Participation of the Surface Structure of Pharaonis Phoborhodopsin, ppR and its A149S and A149V mutants, Consisting of the C-terminal α-helix and E–F Loop, in the Complex-formation with the Cognate Transducer pHtrII, as Revealed by Site-directed 13C Solid-state NMR†
This paper is part of the Proceedings of the 12th International Conference on Retinal Proteins held at Awaji Island, Hyogo, Japan on 4–8 June 2006.
Abstract
We have recorded 13C solid state NMR spectra of [3-13C]Ala-labeled pharaonis phoborhodopsin (ppR) and its mutants, A149S and A149V, complexed with the cognate transducer pharaonis halobacterial transducer II protein (pHtrII) (1–159), to gain insight into a possible role of their cytoplasmic surface structure including the C-terminal α-helix and E–F loop for stabilization of the 2:2 complex, by both cross-polarization magic angle spinning (CP-MAS) and dipolar decoupled (DD)-MAS NMR techniques. We found that 13C CP-MAS NMR spectra of [3-13C]Ala-ppR, A149S and A149V complexed with the transducer pHtrII are very similar, reflecting their conformation and dynamics changes caused by mutual interactions through the transmembrane α-helical surfaces. In contrast, their DD-MAS NMR spectral features are quite different between [3-13C]Ala- A149S and A149V in the complexes with pHtrII: 13C DD-MAS NMR spectrum of [3-13C]Ala-A149S complex is rather similar to that of the uncomplexed form, while the corresponding spectral feature of A149V complex is similar to that of ppR complex in the C-terminal tip region. This is because more flexible surface structure detected by the DD-MAS NMR spectra are more directly influenced by the dynamics changes than the CP-MAS NMR. It turned out, therefore, that an altered surface structure of A149S resulted in destabilized complex as viewed from the 13C NMR spectrum of the surface areas, probably because of modified conformation at the corner of the helix E in addition to the change of hydropathy. It is, therefore, concluded that the surface structure of ppR including the C-terminal α-helix and the E–F loops is directly involved in the stabilization of the complex through conformational stability of the helix E.
Introduction
The archaeal sensory rhodopsins, SRI and SRII, are sensors for positive and negative phototaxis, respectively, on binding with the cognate transducer proteins (1). Pharaonis phoborhodopsin (ppR) is a negative phototaxis receptor and forms a complex with pharaonis halobacterial transducer II protein (pHtrII). The stoichiometry of the ppR–pHtrII complex has been estimated to be 2:2 in cell membrane. When blue light is irradiated, this complex activates the phosphorylation cascade in the cytoplasmic side and regulates the switch of flagella motor (2–5).
The ppR–pHtrII interactions have been well investigated by using X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, etc.: it was shown that the Y199ppR–N74pHtrII and T189ppR–E43pHtrII and –S62pHtrII hydrogen bonds are formed between the binding surfaces of ppR and pHtrII in the transmembrane and extracellular region (6–10). In particular, Tyr199, conserved even in sensory rhodopsin II, is a very important residue when ppR forms a complex with its cognate pHtrII (11,12). In addition, FTIR results strongly indicated that Thr204–Tyr174 forms a hydrogen bond between the retinal pocket and binding surface of ppR–pHtrII and Tyr199 and Thr204 in helix G play an important role for the interaction with pHtrII (13–15). On the other hand, the ppR–pHtrII interactions in the cytoplasmic regions are very important to take part in a signal relay, because the E–F loop in ppR turned out to interact with membrane proximal region in pHtrII as viewed from fluorescent probes (16). Further, pHtrII(1–114) in the cytoplasmic side is tightly bound with ppR inhibiting the proton release, although this activity depends on the length of pHtrII (17). It is also reported that the introduction of mutation into membrane proximal region in HtrI activates the proton release in SRI in spite of HtrI binding with SRI (18).
Fully hydrated membrane proteins are not always rigid solid at ambient temperature but rather flexible, exhibiting a variety of local motions as revealed by site-directed 13C NMR, which are well related with their biological functions (19–22). Site-specific assignments of individual 13C NMR peaks recorded by magic angle spinning (MAS) NMR are essential to locate such portions, as demonstrated for the previous 13C NMR studies on [3-13C]Ala-, [1-13C]Val- and [1-13C]Trp-labeled bacteriorhodopsin (bR) from purple membrane (23–25). To this end, a variety of approaches such as cleavage of the C-terminal residues, site-directed mutation and line-broadenings due to Mn2+ ion-induced transverse relaxation process were utilized. As a result, the organization of the cytoplasmic surface structures, including the C-terminal α-helix and the E–F loop as a function of metal ions, pH, temperature, etc. plays an essential role for the stabilization of the three-dimensional structure of bR at ambient temperature (26–29). It is demonstrated in this study that the 13C NMR signals of the C-terminal α-helical region were also recently identified for [3-13C]Ala- and [1-13C]Val-labeled ppR and D75N mutant in the presence or absence of pHtrII(1–159) in egg phosphatidylcholine (PC) bilayers (see Fig. 1 for the amino acid sequence of ppR) (30–32).
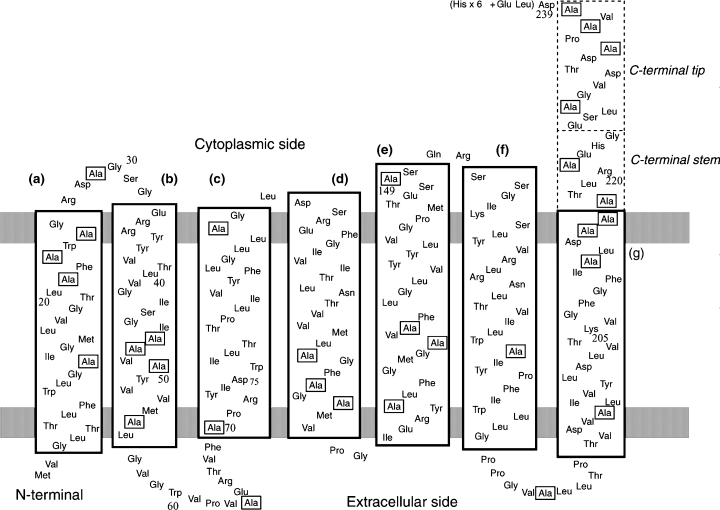
A schematic representation of the secondary structures in pharaonis phoborhodopsin (ppR) based on crystallographic structures reported by H. Luecke et al. (ppR: PDB code 1JGJ) and V. I. Gordeliy et al. (ppR–pHtrII[1–114]: PDB code 1H2S). The surface areas of the lipid bilayer are represented by gray bands. [3-13C]Ala residues are marked with squares.
In this paper, we examined the conformation and dynamics of [3-13C]Ala-labeled ppR, A149S and A149V mutants of ppR with emphasis of a possible role of the E–F loop by means of 13C solid-state NMR techniques with reference to those of bR previously examined (26–28), because Ala149 in ppR is highly preserved on the other archaeal retinal proteins (11). The isotropic 13C CP-MAS NMR peak of Ala149 was initially assigned by comparing 13C NMR peak of [3-13C]Ala-labeled ppR with that of [3-13C]Ala-labeled A149S and A149V. We further examined the corresponding spectral changes of 13C dipolar decoupling (DD)-MAS NMR for [3-13C]Ala-labeled A149S and A149V complexed with pHtrII(1–159). It turned out that mutation of ppR at Ala149 resulted in dynamical changes of the ppR–pHtrII(1–159) complex as far as membrane surface is concerned. It is therefore suggested that residues in the E–F loop regulate dynamics of membrane surface residues in ppR by binding with pHtrII.
Materials and methods
[3-13C]Ala-,[1-13C]Val-labeled A149S and A149V of ppR with His-Tag (6 × His) at the C-terminal were expressed in Escherichia coli BL21(DE3) strain in M9 medium containing [3-13C] l-Ala and [1-13C] l-Val (CIL, Andover, MA) by induction with 1 mM isopropyl-1-thio-b-d-galactoside (IPTG) and 10 μM all-trans retinal. These proteins were solubilized by using n-dodecyl-β-d-maltoside (DM) and purified with a Ni-NTA column (QIAGEN, Hilden, Germany) as described previously (32,33). The truncated transducer, pHtrII(1–159), was prepared by using the above method. Purified proteins in DM micelles were incorporated into a lipid film of egg PC (ppR: egg PC molar ratio of 1:50), followed by gently stirring at 4°C for overnight. DM was removed using Bio-Beads (Bio-Rad, Hercules, CA) to yield egg PC bilayers. Reconstituted suspensions were concentrated by centrifugation and suspended in 5 mM 2-[4-(2-Hydroxyethyl-1-piperizinyl] ethanesulfonic acid (HEPES), 10 mM NaCl buffer solution (pH 7). The pelleted mixture of the complex with ppR : pHtrII ratio of 1:1 in egg PC bilayers was placed in 5.0 mm outer diameter (o.d.) zirconia pencil-type rotor for MAS experiments.
High-resolution 13C solid-state NMR spectra were recorded on a Chemagnetics CMX-400 infinity Fourier transform (FT)-NMR spectrometer at 100.16 MHz for the carbon resonance frequency. Both cross polarization (CP) -MAS and single pulse excitation with DD-MAS were used to record 13C NMR spectra. Mostly, 5000 transients were accumulated and 30 Hz of Gaussian broadening function was applied at the center point of 0.40 prior to Fourier transformation. A doubly tuned MAS probe equipped with a 5.0 mm o.d. rotor was used. The spinning frequency was set to 4 kHz and the duration of 90° pulse for the observed 13C nucleus was 5.5 μs. In CP-MAS experiments, two pulses phase modulation proton decoupling (34) was used and the contact and repetition times were 1 ms and 4 s, respectively. At ambient temperature, fully hydrated ppR undergoes fluctuation motions with frequency range from 102 to 108 Hz depending on its location (30). These wide dynamic ranges allowed us to distinctly observe motional components with the frequency range of 106–108 Hz by DD-MAS and rigid components with the frequency range of 102–106 Hz by CP-MAS methods. When the frequency of incoherent random molecular fluctuations in the region with the order of 104–105 Hz could be interfered with frequency of MAS or proton decoupling frequency, and hence observed 13C NMR signal in such region could be broadened or suppressed as a low efficiency of peak narrowing (35,36). 13C-chemical shifts were externally referred to 176.03 ppm for the carbonyl carbon of glycine from tetramethylsilane (TMS).
Results
Transmembrane α-helical portion of wild-type ppR and mutants at Ala149
Figure 2 illustrates the 13C CP-MAS NMR spectra of [3-13C]Ala-ppR (a) (30), A149S (b) and A149V (c) reconstituted in egg PC bilayers at ambient temperature. The 13C NMR peak at 14.1 ppm was assigned to that of the methyl carbon from egg PC (30). The assignment of the upper field shoulder peak at 14.1 ppm of ppR in egg PC is not clear at present, although this peak arises definitely from the portion of lipids and not present in the 13C NMR spectra of the mutants. It should be noted that many of the 13C NMR peaks of [3-13C]Ala-bR were resonated at the peak position of the αII-helix at 15.9–16.9 ppm which is resonated at lower field portion of the conformation-dependent 13C-chemical shift of α-helical Ala Cβ, in the presence of dynamics-dependent 13C-chemical shifts for α-helix (19,27), rather than the plausibly distorted torsion angles as proposed by Krimm and Dwivedi based on IR spectra (37). It is noteworthy that the peak intensity at 15.9 ppm from the 13C CP-MAS NMR spectra of [3-13C]Ala-A149S and A149V proteins is significantly reduced with reference to that of [3-13C]Ala-ppR owing to the absence of the 13C signals in these mutants (see Fig. 2a–d). In fact, this is consistent with the difference spectrum between [3-13C]Ala-ppR and [3-13C]Ala-A149V (see Fig. 2d). The reduced peak area is 0.06 relative to whole area between 14.5 and 16.9 ppm. This value is twice as large as that of 0.03 as estimated from the number of labeled Ala residues in ppR, namely 1/31. This is because a part of Ala Cβ does not appear in the 13C CP-MAS NMR spectrum. Therefore, the 13C NMR peak at 15.9 ppm can be uniquely assigned to Ala149, which is involved in the α-helix portion with reference to the conformation-dependent 13C-chemical shifts of the α-helix (19,27,38). In particular, Ala149 is located in the αII-helix portion near at the E–F loop in the cytoplasmic side, as judged from the conformation-dependent 13C-chemical shifts (Fig. 1) (19,27,38).
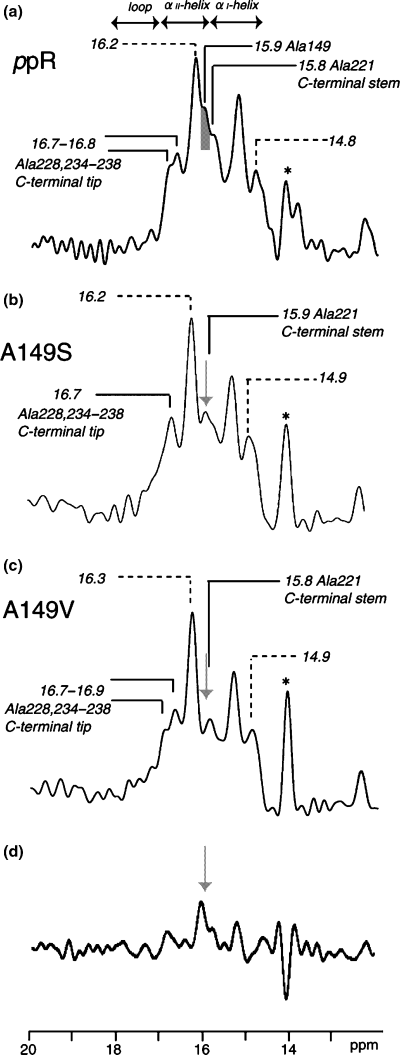
13C cross-polarization-magic angle spinning NMR spectra of uncomplexed [3-13C]Ala-labeled pharaonis phoborhodopsin (ppR) (a), A149S(b) and A149V(c) alone reconstituted in egg PC bilayer. 13C NMR signal at 15.9 ppm corresponding to Ala149 in ppR is shown in the gray (a) and arrows (b, c and d). The resonance peak at 14.1 ppm is ascribed to methyl carbon peak of egg PC as shown as asterisk. Difference spectrum between (a) and (c) is shown in (d).
Figure 3 shows the 13C CP-MAS NMR spectra of [3-13C]Ala-labeled ppR (a) (30), A149S (b) and A149V (c) which are complexed with pHtrII(1–159) in egg PC bilayers. As compared with the spectral patterns in the absence of the transducer (Fig. 2a), the five (or six) well-resolved signals were observed for ppR and mutants at Ala149, when they are complexed with pHtrII(1–159) (Fig. 3). It should be pointed out that the contribution of peaks in the region between 14.5 and 16.9 ppm from residues of natural abundance in ppR–pHtrII complex appeared to be very low. This is because 28Ala Cβ (from pHtrII), 23Ile Cγ2 (from ppR and pHtrII) and 11Met Cε (from ppR and pHtrII) are possibly resonated at this region (39) in the vicinity of Ala Cβ but the relative peak intensity from natural abundance is extremely low (less than 2%) compared with the 13C-labeled peaks from 31Ala Cβ of ppR. In fact, no such contribution was seen for similar preparations of [3-13C]Ala-labeled bR (40). The observed spectral changes in the complex formation mainly from the transmembrane region are obviously explained in terms of the accompanied conformational and dynamics changes due to the presence of van der Waals contact and hydrogen bonds in the binding surface of the transmembrane α-helices between ppR and pHtrII, as the CP-MAS NMR spectra mainly reflect the changes in the transmembrane regions of the ppR–pHtrII(1–159) complex. Naturally, the peak intensity at 15.8–15.9 ppm in the CP-MAS NMR is significantly reduced in the mutants, because no 13C NMR signal from Ala149 is present. The interaction of pHtrII with ppR increases the thermal stability of ppR with the formation of Y199ppR–N74pHtrII hydrogen bond in the transmembrane region (14). In fact, it is confirmed that Ala149 mutants interacted with pHtrII(1–159) increases the thermal stability (data not shown). These results indicate that the structure and dynamics of the transmembrane region are retained in these mutants in which Y199ppR–N74pHtrII hydrogen bond is conserved even in the Ala149 mutants (5,12,13).
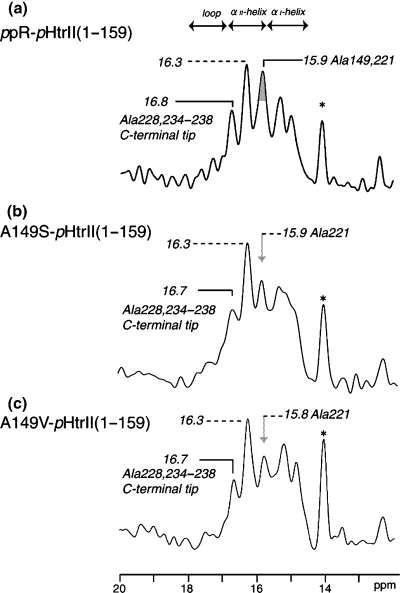
13C cross-polarization-magic angle spinning NMR spectra of [3-13C]Ala-labeled pharaonis phoborhodopsin (ppR) (a), A149S(b) and A149V(c) complexed with pharaonis halobacterial transducer II protein (pHtrII) (1–159) reconstituted in egg PC bilayer. 13C NMR signal from Ala149 in the vicinity of E–F loop in ppR is shown in the gray (a) and arrows (b and c). The resonance peak at 14.1 ppm is the methyl carbon peak of egg PC as shown as asterisk.
Membrane surface residues of ppR and Ala149 mutants
It is noteworthy that the peak intensities of the two kinds of 13C NMR peaks for [3-13C]Ala-labeled ppR and A149V, resonated at 16.7–16.9 and 15.8–15.9 ppm, are substantially suppressed when these two proteins are complexed with the cognate transducer pHtrII, as shown in Fig. 4b,f with reference to Fig. 4a,e, respectively. However, this sort of spectral change turns out to be less pronounced for A149S mutant (Fig. 4c,d). As to the assignment of the above-mentioned peaks, we previously demonstrated that the peaks at 16.7–16.9 and 15.8–15.9 ppm were ascribed to the more flexible tip (Ala228, 234, 236 and 238) and rather static stem (Ala221) regions of the C-terminal α-helix in the cytoplasmic side, respectively. This assignment is made possible on the basis of our recent comparative 13C NMR study on intact ppR and truncated ppR (1–220) (I. Kawamura, H. Yoshida, Y. Ikeda, C. Hasegawa, S. Yamaguchi, Y. Sudo, S. Tuzi, H. Saito, N. Kamo and A. Naito, in preparation) and also in analogy with the data of the similar 13C NMR studies on [3-13C]Ala-labeled bR (23,25,27), as summarized in Table 1.
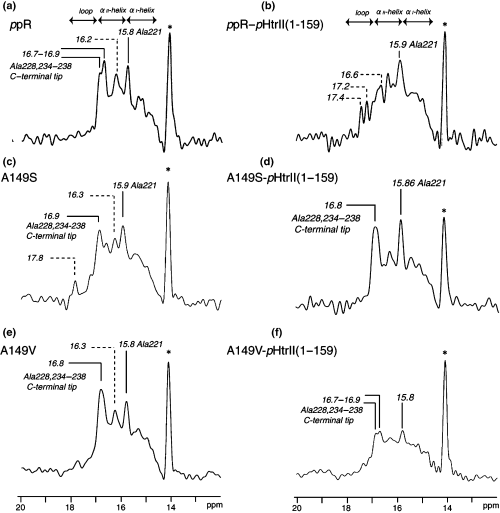
13C dipolar decoupling-magic angle spinning NMR spectra of uncomplexed [3-13C]Ala-labeled pharaonis phoborhodopsin (ppR) (a), A149S (c) and A149V (e) (left panel) and those complexed with pharaonis halobacterial transducer II protein (pHtrII) (1–159) (right panel). And those of ppR (b), A149S (d) and A149V (f) complexed with pHtrII(1–159) reconstituted in egg PC bilayer (right panel).
| Residues | Location | δiso (ppm) | Assignment based on mutants |
|---|---|---|---|
| ppR | |||
| Ala149 | Helix E | 15.9* | A149S, A149V, A149S–pHtrII(1–159), A149V–pHtrII(1–159) |
| Ala221 | C-terminal α-helix (stem) | 15.8* | ppR(1–220) |
| Ala228, 234, 236 238 | C-terminal α-helix (tip) | 16.7–16.9* | ppR(1–220) |
| bR | |||
| Ala160 | E–F loop | 17.38† | A160G |
| Ala228,233 | C-terminal α-helix | 15.91† | Cleavaged C-terminal |
| Ala240,244-246 | C-terminal random coil | 16.88† | Cleavaged C-terminal |
Obviously, such drastic intensity-changes by the complex-formation might be caused by an accompanied dynamics change at the flexible tip and (rather static) stem portions of the C-terminal α-helical region from the fluctuation frequency in the order from 108 and 106 Hz in the uncomplexed state to 105 Hz in the complexed state, respectively. This is a consequence of the peak broadenings due to a failure of the proton decoupling, interfered with frequency of coherent proton decoupling and incoherent fluctuation frequency in the order of 105 Hz in the surface structure (21,27,31,35). Naturally, such a spectral change is more pronounced in the larger dynamics change in the flexible tip portion. It is further noted that the peaks in the loop region at 17.2 and 17.4 ppm, which cannot be usually observed in bR or ppR incorporated as a monomer in lipid bilayers, emerge in the 2:2 complex of ppR–pHtrII (1–159) as a result of an “escape” from such interference leading to the peak-suppression (Fig. 4b).
This finding strongly indicates that the cytoplasmic C-terminal α-helix in ppR and the cytoplasmic α-helix transmembrane helix 2 (TM2) in pHtrII, protruding from the membrane surface, are also participated in the stability of the complex-formation with pHtrII (1–159), besides the role of the above-mentioned transmembrane α-helices as revealed by the CP-MAS NMR experiments. In this connection, it appears that Ala149 or Val149 for ppR and A149V mutant are stably located at the corner of the transmembrane α-helix E in the vicinity of the E–F loop, although its direct evidence as to the mutant is unavailable at present but might play an important role for the stabilization of the complex with pHtrII(1–159) in the cytoplasmic side.
Discussion
We found that the 13C DD-MAS NMR peak intensities of the C-terminal α-helix from [3-13C]Ala-ppR and A149V are substantially reduced when they are complexed with the cognate transducer pHtrII(1–159) (Fig. 4b,f), whereas those of [3-13C]Ala-A149S are almost unchanged (Fig. 4d). This is obviously caused by the characteristic dynamics change leading to reduced fluctuation frequency, in the surface structure including the C-terminal stem and tip portion of the α-helix and the E–F loop of ppR, which is interfered with the proton decoupling frequency (105 Hz). This finding suggests that mutual interaction between the two types of α-helices, the C-terminal α-helix of ppR and cytoplasmic α-helix of pHtrII (1–159) (TM2) is responsible for the stabilization of the complex as viewed from the surface structure. Indeed, we also demonstrated that the C-terminal α-helix of ppR is a very important site to be able to interact with the cytoplasmic α-helix of pHtrII (1–159) (I. Kawamura, H. Yoshida, Y. Ikeda, C. Hasegawa, S. Yamaguchi, Y. Sudo, S. Tuzi, H. Saito, N. Kamo and A. Naito, in preparation), although the surface structure of A149S mutant is not participated in the stabilization of the complex at the membrane surface. This means that the surface structure of the latter could be significantly modified to the extent that such a mutual interaction is geometrically unfavorable. Nevertheless, it should be pointed out that the ppR–pHtrII interaction in the transmembrane region remains unchanged among ppR, A149V and A149S as manifested from the similar 13C CP-MAS NMR spectra (Fig. 2).
The three-dimensional structure of the ppR–pHtrII complex as viewed from the transmembrane region has been shown by XRD study on crystalline sample (8) and electron paramagnetic resonance (EPR) study on spin-labeled reconstituted preparation (6): the straight TM2 (C-terminal side) is oriented parallel to the helix G of the receptor, although the residues (115–159) in the cytoplasmic α-helix of the truncated transducer (1–114) used is absent in the former (8). Therefore, it appears that the transmembrane α-helix G of ppR is in direct contact with the TM2 α-helix of pHtrII(1–159) leading to stabilization of the surface structure. Therefore, it is likely that the E–F loop of ppR interacts with the membrane proximal region in pHtrII(1–159) (14) in an indirect manner, because its direct interaction with the transducer might be stereochemically unfavorable as viewed from the relative orientation of these moieties (6,8). It is probable, therefore, that the location of the hydrophobic Ala149 (and also Val149) at the corner of the helix E, linked to the hydrophilic E–F loop, is very important for the sake of maintaining the surface structure necessary for the mutual interaction between the receptor and transducer. Indeed, Ala149 (and Val149) in ppR is well conserved for the other archaeal retinal proteins (11). On the contrary, the helical structure at the position 149 might be less stable for Ser149 at this corner in A149S mutant, especially at the interface between the hydrophobic and hydrophilic environment. In fact, it is known that Ala, Val and Ser residues are strong α-helix former, α-former and indifferent for α-helix, respectively (41). In addition to the change of stabilization in the corner of the helix E, the change of hydropathy among Ala149, Val149 and Ser149 may be responsible for surface dynamics (42). Therefore, the surface structure might be distorted to some extent from that of wild-type protein and is unfavorable for the stabilization of the complex as viewed from the cytoplasmic surface, although the strong hydrogen bond Y199ppR–N74pHtrII and T189ppR–E43pHtrII and –S62pHtrII in transmembrane region are conserved in ppR and these two kinds of mutants (5,9,12–15). This is the reason why the influence by the mutations is rather limited to the area of cytoplasmic side.
In contrast, it is also interesting to note that a single-mutation in the E–F loop results in significantly modified surface and dynamics structures in bR which also include the C-terminal α-helix and the E–F loop, as manifested from the 13C NMR spectra of A160G and A160V of bR mutants (corresponding to Ala149 in ppR) (Table 1) (25,29), by taking into account the well-known structural homology between ppR and bR. It is, therefore, suggested that dynamics of the membrane surface residues including the E–F loop in the cytoplasmic side in ppR are strongly correlated with the manner of mutual interaction with pHtrII and its resulting biological function in relation to signal relay.
Finally, it is pointed out that site-directed 13C NMR approach is a very valuable means to clarify a missing link between pictures by XRD studies and biological studies: a potential role of the mutual interactions between the cytoplasmic α-helix in ppR and the cytoplasmic α-helix in the C-terminus (TM2) in the cytoplasmic surface.
Conclusion
It was revealed that dynamics of membrane surface residues in ppR complexed with pHtrII (1–159) were greatly changed among ppR, A149V and A149S mutants. Therefore, the surface structure near E–F loop of ppR plays a dominant role to regulate membrane surface dynamics when ppR with pHtrII(1–159) through direct interaction of the C-terminal α-helix region in ppR with the cytoplasmic α-helical region of pHtrII (1–159). These interactions may correlate with the signal relay mechanism.
Acknowledgements— The present work was supported by a Grant-in-Aid for Scientific Research on Priority Area (17048010) from MEXT, Japan and for Scientific Research (17370054) from JSPS and by Research Grant-in-Aid from Magnetic Health Science Foundation. I.K. thanks Yokohama National University for “Research Aid Program for Ph.D. Candidates.”




