Further the quenching of ḃOH was performed by adding mannitol (0.5 m) and sodium benzoate (0.5 m) as specific quenchers.
Photodegradation of 2′-deoxyguanosine (2′-dGuo). To study the radiation effects on guanine base of the nucleic acid, an indirect method involving the photooxidative degradation of 2′-dGuO was used (31). 2′-dGuO was prepared in 0.01 m sodium carbonate buffer (pH = 10) and its optical density was adjusted between 1.2 and 1.4 at 260 nm. A 10 mL solution with or without the drug (20–30 μg mL−1) was irradiated under UV-A (4.104–12.312 J cm−2), UV-B (2.16–6.48 J cm−2) and sunlight (60–180 min). The degradation of 2′-dGuO was monitored by observing the decrease in absorbance at 260 nm. Degradation of 2′-dGuO was further substantiated by NaN3 (2 mm) and DABCO (10 mm) (26,27).
Linoleic acid photoperoxidation. Linoleic acid solution was prepared fresh in phosphate buffered saline (0.01 m, 0.9% NaCl, pH 7.2) using 0.05% Tween-20 as an emulsive agent. The solution containing 0.8 mm linoleic acid and variable concentrations of the drug (0–40 μg mL−1) were irradiated under ambient intensities of UV-A (1.368 J cm−2), UV-B (0.72 J cm−2) and sunlight (20 min). Linoleic acid peroxidation was measured by increase in absorbance at 233 nm (32). The quenching of linoleic acid peroxidation was carried out by SOD (25 and 50 U mL−1).
Cytotoxicity assays. Cytotoxicity assays were performed by MTT (23) and NRU methods (24).
Cell culture. The mouse fibroblast cell lines NIH-3T3 and L-929 were grown in DMEM F-12 HAM culture medium supplemented with 10% FBS and antibiotic-antimycotic solution (1.5%) at 5% CO2 and 95% relative humidity at 37°C.
Phototoxicity assay. Cultured cells were seeded in 25 cm2 tissue culture flasks. The cells were trypsinized by trypsin-EDTA (0.25%) after 36 h at 80–90% confluency, mixed with equal volume of DMEM F-12 HAM and collected in 50 mL sterilized plastic tubes. Cells were washed thrice with HBSS. 2 × 104 cells were seeded per well in 48-well plates. After incubation for 48 h, the medium was aspirated and cells were washed with HBSS, prior to start of the experiment. A basal control (cells only), dark control (drug-treated cells without light exposure) and light control (cells exposed to light only) were also run along with the experimental sets simultaneously under identical conditions. Prior to UV exposure, drug-treated cells were incubated for 30 min for the absorption of the drug by cells. Cells were then exposed to the desired UV-R/sunlight intensities followed by incubation for 30 min in a CO2 incubator. HBSS was replaced by DMEM F-12 HAM and batches were processed further for cytotoxicity assays.
Cell viability assay by MTT: In brief, cells (2 × 104) were seeded per well in 48-well plates and kept in the CO2 incubator for 48 h at 37°C prior to experiment for the proper attachment of the cells. The medium was replaced by HBSS containing the drug for exposure purpose. At the end of irradiation, HBSS was replaced by complete medium containing MTT (20 μL of MTT [5 mg mL−1] with 200 μL complete medium). The culture plates were kept in the CO2 incubator for 4 h. After incubation, the culture plates were washed twice with HBSS and 400 μL of DMSO was added to each well by pipetting up and down to dissolve the content. The absorbance was recorded at 530 nm by using multiwell microplate reader.
Neutral red uptake (NRU) assay. Briefly, after irradiation, the test drug was removed and washed with HBSS. The culture well plates were allowed to incubate for 3 h in complete medium (DMEM F-12 HAM) containing NR dye (50 μg mL−1) followed by a quick wash with fixative (1% wt/vol CaCl2; 0.5% vol/vol formaldehyde) to remove the unbounded dye. The accumulated dye was extracted with 50% ethanol containing 1% (vol/vol) acetic acid and plates were kept for 20 min on a shaker. The absorbance was recorded at 540 nm.
Statistical analysis. The mean and standard error/deviations were calculated. Student’s t-test was used to evaluate the significance.
Results
Ciprofloxacin showed a strong absorption maxima (λmax) in the UV-A (328 nm), UV-B (316 nm) and UV-C (276 nm) regions (Fig. 1). Ciprofloxacin exposed to sunlight (0–120 min) led to slight photo-degradation which increased with time. The absence of isobestic points shows that no new photoproduct was formed. As UV-C does not reach the earth’s surface, phototoxicity study was carried out under UV-A, UV-B and sunlight.
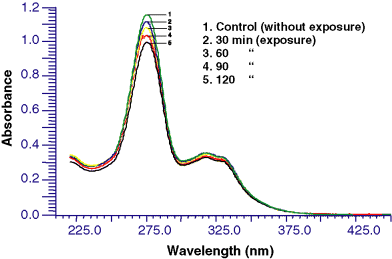
Photo-degradation spectra of ciprofloxacin at different time intervals under sunlight exposure.
Table 1 shows the photochemical generation of 1O2 at various concentrations of ciprofloxacin when exposed to different intensities of UV-A (1.14, 1.6 and 2.2), UV-B (0.6, 0.9 and 1.2 mW cm−2) and sunlight (60 min). Generation of 1O2 was monitored at 10, 20, 40, 60, 80 and 100 μg mL−1 concentrations at different doses of UV-A (4.104–7.92 J cm−2), UV-B (2.16–4.32 J cm−2) and sunlight (60 min). Ciprofloxacin at 100 μg mL−1 generated the highest amount of 1O2 under sunlight followed by UV-B and UV-A. The formation of 1O2 was dependent on the drug concentration and the dose of UV-R. Ciprofloxacin (100 μg mL−1) did not produce 1O2 in the dark. No 1O2 generation was recorded under UV-A, UV-B and sunlight without the drug. The lowest yield of 1O2 was observed 10 μg mL−1 drug concentration under UV-A (4.104 J cm−2) followed by sunlight (60 min) and UV-B (2.16 J cm−2). Similarly, the highest intensity of UV-A (2.2 mW cm−2 or 7.92 J cm−2), UV-B (1.2 mW cm−2 or 4.32 J cm−2) and sunlight (60 min) produced a higher amount of 1O2. The 1O2 producing activity, based on the use of an equivalent concentration of drug and UV-R was found in the following order—sunlight>UV-B>UV-A.
| Drug conc. (μg mL−1) | Radiation exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| UV-A | UV-B | Sunlight | ||||||
| Intensity (mW cm−2) | Dose (J cm−2) | ΔA at 440 nm | Intensity (mW cm−2) | Dose (J cm−2) | ΔA at 440 nm | Time (min) | ΔA at 440 nm | |
| 10 | 1.14 | 4.104 | 0.075 ± 0.003 | 0.6 | 2.16 | 0.226 ± 0.004 | 60 | 0.204 ± 0.022 |
| 20 | 0.161 ± 0.009 | 0.341 ± 0.006 | 0.378 ± 0.003 | |||||
| 40 | 0.216 ± 0.009 | 0.484 ± 0.006 | 0.501 ± 0.005 | |||||
| 60 | 0.274 ± 0.006 | 0.560 ± 0.039 | 0.608 ± 0.007 | |||||
| 80 | 0.338 ± 0.006 | 0.588 ± 0.033 | 0.707 ± 0.009 | |||||
| 100 | 0.356 ± 0.008 | 0.638 ± 0.024 | 0.791 ± 0.002 | |||||
| 10 | 1.6 | 5.76 | 0.173 ± 0.013 | 0.9 | 3.24 | 0.190 ± 0.005 | ||
| 20 | 0.235 ± 0.017 | 0.322 ± 0.030 | ||||||
| 40 | 0.315 ± 0.013 | 0.503 ± 0.017 | ||||||
| 60 | 0.376 ± 0.012 | 0.593 ± 0.045 | ||||||
| 80 | 0.434 ± 0.009 | 0.623 ± 0.007 | ||||||
| 100 | 0.449 ± 0.022 | 0.649 ± 0.002 | ||||||
| 10 | 2.2 | 7.92 | 0.229 ± 0.025 | 1.2 | 4.32 | 0.272 ± 0.024 | ||
| 20 | 0.351 ± 0.006 | 0.411 ± 0.014 | ||||||
| 40 | 0.460 ± 0.007 | 0.532 ± 0.014 | ||||||
| 60 | 0.527 ± 0.013 | 0.630 ± 0.004 | ||||||
| 80 | 0.572 ± 0.015 | 0.685 ± 0.020 | ||||||
| 100 | 0.596 ± 0.003 | 0.746 ± 0.007 | ||||||
- Values are mean of five observations ±SD.
In an attempt to evaluate the possible role of ROS in ciprofloxacin phototoxicity, quenchers (NaN3 and DABCO) were used. Figure 2 shows the percent photochemical quenching of 1O2 by NaN3 (1 and 2 mm) and DABCO (2 and 10 mm) at 100 μg mL−1 drug concentration under UV-A (4.104 J cm−2), UV-B (2.16 J cm−2) and sunlight (60 min). NaN3 and DABCO showed the quenching from 39.1% to 58.6% and 7.6% to 62.5%, respectively. The highest quenching (58.6%) was observed with NaN3 (2 mm) under UV-B and the lowest (7.6%) was observed with DABCO (2 mm) under sunlight.
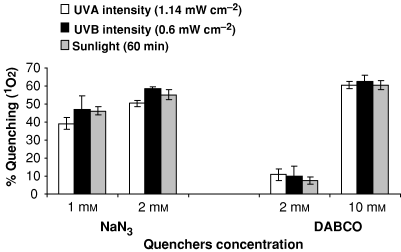
Percent photochemical quenching of 1O2 under UV-A (4.104 J cm−2), UV-B (2.16 J cm−2) and sunlight exposure (60 min) at 100 μg mL−1 ciprofloxacin. Values are mean of three observations ±SD.
Table 2 shows the photochemical generation of O2ḃ− by ciprofloxacin at various concentrations (1–25 μg mL−1) under different intensities of UV-A (1.14, 1.6 and 2.2 mW cm−2), UV-B (0.6, 0.9 and 1.2 mW cm−2) and sunlight (20 min). The highest yield of O2ḃ− was observed under sunlight (20 min) at 25 μg mL−1 drug concentration and the lowest yield by UV-A at 1.14 mW cm−2 (1.368 J cm−2) by 1 μg mL−1 drug concentration. The order of O2ḃ− generating potential of ciprofloxacin at various concentrations was sunlight>UV-A>UV-B. UV-A, UV-B and sunlight itself did not generate O2ḃ− alone. Ciprofloxacin between 1 and 25 μg mL−1 concentrations did not produce O2ḃ− in the dark. The production of O2ḃ− was concentration and dose dependent under UV-A, UV-B and sunlight exposure. Additional evidence of the production of O2ḃ− was obtained by examining the reduction in NBT and concomitantly carrying out O2ḃ− quenching studies with SOD (25 U mL−1). Quenching was 95.3% in sunlight, 92.8% in UV-A and 92.3% in UV-B.
| Drug conc. (μg mL−1) | Radiation exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| UV-A | UV-B | Sunlight | ||||||
| Intensity (mW cm−2) | Dose (J cm−2) | ΔA at 560 nm | Intensity (mW cm−2) | Dose (J cm−2) | ΔA at 560 nm | Time (min) | ΔA at 560 nm | |
| 1 | 1.14 | 1.368 | 0.011 ± 0.003 | 0.6 | 0.72 | 0.019 ± 0.008 | 20 | 0.185 ± 0.009 |
| 5 | 0.133 ± 0.004 | 0.110 ± 0.004 | 0.292 ± 0.011 | |||||
| 10 | 0.210 ± 0.011 | 0.173 ± 0.006 | 0.420 ± 0.024 | |||||
| 15 | 0.254 ± 0.015 | 0.207 ± 0.007 | 0.519 ± 0.008 | |||||
| 20 | 0.290 ± 0.013 | 0.238 ± 0.007 | 0.584 ± 0.018 | |||||
| 25 | 0.319 ± 0.011 | 0.261 ± 0.009 | 0.662 ± 0.018 | |||||
| 1 | 1.6 | 1.92 | 0.040 ± 0.002 | 0.9 | 1.08 | 0.035 ± 0.001 | ||
| 5 | 0.154 ± 0.006 | 0.154 ± 0.005 | ||||||
| 10 | 0.236 ± 0.001 | 0.239 ± 0.016 | ||||||
| 15 | 0.289 ± 0.013 | 0.302 ± 0.017 | ||||||
| 20 | 0.331 ± 0.004 | 0.348 ± 0.013 | ||||||
| 25 | 0.365 ± 0.013 | 0.438 ± 0.006 | ||||||
| 1 | 2.2 | 2.64 | 0.059 ± 0.009 | 1.2 | 1.44 | 0.076 ± 0.003 | ||
| 5 | 0.192 ± 0.013 | 0.208 ± 0.005 | ||||||
| 10 | 0.275 ± 0.020 | 0.323 ± 0.014 | ||||||
| 15 | 0.430 ± 0.013 | 0.412 ± 0.008 | ||||||
| 20 | 0.496 ± 0.009 | 0.492 ± 0.012 | ||||||
| 25 | 0.544 ± 0.009 | 0.525 ± 0.003 | ||||||
- Values are mean of five observations ±SD.
Table 3 shows the photodynamic degradation of 2′-dGuO at two concentrations of ciprofloxacin (20 and 30 μg mL−1) under various intensities of UV-A (1.14, 1.6 and 2.2 mW cm−2), UV-B (0.6, 0.9 and 1.2 mW cm−2) and sunlight (60–180 min). Photo-degradation was carried out at different doses of UV-A (4.104–23.76 J cm−2), UV-B (2.16–12.9 J cm−2) and sunlight (60–180 min). The highest degradation of 2′-dGuO was observed at 180 min under sunlight followed by UV-B (12.96 J cm−2) and UV-A (23.76 J cm−2). The 2′-dGuO degradation was low at lower intensities and increased with higher intensities and doses. The rate of photo-degradation of 2′-dGuO was dose and concentration dependent in the following order—sunlight>UV-B>UV-A.
| Drug conc. (μg mL−1) | Radiation exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| UV-A | UV-B | Sunlight | ||||||
| Intensity (mW cm−2) | Dose (J cm−2) | Percent degradation | Intensity (mW cm−2) | Dose (J cm−2) | Percent degradation | Time (min) | Percent degradation | |
| 20 | 1.14 | 4.104 | 2.82 ± 0.653 | 0.6 | 2.16 | 6.24 ± 0.732 | 60 | 16.1 ± 0.173 |
| 30 | 4.43 ± 1.159 | 13.2 ± 1.05 | 21.5 ± 0.300 | |||||
| 20 | 8.208 | 7.16 ± 0.409 | 4.32 | 11.5 ± 0.395 | 120 | 24.9 ± 0.115 | ||
| 30 | 7.98 ± 0.487 | 17.4 ± 0.171 | 27.6 ± 1.21 | |||||
| 20 | 12.312 | 12.0 ± 1.473 | 6.48 | 13.2 ± 1.16 | 180 | 26.4 ± 1.60 | ||
| 30 | 15.0 ± 0.208 | 19.1 ± 0.459 | 33.5 ± 1.36 | |||||
| 20 | 1.6 | 5.76 | 3.94 ± 0.806 | 0.9 | 3.24 | 9.75 ± 0.95 | ||
| 30 | 4.03 ± 0.339 | 16.1 ± 0.216 | ||||||
| 20 | 11.52 | 11.7 ± 0.400 | 6.48 | 13.8 ± 0.152 | ||||
| 30 | 14.9 ± 0.603 | 22.3 ± 0.405 | ||||||
| 20 | 17.28 | 16.8 ± 0.473 | 9.72 | 18.5 ± 1.42 | ||||
| 30 | 19.0 ± 0.208 | 26.4 ± 0.803 | ||||||
| 20 | 2.2 | 7.92 | 5.17 ± 0.123 | 1.2 | 4.32 | 6.62 ± 0.406 | ||
| 30 | 11.8 ± 0.961 | 15.7 ± 1.23 | ||||||
| 20 | 15.84 | 13.4 ± 0.346 | 8.64 | 18.9 ± 0.512 | ||||
| 30 | 18.9 ± 0.839 | 24.5 ± 1.93 | ||||||
| 20 | 23.76 | 22.1 ± 0.964 | 12.96 | 22.4 ± 1.13 | ||||
| 30 | 26.2 ± 1.138 | 28.7 ± 1.37 | ||||||
- Values are mean of five observations ±SD.
Figure 3 shows the percent quenching of photodynamic degradation of 2′-dGuO by 1O2 quenchers NaN3 (2 mm) and DABCO (10 mm) under UV-A (4.194 J cm−2), UV-B (2.16 J cm−2) and sunlight (60 min). NaN3 has greater quenching ability than DABCO. The highest quenching was observed under UV-B (95.83%) in comparison with sunlight (68.5%) and UV-A (69.9%). Quenching under UV-A and sunlight was more or less the same.
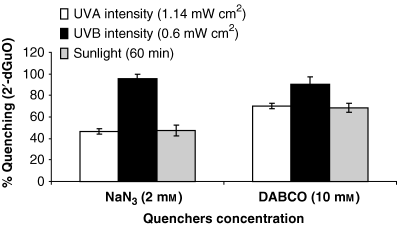
Percent quenching of photodynamic degradation of 2′-dGuO by specific 1O2 quenchers under UV-A (4.194 J cm−2), UV-B (2.16 J cm−2) and sunlight exposure (60 min). Values are mean of three observations ±SD.
Figure 4a–d shows the photosensitizing effects of ciprofloxacin at various concentrations from 50 to 300 μg mL−1 on the L-929 cell line by recording percent cell viability. The phototoxicity of ciprofloxacin in the L-929 cell line was assessed using MTT assay after 30 min of irradiation. Cytotoxicity was also observed with different drug concentrations without irradiation. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. The highest decrease in percent cell viability was observed at 90 min while the lowest decrease was observed at 30 min exposure at all the drug concentrations from 50 to 300 μg mL−1.
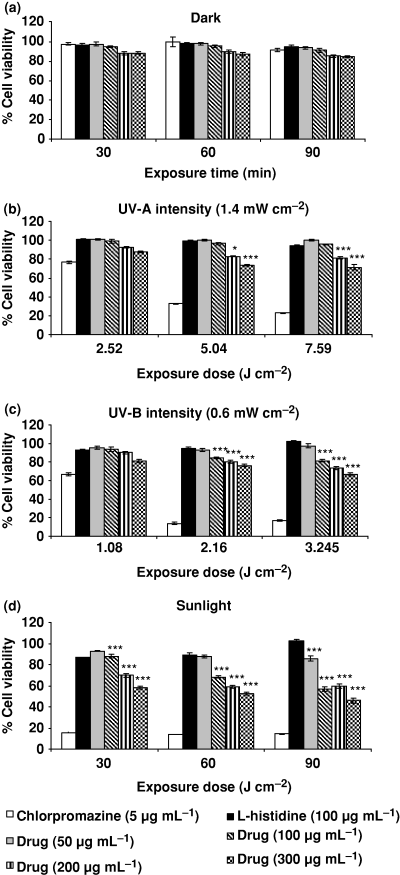
Photosensitizing effect of various concentrations of ciprofloxacin on mouse fibroblast L-929 cell line as percent cell viability by recording mitochondrial dehydrogenase activity (MTT assay) under exposure of (a) dark, (b) UV-A, (c) UV-B and (d) sunlight. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. Data expressed are mean ± SE for four independent experiments. *P < 0.02, ***P < 0.001 as per Student’s t-test.
Figure 4a shows percent cell viability at different concentrations (50–300 μg mL−1) of ciprofloxacin under dark conditions. There was no significant reduction in percent cell viability at 50 and 100 μg mL−1 at 30, 60 and 90 min but at higher concentrations (200 and 300 μg mL−1) a decrease in cell viability was observed. 200 μg mL−1 drug concentration showed 12%, 13% and 15% reduced cell viability in dark at 30, 60 and 90 min, respectively. Similarly, 300 μg mL−1 drug concentration showed reduction in cell viability by 12%, 13% and 16% at 30, 60 and 90 min, respectively.
Figure 4b shows the effect of drug concentrations (50–300 μg mL−1) on percent cell viability at 2.52, 5.04 and 7.56 J cm−2 doses of UV-A (1.4 mW cm−2).The drug concentrations at 200 and 300 μg mL−1 caused significant (P < 0.001) reduction in cell viability by 27% and 29%, respectively, at 7.56 J cm−2 UV-A exposure.
Figure 4c shows the percent cell viability of the L-929 cell line under UV-B (0.6 mW cm−2) exposure at 1.08, 2.16 and 3.245 J cm−2. At 1.08 J cm−2, cell viability was reduced. At 2.16 J cm−2, cell viability reduction was significant (P < 0.001) at 100, 200 and 300 μg mL−1 drug concentrations. The highest reduction (34%) was observed under 3.245 J cm−2 at 300 μg mL−1 drug concentration.
Figure 4d shows the photosensitizing potential of ciprofloxacin under sunlight exposure from 30 to 90 min at different concentrations. The highest reductions in cell viability were 42%, 48% and 54% at 300 μg mL−1 drug concentration (P < 0.001) at 30, 60 and 90 min, respectively.
Figure 5a–d shows the relative photosensitizing effect of ciprofloxacin at various concentrations (50–300 μg mL−1) on the L-929 cell line by recording NRU as percent cell viability. L-929 was exposed to the drug for various radiation doses of UV-A (1.4 mW cm−2) from 2.52 to 7.56 J cm−2, UV-B (0.6 mW cm−2) from 1.08 to 3.245 J cm−2 and sunlight exposure (30–90 min). Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. It is evident from the data that the decrease in cell viability was concentration and dose dependent.
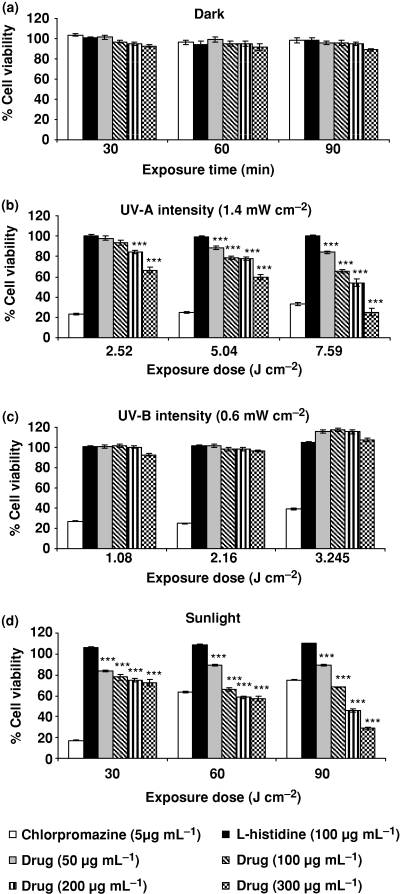
Photosensitizing effect of ciprofloxacin at various concentrations (50–300 μg mL−1) on L-929 mouse fibroblast cell line by recording NRU in lysosomes as percent cell viability. (a) Dark, (b) UV-A, (c) UV-B and (d) sunlight exposure. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. Values expressed are mean ± SE for four independent experiments. ***P < 0.001 as per Student’s t-test.
Figure 5a shows percent cell viability of L-929 at different drug concentrations (50–300 μg mL−1) under dark conditions. No significant reduction in percent cell viability was observed.
Figure 5b shows the effect of different doses of UV-A (2.52, 5.04 and 7.56 J cm−2) on percent cell viability. At 2.52 J cm−2 exposure dose, a significant (P < 0.001) reduction in cell viability was observed at 200 and 300 μg mL−1 drug concentrations. Exposure to UV-A at 5.04 and 7.56 J cm−2 showed significantly (P < 0.001) reduced cell viability at all the drug concentrations. The cell viabilities at 5.04 J cm−2 dose level were 78.0% and 59.5% at 200 and 300 μg mL−1 drug concentrations, respectively. Similarly, at 7.56 J cm−2 dose level the cell viabilities were 54.1% and 25.2% at 200 and 300 μg mL−1 drug concentrations, respectively. The data clearly show that the decrease in cell viability was dose and concentration dependent.
Figure 5c shows the percent cell viability of the L-929 cell line under UV-B exposure at 1.08, 2.16 and 3.245 J cm−2. No significant change in percent cell viability was observed.
Figure 5d shows the photosensitizing potential of ciprofloxacin under sunlight exposure from 30 to 90 min. Dose- and concentration-dependent significant (P < 0.001) reduction in cell viability was observed at all the drug concentrations.
Figure 6a–d shows the relative photosensitizing response of ciprofloxacin on the NIH-3T3 cell line by using mito-chondrial dehydrogenase activity as percent cell viability. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. NIH-3T3 was exposed with drug under UV-A (1.4 mW cm−2 intensity) at 2.52, 5.04 and 7.56 J cm−2, UV-B (0.6 mW cm−2 intensity) at 1.08, 2.16 and 3.24 J cm−2 and sunlight at 30, 60 and 90 min.
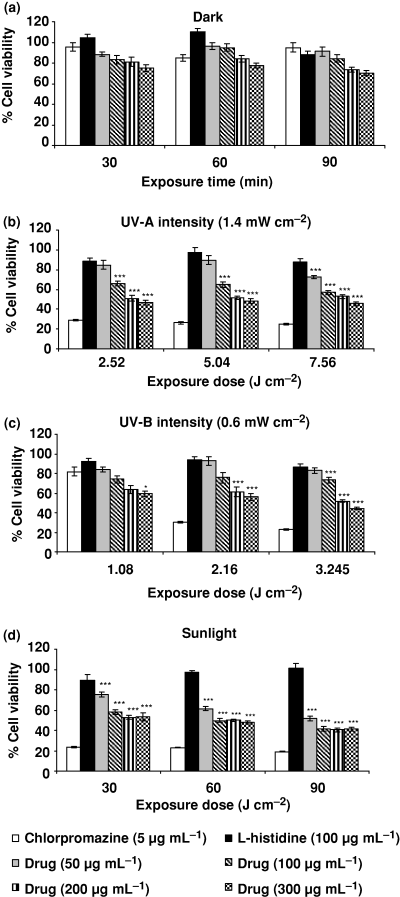
Photosensitizing response of ciprofloxacin on the NIH-3T3 cell line by using mitochondrial dehydrogenase activity (MTT assay) as percent cell viability. (a) Dark, (b) UV-A, (c) UV-B and (d) sunlight. Chlorpromazine (5 μg mL−1) and l-histidine (100 μg mL−1) were used as positive and negative photosensitizers, respectively. Values expressed are mean ± SE for four independent experiments. ***P < 0.001 as per Student’s t-test.
Figure 6a shows the percent cell viability at different concentrations (50–300 μg mL−1) of ciprofloxacin under dark conditions. There were mild reductions in percent cell viability by 19%, 16% and 27% at 200 μg mL−1 and 25%, 23% and 30% at 300 μg mL−1 concentration under 30, 60 and 90 min exposure, respectively. Lower concentrations of drug did not have any impact on cell viability.
Figure 6b shows the effect of UV-A (1.4 mW cm−2 intensity) exposure from 2.52 to 7.56 J cm−2 on percent cell viability. UV-A alone did not show any effect. A significant (P < 0.001) reduced cell viability was observed at all drug concentrations under UV-A irradiation of 2.52, 5.04 and 7.56 J cm−2. The maximum reduced cell viability was observed at 300 μg mL−1 concentration at all the doses of UV-A.
Figure 6c shows the percent cell viability under UV-B exposure at 1.08 to 3.24 J cm−2. A significant reduction (P < 0.001) in percent cell viability was observed at all the drug concentrations at 3.24 J cm−2. The highest reduction (56%) in cell viability was observed at 300 μg mL−1. At 2.16 J cm−2 dose level, a significant (P < 0.001) reduction in percent cell viability was observed at 200 and 300 μg mL−1 drug concentrations. At 1.08 J cm−2 dose level a significant (P < 0.02) reduction in cell viability was observed only at the highest concentration (300 μg mL−1).
Figure 6d shows the photosensitizing potential of cipro-floxacin under sunlight exposure. A significant reduction in percent cell viability was observed at all drug concentrations from 50 to 300 μg mL−1. The highest reduction (59%) was observed at 300 μg mL−1 for 90 min exposure.
Figure 7 shows the photo-peroxidation of linoleic acid by ciprofloxacin under aerobic conditions in aqueous buffer (pH 7.2) solution by UV-A (1.14 mW cm−2) 1.368 J cm−2, UV-B (0.6 mW cm−2) 0.72 J cm−2 and sunlight (20 min). Different drug concentrations from 5 to 40 μg mL−1 were selected for exposure. Lipid peroxidation was higher under sunlight followed by UV-B and UV-A. Lipid peroxidation was dose and concentration dependent under all radiation doses. Linoleic acid itself caused lipid peroxidation under UV-R/sunlight but higher yield was observed with different drug concentrations.
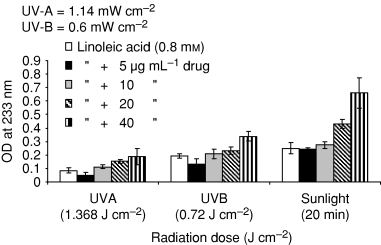
Photoperoxidation of linoleic acid by ciprofloxacin under UV-A (1.368 J cm−2), UV-B (0.72 J cm−2) and sunlight exposure (20 min). The formation of dienic hydroperoxides was followed by the increase in absorption at 233 nm. Values presented are mean of four observations ±SD.
Figure 8 shows the percent quenching of linoleic acid peroxidation caused by ciprofloxacin (20 μg mL−1) through SOD (25 and 50 U mL−1) at UV-A (1.368 J cm−2), UV-B (0.72 J cm−2) and sunlight (20 min). The percent quenching of linoleic acid peroxidation by SOD (25 U mL−1) were 23.3%, 25.3% and 24.0% under UV-A, UV-B and sunlight, respectively. The percent quenching by SOD (50 U mL−1) were 51.6%, 34.9% and 43.3% under UV-A, UV-B and sunlight, respectively. The highest quenching was observed under UV-A and the lowest under UV-B while sunlight showed moderate quenching.
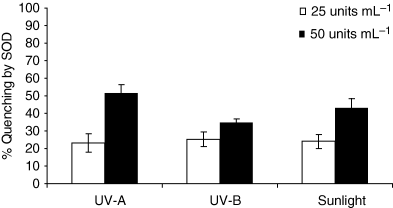
Photochemical quenching of linoleic acid (0.8 mm) peroxidation by SOD (25 and 50 U mL−1) under UV-A (1.368 J cm−2), UV-B (0.72 J cm−2) and sunlight exposure (20 min). Values presented are mean of four observations ±SD.
Figure 9 shows the photochemical generation of ḃOH at various concentrations of ciprofloxacin (25–100 μg mL−1) under UV-A (4.32 J cm−2), UV-B (2.16 J cm−2) and sunlight (60 min). The drug-induced ḃOH generation was concentration dependent. The maximum generation of ḃOH was observed under sunlight followed by UV-A and UV-B.
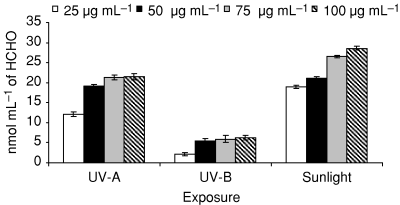
Photochemical generation of ḃOH at various concentrations of ciprofloxacin under UV-A (4.32 J cm−2), UV-B (2.16 J cm−2) and sunlight exposure (60 min). Values presented are mean of three observations ±SD.
Figure 10 shows the percent quenching of ḃOH under UV-A (4.32 J cm−2) at 100 μg mL−1 ciprofloxacin by mannitol (0.5 m) and sodium benzoate (0.5 m). Both the quenchers caused 20% to 30% quenching of ḃOH. Higher quenching was observed by sodium benzoate than by mannitol.
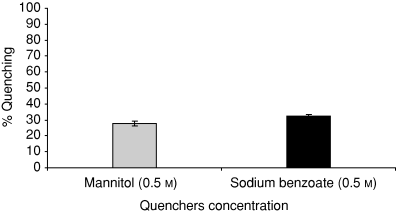
Percent quenching of ḃOH at 100 μg mL−1 ciprofloxacin by mannitol and sodium benzoate under UV-A (4.32 J cm−2). Values presented are mean of three observations ±SD.
Discussion
Ciprofloxacin is a quinolone carboxylic acid derivative and has gram-negative and gram-positive bactericidal activities. It is rapidly absorbed in the body. Three major metabolites, desethylene-ciprofloxacin (metabolite M1), sulfo-ciprofloxacin (metabolite M2) and oxo-ciprofloxacin (metabolite M3) are formed in the body fluids (33). Its irradiation under sunlight leads to slight photodegradation. The absence of isobestic points shows that no new photoproduct was formed under sunlight irradiation. This study suggests that ROS generation and DNA damage by photoilluminated ciprofloxacin were mainly responsible for phototoxicity on L-929 and NIH-3T3 cell lines. Perhaps, this is the first report demonstrating the phototoxicity of ciprofloxacin and its mechanism under ambient levels of UV-A, UV-B and sunlight. Sunlight is a broad spectrum of different wavelengths containing UV-A, UV-B and many other radiations, therefore the effect of sunlight may be cumulative and higher. Earlier studies were performed at higher doses of UV-R (34,35). The fluoroquinolones absorb light in the UV-A wavelength from 320 to 330 nm and produce ROS such as 1O2, O2ḃ−, H2O2 and ḃOH. Thus the photodynamic generation of ROS may be the basis of phototoxicity of quinolones in human beings and animals (36). Our study indicates that the commonly used ciprofloxacin was generating 1O2, O2− and ḃOH through photosensitized mechanisms, in which the photoexcited drug (triplet state) transfers its energy to O2 and generates energy-rich 1O2 (via Type II photodynamic reaction), an active oxidizing agent. Another oxygen-dependent reaction involves the photoreaction of an electronically active photosensitizer, releases the electron or by hydrogen transfer from a compound to O2 and produces O2ḃ− and ḃOH (via Type I photodynamic reaction). Quenching studies with specific quenchers of 1O2 and ḃOH showed that 1O2 and ḃOH were predominant in photo-degradation of 2′-dGuO. Sodium azide and DABCO were able to control the generation of 1O2 and mannitol, and sodium benzoate was able to quench ḃOH. Quenching with SOD confirms the generation of O2ḃ− by ciprofloxacin. Rosen et al. (37) have worked out the mechanism of ROS generation under UV-A-exposed lomefloxacin and ciprofloxacin at the exposure of 20 J cm−2 and found that the phototoxicity of these drugs was mediated through photodynamic reactions. In our study ambient levels of UV-A and UV-B generated ROS through photodynamic action. Sunlight (60 min) exposure also produced similar effects which were not reported earlier. Our earlier studies have shown that the ROS generation and photodegradation of 2′-dGuO are important factors in phototoxicity (25,28,38,39). Photosensitization appears to be the main cause of phototoxicity of quinolones, particularly in older patients with long-term use (40). Double-blind placebo and skin phototesting potential in human beings were investigated by systemically administered fluoroquinolones (41) but these studies did not offer any mechanistic approach. Due to the complexity of the biological system, it is difficult to demonstrate clearly the specific role of ROS in the manifestation of phototoxicity in in vivo studies. It is clear that 1O2 is an important ROS in DNA damage under in vitro conditions (42,43). In photosensitization reactions 1O2, O2ḃ− and ḃOH are formed which on interconversion lead to the generation of H2O2 (9). These reactive forms of oxygen may be responsible for linoleic acid peroxidation (32).
2′-dGuO was used to assess the nuclear damage caused by sensitivity of the guanine base to UV-R/sunlight. Significant photo-degradation of 2′-dGuO was observed by the photosensitized ciprofloxacin. Lomefloxacin and ciprofloxacin showed the photodynamic ability of 8-oxo, 7-8-dihydro-2′-deoxyguanosin formation in adult rat liver-18 cells under UV-A exposure. Our observation is in accordance with that of Rosen et al. (37). Commonly used antibiotics such as cephaloridine, cephalexin and cephradine were found to generate significant amounts of 1O2 and caused photo-degradation of 2′-dGuO under UV-R (25). Antibiotics like lomefloxacin and enoxacin generated 1O2 and O2ḃ− under UV-R and sunlight, caused lipid peroxidation in human blood and photodegraded 2′-dGuO in vitro (39). The highly photoreactive lomefloxacin and BAYy3118 have caused photo-induced DNA strand breaks (3). Photoirradiation of neuroleptic drugs with cells released lactate dehydrogenase and caused the loss of mitochondrial NADH dehydrogenase activity, indicating that plasma membrane and mitochondria were the targets of phototoxicity (35).
UV-R-induced impairment of proteasome function has been investigated in human keratinocyte culture (44). UV-R caused delayed mutations and chromosomal instability in fibroblasts (45). Ciprofloxacin significantly reduced the cell viability of the NIH-3T3 cell line compared with the L-929 cell line. The MTT assay results were comparatively significant in the NIH-3T3 cell line. A high reduction of cell viability in the NIH-3T3 cell line under sunlight exposure had demonstrated that the damaging potential of sunlight was more lethal compared with UV-A and UV-B. The selection of different intensities of UV-A and UV-B radiation prove our viewpoint that higher intensities of UV-A and UV-B radiation in sunlight (in view of ozone depletion) would be more deleterious. Therefore, the measurement of ambient intensity and the total dose is an important factor for phototoxicity assessment.
The results show that MTT and NRU assays are high quality and suitable for identifying phototoxicity. Lomefloxacin-treated normal human skin cells showed UV-A-induced DNA strand breaks and pyrimidine dimmers in genomic DNA. The phototoxicity of the drug can be drawn by different mechanisms. The photobiological impact of the drug varies according to cell types (46). The effect on L-929 and NIH-3T3 cell lines point toward the involvement of ROS and DNA damage in phototoxicity. It may suggest a link between the phototoxic and photocarcinogenic potential of ciprofloxacin. The phototoxicity of the drug on cell lines and lipid peroxidation of linoleic acid suggest that the ROS might initiate cellular lipid peroxidation and DNA damage which finally leads to cell death.
To examine the validity of this hypothesis, linoleic acid photo-peroxidation was used as a model system (47,48). The ability of ciprofloxacin to induce photodynamic lipid peroxidation and cell damage may be expected in vivo. SOD was used as an antioxidant/quencher for the protection/quenching of linoleic acid peroxidation. In an earlier study SOD was used as a specific quencher of O2ḃ− (28). Percent quenching of linoleic acid peroxidation by SOD accorded the involvement of ROS in ciprofloxacin phototoxicity. The generation of the ḃOH radical by ciprofloxacin under sunlight and UVR is a significant indication of the reactivity of photosensitized ciprofloxacin toward nucleic acid bases. This was further confirmed by the quenching of the ḃOH radical by specific quenchers (mannitol and sodium benzoate). The production of ROS, photo-degradation of 2′-dGuO and photodamage in cell lines (in vitro) appeared to be the possible mechanisms of ciprofloxacin phototoxicity and photogenotoxicity (49).
In conclusion, the study suggests that ciprofloxacin generates oxygen-dependent Type I and Type II photosensitizing reactions, producing 1O2, O2ḃ− and ḃOH which leads to photo-degradation of 2′-dGuO, linoleic acid peroxidation and cell damage. The drug is generally used for a period of 1 week and above for the treatment, so the use of drug and sunlight exposure may be harmful and lead to phototoxic/photogenotoxic effects. This advocates avoiding outdoor activities (especially in peak hours) during ciprofloxacin treatment.
Acknowledgments
Acknowledgements— The authors are thankful to the Director, ITRC, for his keen interest in the study and to Mr. B. D. Bhattacharji for editing the manuscript. This study was financially supported in part by the Council of Scientific & Industrial Research, New Delhi, under Network project (CMM-0018).




