Chemiluminescence Associated with Singlet Oxygen Reactions with Amino Acids, Peptides and Proteins†
From coauthors Emilio Alarcón, Carola Henríquez and Alexis Aspée: This paper is part of a symposium-in-print dedicated to Professor Eduardo A. Lissi on the occasion of his 70th birthday.
Abstract
Low level chemiluminescence (CL) is observed after protein oxidation mediated by singlet oxygen produced in Rose Bengal (RB) irradiation. This CL lasts for several minutes after the end of the photolysis. In this work, the mechanism of the process was assessed from the spectral characteristics of the CL and the effect of antioxidants (Trolox or ascorbate), Ebselen (a compound with peroxidase-like activity), azide (a singlet oxygen scavenger) and D2O, added prior to or after RB irradiation. It is concluded that most of the light emission is due to formation of excited states generated in the decomposition of peroxides and/or hydroperoxides accumulated during the photolysis. Experiments carried out in the presence of several amino acids (Cys, Met, His, Tyr and Trp) and di- and tripeptides suggest that peroxides (and/or hydroperoxides) of Trp residues are mainly responsible for the CL observed after singlet oxygen-mediated protein oxidation. The much weaker CL observed after the oxidation of proteins without Trp residues supports this conclusion. A comparison of the results obtained employing free Trp, Ala-Trp and Trp-Ala dipeptides, Ala-Trp-Ala tripeptide and Trp-containing proteins supports the conclusion that blocking the amino group of the Trp moiety strongly increases the efficiency of the chemiluminescent process, producing ≈2.5×10−8 photons per oxidized Trp group in Ala-Trp. A mechanism comprising two chemiluminescent oxidation pathways of Trp residues is proposed to explain the results.
Introduction
The oxidation of organelles, cells, tissues or whole animals leads to the emission of low level chemiluminescence (CL) (1–6). This phenomenon is also a general feature of the oxidation of most biomolecules. In particular, the oxidation of amino acids and proteins by peroxyl radicals (7,8), hypochlorite (9–11) or peroxynitrite (12,13) leads to CL emission in the visible region. Similar emissions are observed in the Cu(II), Fe(II) and peroxyl radical-promoted oxidation of lipoproteins (14,15). From the time profile of the emission, as well as the response of the emitted intensity to the addition of free radical scavengers and compounds with peroxidase-like activity, it has been concluded that this emission is due to the decomposition of peroxides and/or endoperoxides formed during the oxidation process (7–9,15,16). The thermal decomposition of these intermediates would lead to the formation of the excited carbonyls responsible of the emitted CL (7,9).
In a series of works, Davies et al. (17–20) have shown that the interaction of singlet oxygen with proteins leads to the formation of unstable peroxidated intermediates, mostly associated with the oxidation of tyrosyl, histidyl and triptophanyl groups. These compounds could be potential precursors of excited carbonyls. In order to test this possibility, we have evaluated the intensity and characteristics of the visible CL that follows the interaction of singlet oxygen with free amino acids, peptides and proteins.
Materials and Methods
Materials. Rose Bengal (RB); 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox); 2-phenyl-1,2-benzisoselenazol-3[2H]-one (Ebselen); Xylenol Orange; reduced glutathione; l-tryptophan (98%); l-tyrosine (Tyr) (>99%); l-cysteine (>99%); l-methionine (Met) (>99%); l-histidine (His) (>99%); Ala-Trp (>99%); Trp-Ala (>99%); Ala-Tyr (>99%); Tyr-Ala (>99%); Gly-His (>99%); His-Gly (> 99%); ascorbic acid; lipid-free albumin from human serum (HSA); lipid-free albumin from bovine serum (BSA); phosphatase alkaline type I-S from bovine intestinal mucous (AP); lysozyme from chicken egg white (LYZ); ribonuclease A, type III-A from bovine pancreas (RA); insulin from bovine pancreas (INS); catalase from bovine liver; and Poly-l-Trp were purchased from Sigma (USA). Ala-Trp-Ala (94%) and Ala-Tyr-Ala (>99%) were from Bachem Feinchemikalien AG (Switzerland). Solutions were prepared employing twice-distilled water pretreated with Chelex-100 (Sigma), 1 g L−1 for 12 h.
Singlet oxygen reactions. Singlet oxygen was generated in the irradiation of RB in air-saturated solutions. To this purpose, light from a halogen lamp was filtered through a 400 nm cut-off glass filter. The solution containing the RB (5 μM) and the target molecule (amino acids, peptides or proteins) in 10 mM phosphate buffer, pH 7.0, were kept at fixed temperature (4°C or 25°C) during the irradiation (5 min.). Peroxide formation was evaluated by a FOX methodology based on the formation of a Xylenol Orange/Fe(III) complex following the peroxide promoted oxidation of Fe(II) in a 1 mM phosphate buffer solution. The formation of the complex was quantified by the absorption of the sample at 560 nm (21). Calibrations carried out in the presence of the buffer were indistinguishable from those obtained in distilled water.
Measurement of CL from pre-irradiated samples. CL intensities were measured in a Beckman LS 6500 scintillation counter working in the out-of-coincidence mode, using the narrow tritium iso-set module. The photomultiplier had a nearly constant response up to 650 nm. The equipment was calibrated employing the hemin-hydrogen peroxide-luminol reaction as a standard (9), rendering an efficiency of 2.3 × 1021 counts/Einstein.
After irradiation of solutions containing RB and the substrates, 3 mL of the sample was transferred to a polyethylene vial and introduced into the scintillation counter. The elapsed time from irradiation to the start of the CL measurement was about 30 s. When the photolysis was carried out at 4°C, the CL was measured in solutions kept at near 0°C by their introduction into previously frozen vials containing 0.5 g of ice (buffer solution). Each one of these vials was placed in a larger glass vial to minimize its heating rate during the recording time.
The spectral distribution of the CL was assessed from the changes in intensity observed when different filtering solutions were introduced between the polyethylene vials containing the solution and the external glass vials. Three filters were employed: a concentrated solution of RB (cut-off 580 nm), and solutions of potassium dichromate (0.05 M) in either 6 M HCl (cut-off 540 nm) or 3 M NaOH (cut-off 490 nm).
In order to establish the mechanism of the CL, antioxidants (700 μM Trolox or 100 μM ascorbic acid), a peroxidase-like compound (120 μM Ebselen), Fe(II) (5 mM FeSO4 ) or a metal chelator (0.1 mM dietylentriaminepentaactic acid [DTPA]) were added after the irradiation. In order to evaluate the role of singlet oxygen in the formation of the CL precursors and in the CL process, azide (1 or 5 mM) was added prior to or after the RB irradiation. To further assess the role of singlet oxygen in the production of the CL precursors, irradiations were also performed in deuterated phosphate buffer, pD 7.0, or in enriched deuterium oxide solutions. In some experiments, photolyzed solutions were diluted in heavy water (10:1) in order to assess the role of singlet oxygen in the after-photolysis emission.
Results and Discussion
CL following the reaction of free amino acids with singlet oxygen
The CL profiles of amino acid solutions after irradiation (500 μM, 5 min irradiation) are shown in Fig. 1. These data show that only pre-irradiated solutions containing Tyr and Trp, and to a minor extent those containing His, produce a CL significantly larger than that of the control sample (RB irradiated alone). The CL profile can be well fitted to biexponential decays. These decays are dominated by the fast component, whose lifetimes and amplitudes are included in Table 1. These values show that the CL precursors generated in the three systems have lifetimes shorter than 5 min.
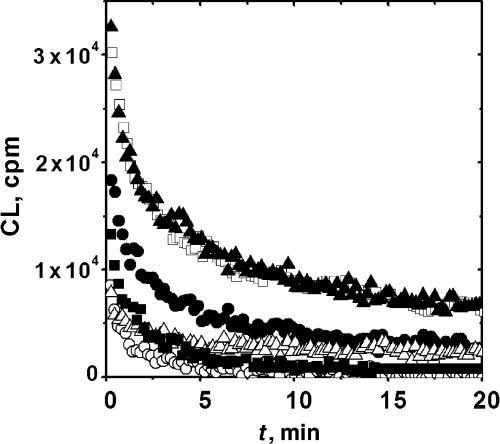
Time profile of the chemiluminescence observed after the irradiation (5 min) of Rose Bengal in the presence of different amino acids. Data obtained employing 5 μM RB and 500 μM amino acid: Trp (), Tyr (□), Met (○), Cys (Δ) and His (•). Chemiluminescence in control experiments in the absence of amino acids (). Chemiluminescence measurements were started 30 s after irradiation. Irradiation and CL measurements were carried out at 25°C.
| Compound | Amplitude (104 counts min−1) | Lifetime (min) |
|---|---|---|
| Histidine | 1.1 | 0.79 |
| Tryptophane | 2.14 | 0.47 |
| Tyrosine | 1.72 | 0.84 |
| Gly-His | 1.4 | 0.6 |
| Hist-Gly | 0.9 | 0.6 |
| Tyr-Ala | 1.4 | 1.45 |
| Ala-Tyr | 1.3 | 0.86 |
| Ala-Tyr-Ala* | 3.0 | 5.0 |
| Ala-Trp* | 4900 | 1.14 |
| Ala-Trp* (0°C) | 4100 | 5.1 |
| Trp-Ala | 1.8 | 1.8 |
| Trp-Ala* (0°C) | 6.3 | 1.7 |
| Ala-Trp-Ala | 28 (6.5) | 0.53 (3.2) |
| Ala-Trp-Ala (0°C) | 1300 (14.3) | 0.95 (11) |
| Poly-l-Trp† | 356.7 | 1.6 |
- *Data fitted to a monoexponential decay. †75 μM. Values given are averages of at least two independent measurements. Reproducibility was better than 10%. Values in parenthesis represent the amplitude and lifetime of the slow component. Chemiluminescence profiles were fitted to a biexponential decay. CL, chemiluminescence; RB, Rose Bengal.
CL following the reaction of peptides with singlet oxygen
In order to evaluate the effect of the peptide linkage upon the formation of CL precursors, the emission from several peptides containing either Trp, His or Tyr moieties were measured. The values obtained are included in Table 1. The data show that the presence of the peptide linkage produces only minor changes in the CL of Tyr or His groups, although the decay seems to be slower in the Tyr tripeptide than in the corresponding dipeptides.
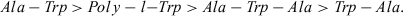
In order to see if these large differences were due to a fast decay during the time elapsed between photolysis and CL measurements, we carried out photolysis and CL measurements at lower temperatures. As expected, slower decays were observed, allowing a more precise estimation of the “initial” CL. The values obtained under these conditions showed a much smaller difference between Ala-Trp and Ala-Trp-Ala. This indicates that the large difference observed between these compounds when the measurements were carried out at 25°C was mostly due to a faster decay of the CL precursors in the tripeptide. On the other hand, a difference of ca three orders of magnitude remained in the emissions of Ala-Trp and Trp-Ala, reflecting true differences in the efficiency of CL precursor formation.
Time profiles of the CL arising from pre-irradiated samples containing Ala-Trp and Trp-Ala are shown in Fig. 2. The data showed a noticeable difference between the behavior of the two peptides, a difference that was emphasized by the data collected in Table 1. In fact, while the behavior of Trp-Ala was similar to that of free Trp, the Ala-Trp dipeptide presented a CL that was nearly three orders of magnitude higher than that of the free peptide. The same difference was observed when the photolysis and the CL measurements were carried out at a lower temperature, a condition that should minimize the decomposition of the intermediates in the time elapsed between the photolysis and the CL intensity measurement. The tripeptide, particularly at the lower temperature, had an emission of the same order of magnitude as the Ala-Trp dipeptide, and much higher than that of the Trp-Ala dipeptide. These results indicate that formation of the peptide linkage at the amino group of the Trp moiety leads to a noticeable increase in emission intensity. This difference is not due to differences in the extent of oxidation, as the consumption of the compounds during the irradiation period, as well as the amount of peroxides, is similar (Table 2).
Time profile of the chemiluminescence observed after the irradiation of Rose Bengal in the presence of Ala-Trp and Trp-Ala. Data obtained employing 5 μM RB and 500 μM dipeptide: Ala-Trp (•), Trp-Ala (○). Chemiluminescence measurements were started 30 s after irradiation. Irradiation and CL measurements were carried out at 25°C.
| Compound | Initial rate (μM min−1) | *Peroxides (μM) |
|---|---|---|
| Trp | 14.0 ± 0.8 | 2.8 |
| Ala-Trp | 13.0 ± 0.5 | 3.1 |
| Trp-Ala | 11.0 ± 0.6 | 5.8 |
| Ala-Trp-Ala | 8.0 ± 0.6 | 1.2 |
| Tyr | 0.59 ± 0.01 | 9.8 |
| Ala-Tyr | 0.69 ± 0.01 | 9.2 |
| Tyr-Ala | 0.38 ± 0.02 | 14.0 |
| Ala-Tyr-Ala | 0.31 ± 0.02 | 9.5 |
- *Values given are averages of at least two independent measurements. Reproducibility was better than 10%. The peroxide concentration was evaluated after 5 min of irradiation by FOXs method.
CL following the reaction of proteins with singlet oxygen
The CL emitted after irradiation of RA and INS, proteins lacking Trp moieties, was considerably smaller than that of the Trp-bearing proteins (Table 3). However, it must be noted that Trp-lacking proteins, and in particular RA, emit a CL that is more than an order of magnitude higher than that of Tyr-containing small peptides.
| Protein | CL initial 106 counts minute | Integrated CL 106 counts | Half-time minutes |
|---|---|---|---|
| HSA | 115 | 375 | 1.9 |
| BSA | 46.3 | 125 | 1.2 |
| AP | 1.2 | 4.4 | 1.2 |
| Lysozyme | 3.9 | 12 | 1.0 |
| Ribonuclease A* | 0.1 | 0.24 | 1.0 |
| Insulin* | 0.05 | 0.37 | 4.6 |
| Control without protein | 0.01 | 0.1 |
- *Proteins without Trp residues. Values given are averages of at least two independent measurements. Reproducibility was better than 10%. CL, chemiluminescence; HSA, human serum albumin; BSA, bovine serum albumin; AP, alkaline phosphatase.
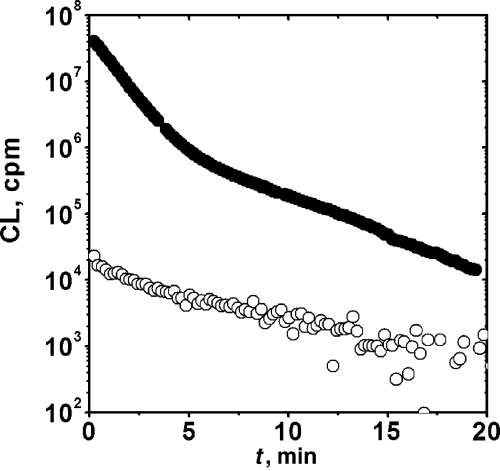
When proteins with Trp groups (HSA, BSA, AP and LYZ) were photolyzed in the presence of RB, their CL showed characteristics similar to those previously described for Ala-Trp and Ala-Trp-Ala peptides. Typical examples are shown in Fig. 3. A common feature of these data is that the decay was more complex than that of the small peptides and could only be fitted to multiexponential decays. We have thus characterized the process by the initial intensity, the integrated intensity (from 0 to 20 min), and the time required for the CL intensity to decay to half of the initial value. These data are summarized in Table 3. It can be concluded that the after-photolysis emission of the protein is even more intense than that of the peptides. Furthermore, these data also show that, in spite of the high CL intensities of all Trp-bearing proteins, there are noticeable differences in the efficiency of the process. In fact, emission from pre-irradiated HSA and BSA was considerably more intense than that of the other proteins.
Decay profiles of the chemiluminescense following irradiation (5 min) in the presence of 5 μM RB, bovine serum albumin (BSA; •), lysozyme (LYZ; ∇), alkaline phosphatase (AP; Δ), insulin (I; ○), ribonuclease A (RA; □) and control (). Chemiluminescence measurements were started 30 s after irradiation. Irradiation and CL measurements were carried out at 25°C.
Spectral distribution of the CL arising from pre-oxidized Trp-containing peptides and proteins
The spectral distribution of the CL arising from preoxidized peptides and proteins was assessed employing cut-off solutions. The results obtained are summarized in Table 4. These data indicate that the observed CL is not due to singlet oxygen dimol emission and that it can be attributed to the emission from carbonyl group containing products derived from the singlet oxygen-mediated oxidation of the parent molecules. We cannot discard a priori a mechanism involving the dark formation of singlet oxygen (from endoperoxides formed during the photolysis) and its reaction with the remaining peptides and/or their products. However, the observed CL was not reduced by the addition of 5 mM sodium azide, a singlet oxygen quencher via a physical mechanism, after the photolysis of Ala-Trp. On the other hand, its addition prior to photolysis almost totally abolished the after-photolysis emission. This would indicate that the CL precursors are produced in singlet oxygen-mediated reactions taking place during the photolysis and not in Type I photooxidation processes. This conclusion is further supported by the increase in after-photolysis CL observed when the irradiation was performed in (partially) deuterated water (Table 5). Furthermore, the production of chemiluminescent precursors is associated with the presence of oxygen as no CL was observed when the pre-irradiation was carried out in nitrogen-purged solutions (data not shown).
| Pre-irradiated compound | λ < 490 | 490 < λ < 580 | λ > 580 |
|---|---|---|---|
| Ala-Tyr-Ala | 11 | 89 | 0 |
| Ala-Trp-Ala | 48 | 37 | 15 |
| Ala-Trp | 34 | 66 | 0 |
| Poly-l-Trp | 47 | 38 | 15 |
| HSA | 5 | 69 | 26 |
| BSA | 10 | 71 | 19 |
| Insulin | 98* | 2 | |
| Lysozyme | 43 | 51 | 6 |
| Ribonuclease A | 18 | 62 | 20 |
| Phosphatase alkaline | 46 | 44 | 10 |
- *Percentage of the light intensity with wavelength shorter than 580 nm. Values given are averages of at least two independent measurements. Reproducibility was better than 20%. Irradiations were performed in 10 mM phosphate, pH 7.0. Wavelength ranges given in nanometer (nm). CL, chemiluminescence; HSA, human serum albumin; BSA, bovine serum albumin.
| Sample | Intensity ratio |
|---|---|
| Trp | 1.5 |
| Tyr | 3.1 |
| His | 2.7 |
| Ala-Trp-Ala | 4.2 |
| Ala-Tyr-Ala | 2.4 |
- Values given are averages of at least two independent measurements. CL, chemiluminescence.
Effect of additives upon the CL following the reaction of Trp containg peptides and proteins
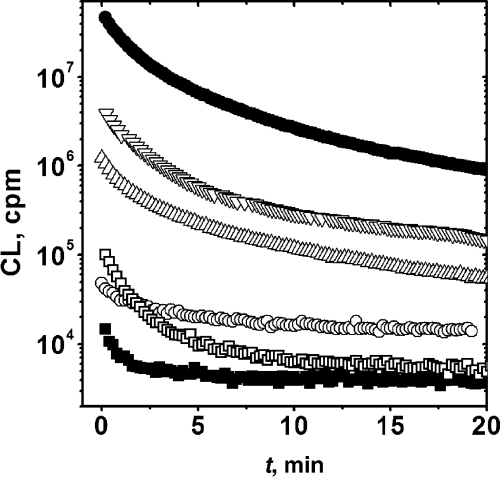
In order to obtain some insight into the characteristics of the products that lead to the post-irradiation CL and the mechanism of the process, several additives were added after irradiation, prior to the CL measurements. Typical results are shown in Fig. 4, and main conclusions are summarized in Table 6. The total suppression of the CL elicited by Fe(II) or Ebselen addition would support the proposal that the emission arises from the cleavage of peroxides. In this regard, the CL in this system behaves as that observed in other protein oxidations (8) and in red cells or brain homogenate oxidations (22–24). However, a peculiar feature of the present data is the lack of effect of free radical scavengers (Trolox or ascorbic acid). This contrast with results obtained in other systems where, at least partially, the CL emitted from oxidized products is enhanced by free radical processes (7–9).
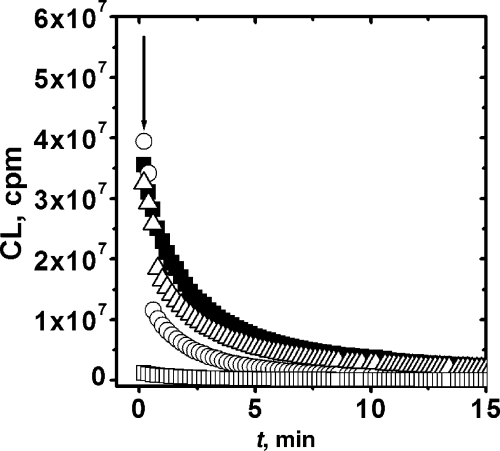
Effect of additives upon the CL profile of pre-irradiated BSA. Additives were added after irradiation at the time indicated by the arrow. Additives: Trolox 700 μM (Δ), Ebselen 100 μM (□), FeCl2 5 mM (○) and control ().
| Additive | Observed effect on the CL | Conclusion |
|---|---|---|
| Sodium azide, 5 mM | None | No role of 1O2 |
| Trolox, 0.7 mM | None | No role of free radicals |
| Ascorbic acid, 0.1 mM | None | No role of free radicals |
| DTPA, 0.1 mM | None | No role of metals |
| Fe(II), 5 mM | Total quenching | Fast dark decomposition of precursors (peroxides) |
| Ebselen, 0.1 mM | Fast drop to nearly background levels | Fast dark decomposition of precursors (peroxides) |
| Catalase, 10 U mL−1 | No change in the initial intensity. Slightly faster decay | Slow dark decomposition of hydroperoxides |
- CL, chemiluminescence.
The effect of catalase is less straightforward. Its addition slightly increases the rate of CL decay, without any effect on the initial intensity. These results could be attributed to a slow suicidal decomposition of hydroperoxide intermediates (see following scheme) by catalase (25).
Estimation of the CL quantum yield
A comparison of the integrated CL with the amount of modified Trp groups allows a rough estimation of the CL quantum yield, defined as the number of photons emitted by the reacted Trp moiety. In the evaluation of the integrated CL it has been assumed that:
- 1
The biexponential decay of the CL can be back-extrapolated to the end of the photolysis and
- 2
Due to the rather short decay times, it is assumed that the CL reaches a steady state value during the photolysis and that this steady state concentration is the same as that estimated at the end of the photolysis. This could slightly overestimate the integrated CL value.
With these assumptions, the quantum yield for Ala-Trp is 2.5 × 10−8, showing, that in spite of the high intensities emitted, the CL pathway corresponds to a very minor fraction of the whole reaction between Trp groups and singlet oxygen. Regarding the multiplicity of the emitting species, it is interesting to note that the CL intensity is very similar in air-saturated or oxygen-saturated solutions (data not shown). This would indicate that it corresponds to fluorescence from a singlet state and/or phosphorescence from a very short-lived triplet.
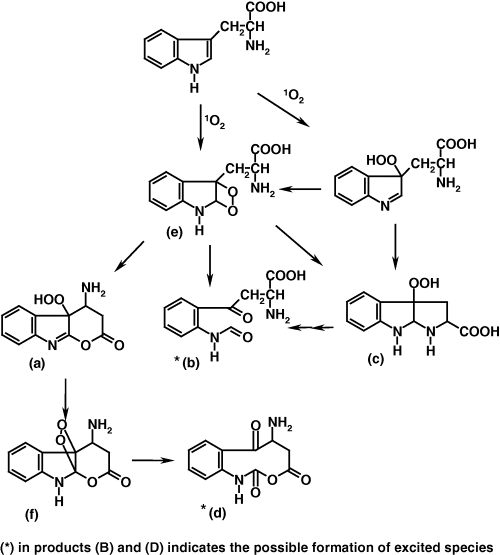
Mechanism of the CL process

Acknowledgments
Acknowledgements— This work was supported by Fondecyt-Chile (1030033 and 1050137). E.A. gratefully acknowledges a CONICYT Doctoral fellowship.




