Involvement of Phosphodiesterase Isozymes in Osteoblastic Differentiation†‡
Presented in part at the 22nd annual meeting of the American Society for Bone and Mineral Research, Toronto, Ontario, Canada, 2000 and printed in J Bone Miner Res 15:S1;S504 (abstract)
The authors have no conflict of interest
Abstract
The cyclic monophosphate nucleotides (cyclic adenosine monophosphate [cAMP] and cyclic guanosine monophosphate [cGMP]) are found ubiquitously in mammalian cells and act as second messenger transducers to effect the intracellular actions of a variety of hormones, cytokines, and neurotransmitters. In turn, these nucleotides also modulate the signal transduction processes regulated by a range of cytokines and growth factors. Previously, we have reported that pentoxifylline, a nonselective phosphodiesterase (PDE) inhibitor, can promote osteoblastic differentiation by elevating intracellular cAMP levels and, consequently, enhance bone formation in vivo and in vitro. In this study, reverse-transcription polymerase chain reaction (RT-PCR) analysis of the osteoblastic cell lines, MC3T3-E1 and ST2 revealed the presence of PDE1, PDE2, PDE3, PDE4, PDE7, PDE8, and PDE9. We examined the effect of selective inhibitors for a respective PDE isozyme on the capacity of bone morphogenetic protein 4 (BMP-4)-induced alkaline phosphatase (ALP) activity, a cellular differentiation marker, in cells with osteogenetic potential. The results indicate that selective inhibitors for PDE2, PDE3, and PDE4 enhanced the BMP-4-induced ALP activity in a dose-dependent manner in ST2 cells but not in MC3T3-E1 cells. Northern blot analysis also revealed that the selective inhibitors for PDE2, PDE3, and PDE4 enhanced the levels of expression of messenger RNAs (mRNAs) of ALP, osteopontin (OP), and collagen type I in ST2 cells but not in MC3T3-E1 cells except for the treatment with PDE4 inhibitor. Given these data, we conclude that PDE isozymes are involved in the modulation of osteoblastic differentiation mainly at an early stage. Additionally, selective inhibitors for PDE2, PDE3, and PDE4 appear to promote the differentiation of osteogenic precursor cells toward an osteoblastic phenotype.
INTRODUCTION
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) act as second messengers in the functional responses of various cells to hormones, neurotransmitters, and other agents. In osteoblasts, for example, cAMP produced in response to parathyroid hormone (PTH) or prostaglandins (PGs) regulates osteoblastic differentiation.1-4 There are corresponding data that show how administration of PTH or PGs also lead to increases in cancellous bone volume in animal models.5-10 The intracellular level of cAMP is regulated by G protein coupled adenylyl cyclase11 and degradation is mediated by the phosphodiesterases (PDEs),12, 13 a superfamily of enzymes that catalyze the hydrolysis of cAMP and cGMP.14, 15 Therefore, controlling the activities of these enzymes would in turn regulate the intracellular level of the second messengers.
Previously, we reported that pentoxifylline, a nonselective PDE inhibitor, had the potential to promote bone formation in normal mice.16 The effects of pentoxifylline on osteoblastic cells were explored also in an in vitro system. Pentoxifylline stimulated osteoblastic differentiation of ST2 cells derived from bone marrow stromal cells and the chondrogenic differentiation of multipotential C3H10T1/2 cells. However, MC3T3-E1 cells derived from osteoblasts of murine calvaria were not affected by pentoxifylline. In cells in which pentoxifylline influenced differentiation, the effects were observed in the presence of bone morphogenetic protein 4 (BMP-4) in a dose-dependent manner.17
PDEs comprise a large group of structurally related isoenzymes derived from at least nine distinct genes and classified into nine groups based on their substrate specificity, selective inhibition or stimulation by cofactors, selective inhibition by standard inhibitors, and gene homology.14 The classification is becoming more complex because of the presence of isoforms for a specific isozyme derived from differences in transcriptional start sites and splicing variant formation. The PDE families (PDE1 to PDE9 and their isoforms) are found in different amounts, proportions, and subcellular locations depending on the cell, tissue, and species.14, 18, 19 Because of this variation, the identification of the PDE isozymes expressed in osteoblastic cells at different stages of differentiation is essential to understand the effects of PDEs in the bone-forming process. This is particularly important for BMP-induced bone formation, given that a nonselective PDE inhibitor pentoxifylline was clearly effective in regulating this process.20
We used two cell lines (MC3T3-E121 and ST222) as osteoblastic lineages at different stages of differentiation. MC3T3-E1 cells express the early stage of osteoblastic phenotype, and ST2 cells represent a bone marrow stromal cell with the ability to differentiate into osteoblasts.23, 24 These cell lines have been used widely as the established model for the identification of many molecular mechanisms and under appropriate conditions, become matrix-forming mineralization osteoblasts.25, 26
In this study, we attempted to identify by reverse-transcription polymerase chain reaction (RT-PCR) the PDE isozymes expressed in osteoblastic cell lines and their ability to regulate the biological action of BMP-4 with the use of selective inhibitors for PDE isozymes.
MATERIALS AND METHODS
Cell culture
A mouse osteoblastic cell line MC3T3-E1 and a mouse bone marrow stromal cell line ST2 were obtained from the RIKEN Cell Bank (Tsukuba, Japan). These cells were inoculated at a cell density of 3 × 105 cells/100-mm plastic dish. MC3T3-E1 was cultured with α-minimal essential medium (α-MEM; Gibco, Grand Island, NY, USA) and ST2 was cultured with RPMI1640 (Gibco). Both media contained 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Gibco) and the cells were cultured at 37°C in a humidified 5% CO2 incubator.
RNA preparation and RT-PCR
Total RNA was isolated from two cell lines and multiple tissues of mice (ddY, 6 weeks old) using Isogen (Nippon Gene Co., Tokyo, Japan) according to the manufacturer's instructions. After treating with RNAse-free deoxyribonuclease I (Gibco), 1 μg of total RNA was reverse-transcribed using an RNA PCR kit (TaKaRa Shuzo Co., Shiga, Japan) according to the instruction manual. The reaction time was 30 minutes at 42°C. Aliquots of the obtained complementary DNA (cDNA) pool were subjected to PCR and amplified in a 20-μl reaction mixture using Taq polymerase (TaKaRa Shuzo). Amplifications were performed in a Program Temp Control System (PC-800; ASTEC, Fukuoka, Japan) for 35 cycles after an initial denaturation step at 94°C for 3 minutes, denaturation at 94°C for 30 s, annealing for 30 s at the specified temperature, and extension at 72°C for 90 s, with a final extension at 72°C for 10 minutes. Positive standards and reaction mixtures lacking reverse transcriptase were used routinely as controls for each of the RNA samples. No PCR product was detected in the absence of reverse transcriptase during the RT step, indicating that the RNA preparations were free from intact genomic DNA. Amplification reactions specific for the following cDNAs were performed: PDE1A, PDE1B, PDE1C, PDE2, PDE3A, PDE3B, PDE4A, PDE4C, PDE4D, PDE5, PDE7, PDE8, and PDE9. PDE4B, which had not been identified in mouse, and PDE6, which is expressed selectively in retina, were excluded. The PCR primer sequences are given in Table 1. Reaction products were electrophoresed in 1.5% agarose gel, and the amplified DNA fragments were visualized by ethidium bromide staining under UV light. All PCR products were subcloned and sequenced using the DNA sequencing kit (Applied Biosystems, Warrington, UK). The nucleotide sequences of the cloned PCR products were compared with the European Molecular Biology Laboratory (EMBL) and the GenBank databases.
Selective inhibitors for PDE isozymes
The selective PDE inhibitors vinpocetine (Sigma Chemical Co., St. Louis, MO, USA), erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA; Sigma Chemical Co.), milrinone (Sigma Chemical Co.), rolipram (Sigma Chemical Co.), Zaprinast (Sigma Chemical Co.), and dipyridamole were gifts from JT, Inc. (Osaka, Japan). All reagents were diluted in dimethylsulfoxide (DMSO). At concentrations of 0.2%, DMSO had no influence on cell viability (data not shown).
Assay for alkaline phosphatase activity
Both cell lines were plated at a cell density of 5 × 104 cells/well in 6-well plates. At confluency, the medium was changed to one containing various concentrations of each selective PDE inhibitor with or without conditioned medium (20%, vol/vol) from Chinese hamster ovarian (CHO) cells transfected with mouse BMP-4 (mBMP-4) cDNA27 on culture days 1 and 4. These conditioned media were prepared by collecting media of CHO cells transfected with mBMP-4 cDNA at a density of 3 × 105 cells/100-mm plastic dish with 10% FBS-added α-MEM (Gibco) after 5-day culture. As control conditioned media, culture media from mock vector-transfected CHO cells were prepared in the same manner.
After 6 days of exposure to each selective PDE inhibitor with the BMP-4 or mock conditioned media, the osteoblastic cells were washed twice with phosphate-buffered saline (PBS; −), scraped off into 0.3 ml of 0.5% NP-40 containing 1 mM of MgCl2 and 10 mM of Tris (pH 7.5), and sonicated twice for 15 s each with a sonicator (model W-220; Wakenyaku Co., Kyoto, Japan). Then, the cell lysates were centrifuged for 10 minutes at 3000 rpm and the supernatants were used for the enzyme assay. Alkaline phosphatase (ALP) activity was assayed with the method of Kind-King, using a test kit (Iatron Laboratories, Inc., Tokyo, Japan) with phenylphosphate as a substrate.28 The enzyme activity was expressed in King-Armstrong (K-A) units, normalized to the protein content of the sample. The protein content was determined with the bicinchoninic acid (BCA) protein assay kit (Pierce Chemical Co., Rockford, IL, USA) using bovine serum albumin (BSA) as the standard.
Northern blots
Twenty micrograms of total RNA were electrophoresed in 1.0% agarose gels containing 18% formaldehyde and transferred to Hybond-N+ membrane (Amersham International, Amersham, UK). The membranes were prehybridized in hybridization buffer (50 mM of Tris-HCl, pH 7.5, 1 mg/ml of denatured salmon sperm DNA, 1% SDS, 1 M of NaCl, 10 mM of EDTA, 0.2% Ficol 400, 0.2% polyvinylpyrrolidone, and 0.2% BSA) for 3 h at 65°C. ALP, osteopontin (OP), collagen type I, osteocalcin (OC), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA were labeled with [α-32P]deoxycytosine triphosphate (dCTP) using the BcaBest Labeling Kit (TaKaRa Shuzo). Hybridization was performed at 68°C overnight, and the membranes were washed three times at 68°C for 1 h with 0.1× SSC and 0.5% SDS. The membranes were stripped using boiled distilled water containing 0.5% SDS and then rehybridized. The signals were detected with a Fuji BAS 1500 BioImaging Analyzer (Fuji Photo Film Co., Tokyo, Japan). Quantification was performed with a Science Lab 98 Image Gauge (Fuji Photo Film Co.), and each value was normalized against that of the GAPDH band in the corresponding lane. Then, the normalized values were compared with the calculated fold induction.
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay
Both cell lines were plated at a cell density of 5 × 103 cells/well in 96-well plates and the medium was changed to one containing various concentrations of each selective PDE inhibitor on culture day 1 and day 4. On culture days 2, 4, and 6, the viability of the cells was monitored with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Dojindo Laboratories, Kumamoto, Japan). The MTT assay is an indirect method that measures the metabolic activity of cellular enzymes. In this assay, the quantity of formazan products as measured by the amount of 570-nm absorbance is directly proportional to the number of living cells in culture.
Statistical analysis
Data are expressed as the mean ± SD for each group. Statistical differences among treatment groups were evaluated with analysis of Student's t-test. The values of p < 0.05 were considered to be significant.
RESULTS
Expression of mRNAs for the PDE isozymes
First, we examined the expression of each PDE messenger RNA (mRNA) by RT-PCR in MC3T3-E1 and ST2 cells. Most of the PDE isozymes are comprised of more than one isogene (subtype) and numerous subtype splice variants (isoforms). We designed subtype specific primer sets, which covered all subtype splice variants. Both of the cell types expressed PDE1, PDE2, PDE3, PDE4, PDE7, PDE8, and PDE9 mRNAs (Fig. 1). No dominant PDE isozyme specific to osteoblastic cells could be identified.
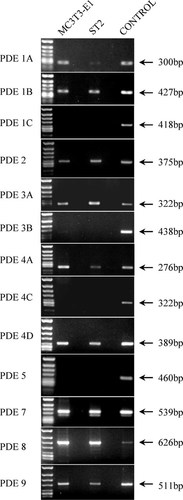
RT-PCR analysis of osteoblastic lineage cell mRNA using specific primer for PDE isozymes or subtypes. Total RNA was prepared from two cell lines that were sampled on reaching confluency. Then, the samples were analyzed with an RT-PCR procedure as described in the Materials and Methods section. An aliquot of each of the PCR reactions was electrophoresed on 1.5% agarose gels. The data shown are typical of the results of experiments performed three times using cell preparations from different explants. A positive control and each sample performed in the absence of reverse transcriptase (next lane) also are shown in the figure.
Effect of selective PDE inhibitors on ALP activity
The effects of the selective inhibitors for the PDE isozyme on the induction of ALP, an early marker of osteoblastic differentiation,29 in MC3T3-E1 and ST2 cells are illustrated in Figs. 2A and 2B. ALP activity significantly increased in both MC3T3-E1 and ST2 cells by BMP-4 as previously reported.24, 30 Treatment of ST2 cells with EHNA (PDE2 inhibitor), milrinone (PDE3 inhibitor), and rolipram (PDE4 inhibitor) enhanced the BMP-4-induced ALP activity in a dose-dependent manner. The enhancement of ALP activity without BMP-4 was observed only in the treatment of rolipram at concentrations 10 μM (1.4-fold above control). Although expression of PDE1, PDE7 (inhibitor not available), PDE8, and PDE9 were confirmed by RT-PCR analysis in ST2 cells, treatment with vinpocetine (PDE1 inhibitor), dipyridamole (PDE5 inhibitor, but proved to inhibit PDE831), and Zaprinast (PDE5 and PDE932 inhibitor) did not affect the BMP-4-induced ALP activity. In contrast, the BMP-4-induced ALP activity in MC3T3-E1 cells was not influenced by any of the selective PDE inhibitors.
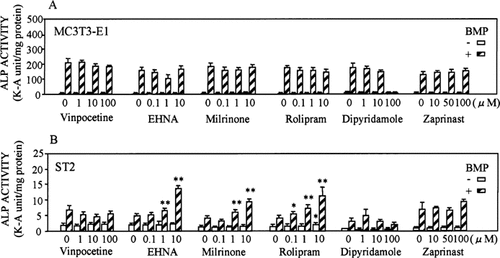
Dose-response effect of selective PDE inhibitors with or without BMP on ALP activity in (A) MC3T3-E1cells and (B) ST2 cells. ALP activity was measured as described in the Materials and Methods section. Data are means ± SD of three culture wells. Data shown are typical of the results of three different experiments. Significantly different from the control at *p < 0.05 and **p < 0.01. Vinpocetine, PDE1 inhibitor (IC50, 20 μM); EHNA, PDE2 inhibitor (IC50, 1 μM); milrinone, PDE3 inhibitor (IC50, 0.4 μM); rolipram, PDE4 inhibitor (IC50, 2 μM); dipyridamole, PDE8 inhibitor (IC50, 9 μM); Zaprinast, PDE9 inhibitor (IC50, 35 μM); IC50, half-maximal inhibitory concentration.
Effect of selective PDE inhibitors on expression of BMP-4-induced osteoblastic differentiation marker genes
Compared with the controls, treatment with EHNA, milrinone, and rolipram increased gene expression of ALP, OP, and collagen type I in ST2 cells but not in MC3T3-E1 cells, except for the treatment with rolipram. There were no appreciable changes in the expression of OC mRNA in both cell types (Figs. 3A-3C). On the other hand, there were no appreciable changes in the expression of these genes in the treatment without BMP-4 (data not shown).
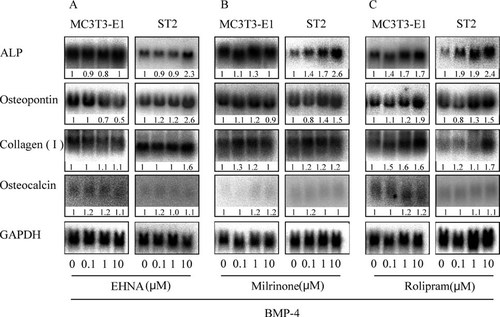
Expression of osteoblastic differentiation marker genes in MC3T3-E1 and ST2 cells. Both cell lines were plated at a cell density of 30 × 104 cells/100-mm plastic dish and exposed to each selective PDE inhibitor with the BMP-4 conditioned media. Total RNA was isolated from control and selective PDE inhibitor-treated samples at the same time point, and the gene expression of osteoblastic differentiation marker was analyzed by Northern analysis. The effect of (A) EHNA, (B) milrinone, and (C) rolipram on the induction of BMP-4-induced mRNAs for ALP, OP, collagen type I, and OC. Data shown are typical of the results of three different experiments. The number shown to below each signal indicates the ratio of expression level between the respective mRNA and GAPDH mRNA compared with those in the control culture.
MTT assay
Viability of cells after treatments with selective inhibitors for PDE2, PDE3, and PDE4 was monitored with the MTT assay. Only on day 2, viability of cells treated with PDE2 and PDE3 significantly increased but thereafter remained unchanged. Therefore, we concluded that none of the selective inhibitors at concentrations used in the experiments had altered the viability of the cells throughout the culture period (Figs. 4A-4C).

Effect of selective PDE inhibitors on the growth of ST2 cells. Cells were cultured in 96-well plates containing various concentrations of each selective PDE inhibitor for 6 days. The number of viable cells was quantitated by the MTT assay as described in the Materials and Methods section. The means ± SD of the quadruple wells are shown. Data shown are typical of the results of three different experiments. (A) EHNA, (B) milrinone, and (C) rolipram. Significantly different from the control at *p < 0.05 and **p < 0.01.
DISCUSSION
This study provides evidence that osteoblastic lineage cell lines qualitatively express seven PDE isozymes at the mRNA level. Most of the PDE isozymes are known to comprise more than one isogene product called a “PDE subtype.” Furthermore, each of the PDE subtype products has one or more mRNA splice variants (PDE isoforms) resulting from either alternative splicing of mRNA or alternative transcription initiation site. Therefore, variation of PDE isozymes must be extensive and each PDE isoform might exhibit a tissue-specific distribution pattern. In this study, we examined PDE isozymes (or subtypes) but PDE isozymes specific to osteoblastic lineage were not identified. However, functional analysis involving the use of the selective inhibitors for PDE isozymes revealed that PDE2, PDE3, and PDE4 were shown to regulate the differentiation of early stage osteoblastic cells. Our findings point to a number of possibilities; either the mRNA of PDEs other than PDE2, PDE3, and PDE4 are not translated or the translated protein is either inactive, unstable,33 or not involved in regulation of cytodifferentiation.
The PDE2 inhibitor EHNA, the PDE3 inhibitor milrinone, and the PDE4 inhibitor rolipram stimulated BMP-4 action as indicated by ALP activities. Only the PDE4 inhibitor rolipram enhanced the BMP-induced expression of osteoblast markers except OC in both ST2 and MC3T3-E1 cells. Because PDE2 and PDE3 act on both cAMP and cGMP but PDE4 is a cAMP-specific PDE, the elevated intracellular level of cAMP might stimulate differentiation of both cells. Previous studies from our laboratory indicated a positive effect of rolipram on bone formation in an in vivo model,16 and other corresponding studies were reported.34, 35 These results indicate that PDE4 is a key functional enzyme in the regulation of BMP-4 action at an early stage of osteoblastic differentiation because the effect of ALP activity was not seen in well-differentiated MC3T3-E1 cells.
cAMP/protein kinase A (PKA)-dependent pathways are reported to exert antiproliferative effects in osteoblasts.36 However, regulation of differentiation by cAMP-mediated signaling through cAMP inactivation by PDEs is poorly understood in the osteoblast.37, 38 We found that the use of the nonselective PDE inhibitor pentoxifylline induced mild accumulation of cAMP (data not shown), enhanced BMP-4-induced ALP expression in vitro, and stimulated systemic bone formation. This study showed that selective inhibitors for PDE2, PDE3, and PDE4 reproduced the pentoxifylline effects in bone marrow stromal cells ST2. Furthermore, replacement of pentoxifylline by forskolin, a universal adenylyl cyclase stimulator, and by dibutyryl cAMP, a cAMP analog, resulted in an increase in BMP-4-induced ALP activity.17 These results support the notion that signal transduction via the PKA pathway is necessary but not sufficient. Because the PDE inhibitors alone did not affect the differentiation of the ST2 cells, the effect of the PDE inhibitors or cAMP/PKA-mediated signaling is most likely mediated via BMP-4 signaling through the Smad cascade at an early stage of differentiation in osteoblasts. BMP-4 induced the expression of mRNA for core binding factor a1 (Cbfa-1),39 which acts as a transcription activator in osteoblastic differentiation and a positive regulator for bone formation.40, 41 Therefore, we examined the induction of Cbfa-1 in cells treated with the PDE inhibitor and BMP-4. However, no change in Cbfa-1 mRNA levels was observed (data not shown). A more detailed examination of this mechanism will be the subject of future studies.
The potential use of PDE inhibitors as drugs to stimulate new bone formation would be another target of this study. For this purpose, identification of osteoblastic cell-specific PDE isoforms and their selective inhibitors will be required to avoid undesired side effects in patients.
Currently, BMP molecules (BMP-2) produced by DNA recombination techniques42 are ready for use in clinical practice. However, one of the issues associated with the use of these proteins is the high dose required (milligrams for 1 mm3 of new bone mass) to induce new bone in humans. If some PDE inhibitors enhance BMP action, they stand to improve the efficacy of BMP.
In conclusion, we have found that PDE isozymes are involved in the modulation of differentiation processes in osteogenic precursor cells. Further, selective inhibitors for PDE2, PDE3, and PDE4 favorably influenced osteoblastic differentiation in these cells. Further studies of osteoblastic precursors and cAMP-mediated signaling involving PDE isoforms and their corresponding inhibitors are needed. We propose that this enzyme is a potentially significant target for the development of new signal transduction pharmacotherapies for the treatment of metabolic bone diseases.
Acknowledgements
This work was supported in part by a grant-in-aid for Scientific Research on Priority Areas (12137203) from the ministry of Education, Culture, Sports, Science, and Technology of Japan.





