The Relationship of Biochemical Markers of Bone Turnover to Bone Density Changes in Postmenopausal Women: Results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial
Abstract
We assessed the associations of eight bone turnover markers (BTMs) with baseline and 1-year percentage changes in lumbar spine and hip bone mineral density (BMD) of 293 postmenopausal women undergoing treatment with hormone replacement therapy (HRT) or placebo using squared correlation coefficients (R2). In 239 women assigned to treatment with estrogen alone or with with estrogen plus progestins (active treatment), mean percentage changes for all markers decreased significantly and remained below baseline values through 3 years of study, whereas mean percentage changes for 54 women assigned to the placebo group showed no significant change from baseline in any marker. At baseline, age and body mass index (BMI) together accounted for 16% and 25% of the variance in spine and hip BMD, respectively. The telopeptide resorption marker, cross-linked N-telopeptide of type I collagen (NTX), alone accounted for 12% and 8% of variance, respectively. Another telopeptide, carboxy-terminal telopeptide of type I collagen (Crosslaps), accounted for 8% and 7% of variance, respectively. A bone-specific alkaline phosphatase (BALP-2) accounted for 8% of variance at the spine and 5% at the hip. No other marker accounted for more than 5% of total variance at either site; adding either baseline NTX, Crosslaps, or BAP-2 to regressions containing age and BMI increased R2 values at the spine and hip to about 22% and 28%, respectively. In the placebo group, baseline spine BMD accounted for 4% of the variance in 1-year spine BMD percentage change, whereas baseline values for age and BMI accounted for 1% and 0% of the variance, respectively; none of the three accounted for more than 0% of hip BMD percentage change; Crosslaps and NTX contributed 5% and 4% to the variance in 1-year spine BMD percentage change, but other markers accounted for < 2% of variance at the spine. At the hip, another BALP (BALP-1) accounted for 4% of variance, but no other baseline marker except NTX accounted for more than 1% of variance. In the active treatment group, baseline values for age, BMI, and spine BMD together accounted for 13% of the percentage change in spine BMD and for 4% of the BMD change at the hip. No individual or pair of baseline markers significantly enhanced these R2 values, but addition of 1-year percentage changes in some individual markers did significantly increase it. The largest R2 value was obtained by adding the percentage change in BALP-2, which increased the R2 in spine BMD percentage change to 20% and that at the hip to 8%. Adding baseline and change variables for all eight markers to the regression increased R2 to 28% at the spine and 12% at the hip. Restricting the set of analyses to individuals who suppressed marker activity beyond the precision error for the measurement did not improve R2s for the regressions. When baseline marker values were stratified into quartiles, only NTX and osteocalcin showed significant relationships between quartile and change in spine BMD, and these did not reach significance at the hip. When the 1-year change in markers was stratified into quartiles, significant relationships with percentage change in spine BMD were observed only for BALP phosphatases. We conclude that BTMs are not a surrogate for BMD to identify women with low bone mass and that they offer little useful information for predicting BMD changes for individual untreated or HRT-treated postmenopausal women.
INTRODUCTION
BIOCHEMICAL MARKERS of bone turnover are chemical entities whose appearance in the circulation or excretion in the urine mirrors the contemporaneous whole body rate of bone turnover. Introduction of these markers as accurate, noninvasive, and relatively simple diagnostic tests has routinized the assessment of bone turnover and its components, resorption and formation.1 Proper use of these tests in the clinical setting, rather than in population studies, remains an issue of great interest and some controversy. Several potential applications for turnover markers have already been proposed. These include the diagnosis of high remodeling states; characterizing the pathogenesis of skeletal disorders (e.g., defining the relative activity of resorption vs. formation); obtaining information about remodeling dynamics that might otherwise require a bone biopsy; monitoring the response to therapy; predicting fracture risk; and early prediction of changes in bone mass. The latter application, if validated, would be particularly useful to physicians who currently must wait to determine by densitometry whether bone is being conserved or lost. This is particularly true in the case of women at the time of menopause, in whom the time required for bone density follow-up may exceed 1 year because the actual rate of change in bone mineral density (BMD) may not be much greater than the precision error of the measurement itself. To date, only limited evaluation of turnover markers for this application has been reported, and the utility of bone markers, particularly for individual patients, remains uncertain. The recent Postmenopausal Estrogen/Progestin Interventions Trial (PEPI) allowed us to examine relationships between bone mass and several different bone turnover markers (BTMs), either alone or in combination, in a large group of women who underwent menopause 1–10 years previously. In this paper, we focused on three issues: the cross-sectional relationship between baseline turnover marker concentrations and baseline BMD, the degree to which baseline values of turnover markers predict rates of change in BMD for women assigned either to placebo or to active hormonal therapy, and the relationship between changes in turnover markers and changes in BMD over time.
MATERIALS AND METHODS
Design, overview, and participants
The rationale, design, and background of the PEPI trial have been described in detail elsewhere.2-5 Briefly, PEPI was a 3-year randomized, double-blinded, placebo-controlled clinical trial conducted in 7 United States clinical centers. The primary goal of PEPI was to compare the effects of selected hormonal regimens on coronary heart disease risk factors in healthy postmenopausal women.2, 3 A second purpose was to assess long-term effects of these hormonal regimens on BMD at clinically relevant sites.
Between December 1989 and February 1991, 875 women between the ages of 45 and 64 years were randomized. Eligible women were stratified by clinical center and hysterectomy (with or without uterus) status, and randomized at baseline to one of five treatment groups. Women had to be surgically or naturally menopausal (longer than 1 year but less than 10 years since the last menstrual period), not taking estrogen or progestin for at least 2 months prior to the first screening visit (4 months before randomization), and, if treated with thyroid hormone replacement, to have been on a stable dose for at least 3 months prior to initial screening. Medical exclusions included one or more of the following: extreme hyperlipidemia, marked obesity, severe hypertension, recent myocardial infarction, congestive heart failure, stroke (or transient ischemic attack), antiarrhythmia medication use, diabetes mellitus requiring insulin, prior breast or endometrial cancer, melanoma, any nonbasal cell skin cancer in the previous 5 years, an elevated highly sensitive thyroid-stimulating hormone concentration, a history of trauma to the lower spine or hip fracture, chronic glucocorticosteroid use, and severe menopausal symptoms.
Participants returned biannually for 3 years. The following were assessed at every clinic visit: symptoms; medication use; adherence to assigned medication; adverse experiences, including fractures; blood pressure; weight; and height. BMD was assessed three times during the trial: prior to or at the randomization visit, at the 12-month visit, and at the 36-month visit.
Three PEPI clinical centers (University of California, Los Angeles, CA, George Washington University, Washington, DC, and Stanford University, Palo Alto, CA) were designated for special studies which required the collection and repository storage of additional urine and blood specimens. Three hundred and eighty-three participants were randomized at these centers. Most of the analyses reported here represent 293 of these women who took more than 80% of their assigned study medication and for whom complete bone turnover and BMD data are available for the baseline and 1 year of observation (the study group). A few analyses apply to 212 of those adherent women with complete bone turnover and BMD data available for the entire 3-year trial.
Treatment
Participants were assigned to one of the following treatment arms in 28-day cycles: placebo; conjugated equine estrogens (CEE), 0.625 mg daily (estrogen only); CEE, 0.625 mg/day plus medroxyprogesterone acetate (MPA), 10 mg/day for days 1–12; CEE, 0.625 mg/day + MPA, 2.5 mg/day (MPAC); and CEE, 0.625 mg/day daily + micronized progesterone (MP), 200 mg/day for days 1–12. All study medications were taken orally as pills (CEE and MPA) or capsules (MP). Active medications and placebo were prepared to appear identical; additionally, the two doses of MPA were prepared to be indistinguishable in appearance. Further details on the design, rationale, conduct, and safety monitoring are provided elsewhere.3, 4
Measurements
Bone mineral density:
BMD in the lumbar spine (L2–L4) and hip was measured using dual-energy X-ray absorptiometry (QDR 1000; Hologic, Waltham, MA, U.S.A.). With few exceptions, scans were performed in duplicate at baseline, 12 months, and 36 months. Calibration of densitometers, scan quality control, and scan review were centrally coordinated by the PEPI Bone Scan Quality Control Center at the Mayo Clinic, as detailed previously.4, 5
Bone turnover markers:
Participants in the study group provided early morning fasting samples of urine and blood at baseline and periodically thereafter. Aliquots of urine and serum were stored at –70°C in a central facility and shipped at the conclusion of the study to the laboratory for analysis. Both urine and serum markers were assayed at baseline, 12 months, and 36 months; serum markers were also assayed on 24-month samples. All turnover measurements were made in the laboratory of R.M. (VA Medical Center Palo Alto, CA, U.S.A.). All specimens for each participant were thawed only once and processed batchwise in single assay runs. Specimens were identified by a study number without reference to treatment assignment. Assay results were forwarded to the PEPI Coordinating Center (B.W.) for entry into the PEPI database and statistical analysis. The following bone turnover markers were assayed.
Bone resorption markers:
Intra/interassay coefficients of variation (CVs) in our laboratory are expressed as percent in the parentheses. Carboxy-terminal telopeptide of type I collagen (Crosslaps; Osteometer, obtained through Diagnostic Systems Laboratories, Inc., Webster TX, U.S.A.; 7.1/7.2%); amino-terminal telopeptide of type I collagen (NTX) (Ostex, Seattle, WA, U.S.A.; 8.1/9.0%); total and deoxypyridinolines (Pyrilinks 3.4/9.5%, Pyrilinks-D 1.3/8.3%; Metra Biosystems, Mountain View, CA, U.S.A.) (abbreviated Pyr and Dpyr).
Bone formation markers:
Two assays for bone-specific alkaline phosphatase (termed BALP-1 and BALP-2, respectively): Ostase (Hybritech, San Clemente, CA, U.S.A.; 6.8/7.5%) and Alkphase-B (Metra Biosystems; 8.0/8.5%), carboxy-terminal extension peptide for type I collagen (Prolagen-C; Metra Biosystems; 4.2/8.5%) (abbreviated C1CP), human intact osteocalcin (immunoradiometric assay; Diagnostic Systems Labs, Inc.; 4.7/4.9%). All reagents were the generous gifts of the manufacturers, and all assays were run precisely according to written manufacturer specifications.
Statistical methods
Observed repeated measures of BMD (means of replicate measurements for spine and total hip) and single BTM measures (four formation and four resorption markers) for each woman were used to calculate the following response variables at each follow-up assessment: annualized actual change (ΔBMD) and annualized percentage change (%Δ) from baseline values. The focus here is on %Δ for BMD and BTM measures and on their longitudinal relationships. Cross-sectional product moment correlations at baseline for the BTM cohort were also examined, as well as cross-sectional correlations within placebo and active treatment groups at 1 year and 3 years. For correlations and as baseline covariates, natural logarithms of observed BTMs were used because observed BTM distributions were skewed toward high values.
Baseline and %Δ BTM measures were modeled both as response variables to examine the effect of adhering to study regimens on BTMs and as time-dependent “explanatory” covariables for %Δ BMD measures in general linear models. SAS software (SAS Institute, Cary, NC, U.S.A.) was used for computation.6 Squared correlation coefficients, R2, both simple and partial, were used to estimate strength of relationships.
The basic linear model for testing the strength of added variables included baseline age, BMD (spine or hip), and body mass index (BMI, weight/height2) as independent variables. The five protocol treatment regimens were added to the basic model to test pairwise differences among regimens on the response variables. %ΔBMD (spine and hip) and %ΔBTM (eight variables) were each analyzed as single response (dependent) variables. For each of the 10 variables, every active arm had a significantly different effect from placebo and no active arm differed significantly from another. Excluding the placebo group and rerunning the analysis with the four active arms yielded the same result. Hence, all active arms were pooled into a single “active” group.
For each group (active and placebo), the logarithms of baseline BTMs were added to the basic model, first singly and then in combinations to test the significance of the additional R2 for “explaining” %ΔBMD at 1 year. Then, for the active group, %ΔBTM values at 1 year were added in a similar manner to obtain estimates of additional R2 as a measure of the “strength” of the relationship of %ΔBTM as time varying covariates with %ΔBMD.
t-tests were used to test placebo versus active group pairwise comparisons at baseline. “F” or t-tests were used to test the significance of additions to R2. In general, the probability value, p ≤ 0.05 was considered significant when testing the hypothesis that R2 addition(s) = 0. No adjustments were made for multiple comparisons.
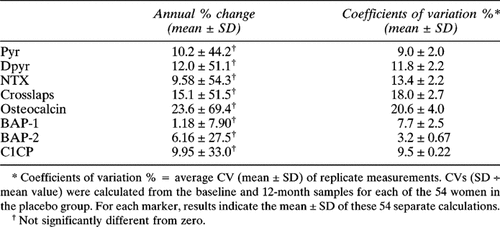
Precision characteristics were estimated for each of the BTM assays by two methods. To determine the interassay CV (or index of precision) for these assays in our laboratory, daily blood and urine samples were collected from 0600–0800 h for 6 successive days for a group of 10 postmenopausal women participants in an inpatient metabolic protocol. These women had been equilibrated to a constant diet for 7 days prior to initiating the specimen collections. The interassay CVs from this analysis are given above under the Bone Turnover Markers section. To test the long-term stability of BTMs in the PEPI study group, CVs were calculated using the baseline and 1 year specimens from the 54 women assigned to the placebo group. These results are presented in Table 3.
RESULTS
Baseline characteristics of the study group are presented in Table 1. Except for a lower prevalence of hysterectomy in the study group, no significant difference in any baseline variable distinguished these women from those in other PEPI clinics. Within the study group, there were no significant differences between the 54 women assigned to placebo and the 239 women assigned to estrogen.
BMD response to treatment
For women assigned to placebo, loss of BMD from the spine (mean ± SEM) averaged 1.43 ± 0.34% at year 1 and 3.31 ± 0.64% by year 3. By contrast, women receiving estrogen gained 3.23 ± 0.16% at year 1 and 5.40 ± 0.30% by year 3. At the hip, women assigned to placebo lost 1.59 ± 0.35% and 2.61 ± 0.36% at 1 and 3 years, whereas women assigned to active treatment gained 1.12 ± 0.14% and 2.76 ± 0.24% at the same time points. Differences between placebo and active treatment were significant at both time points, and the results did not differ from BMD changes reported for the entire cohort of PEPI women who had been compliant with at least 80% of assigned study medication.5
Bone turnover markers at baseline and response to treatment
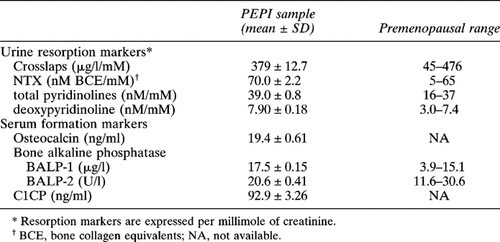
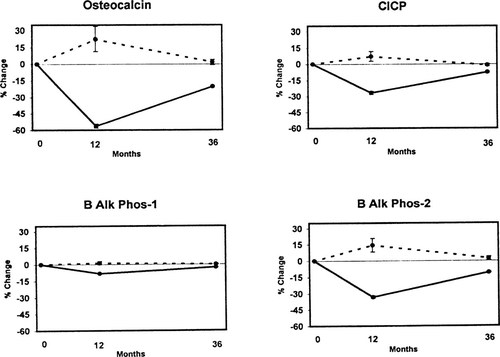
Baseline values for all marker variables are presented in Table 2. For all resorption markers except Crosslaps, the average value exceeded the manufacturer's designated upper limit of normal for premenopausal women. Marker responses to treatment are shown in Fig. 1 (resorption markers) and Fig. 2 (formation markers). Women assigned to placebo showed no significant change from baseline in any turnover marker for any time period. As a measure of their long-term stability, the annual percentage mean change and estimates of interassay precision were made for each marker using single samples obtained at baseline and at year 1 from women in the placebo group. These are presented in Table 3.
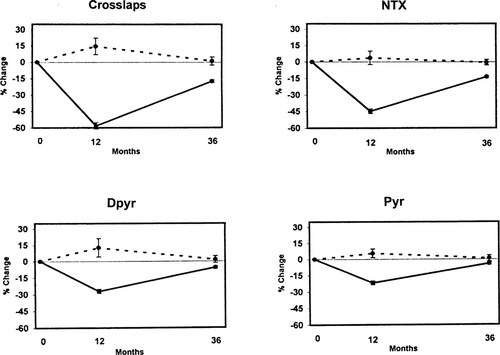
Change over time in resorption markers. Dotted lines = placebo; solid lines = active treatments.
Change over time in formation markers. Dotted lines = placebo; solid lines = active treatments.
Women assigned to active treatments showed significant reductions in all markers (Figs. FIG. 1., FIG. 2.). Maximum suppression was observed at 12 months with subsequent return toward baseline by 3 years in all cases. Because urine and serum were not available between baseline and the 12-month visit, no statement can be made regarding the tempo of response during the first year. The magnitude of marker suppression varied highly among markers. For resorption markers, the greatest suppression was obtained with the two telopeptides, Crosslaps (60%) and NTX (50%), with less suppression for the pyridinolines (30% Dpyr, 25% Pyr). Similar variability was observed with formation markers, ranging from 60% suppression for osteocalcin to 8% for BAP-1. For an apparent change in turnover marker for an individual to be statistically significant with 95% confidence the degree of suppression must exceed twice the SD of the compounded precision error for change (i.e., 2 × √2 × CV). The following proportion of women in the active treatment groups suppressed marker activity to that degree or greater: Pyr = 45.7%; Dpyr = 48.4%; NTX = 59.0%; Crosslaps = 72.2%; osteocalcin = 47.6%; BAP-1 = 30.2; BAP-2 = 62.5%; C1CP = 49.0%.
Relationship of baseline markers to baseline BMD
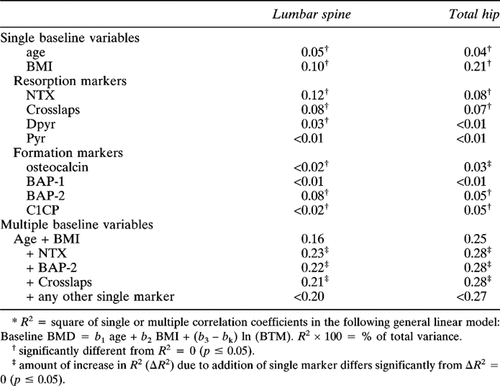
Age and BMI together accounted for 16% of the variance in baseline spine BMD and 25% of the variance in hip BMD, respectively (Table 4). NTX alone contributed 12% of R2 for spine BMD and 8% at the hip, with values for Crosslaps of 8% and 7%, respectively. BAP-2 accounted for 8% of variance at the spine and 5% at the hip. No other resorption or formation marker by itself accounted for more than 5% of total variance at either site. Adding either baseline NTX or Crosslaps to regressions containing age and BMI increased R2 values at the spine and hip to about 22% and 28%, respectively (Table 4).
Relationship of bone turnover markers to 1-year BMD change
Placebo group:
Baseline spine BMD accounted for 4.4% of the variance in 1-year spine BMD change, whereas baseline values for age and BMI accounted for 1.3% and 0.2% of the variance, respectively (Table 5-IA). Individual values for Crosslaps and NTX accounted for 5.3% and 4.1% of the variance in 1-year spine BMD change (Table 5-IB). No other single marker accounted for as much as 2% of variance at the spine. At the hip, BAP-1 accounted for 3.6% of variance, but no other baseline marker except NTX accounted for as much as 1.3% of variance (Table 5-IB).
Baseline values for BMI, age, and spine BMD together accounted for 7% of the variance in spine BMD percentage change, whereas baseline BMI, age, and hip BMD accounted for 0.3% of the variance in hip BMD percentagechange (Table 5-IIC). Addition of Crosslaps + ΔBAP-1 to this regression significantly increased the spine R2 to 19%. Addition of NTX + Δosteocalcin to the regression increased R2 for hip BMD change to 11%. No other single baseline variable added significantly to the regression. When all baseline and %Δ markers were forced into a single regression with age, baseline (spine or hip) BMD, and BMI, R2 rose to 44% for the spine and 33% for the hip (Table 5-IIC). At best, these R2s and ΔR2s were of borderline significance (Table 5).
Active treatment group:
Baseline values for age, BMI, and spine BMD together accounted for 12.6% of the change in spine BMD and for 4.3% of the BMD change at the hip (Table 6-IIC). No individual or pair of baseline markers significantly enhanced this regression. However, the addition of percentage changes in some individual markers did significantly increase it. The largest R2 was observed by adding the change in BAP-2, which increased the R2 in spine BMD change to 19.6% and that at the hip to 8%. Adding the baseline and change values for any resorption marker to this regression gave no further significant increase in R2 at the spine, but slightly increased R2 to about 9% for the hip. Adding all baseline and change variables to the regression increased R2 to 28% at the spine and 12% at the hip.
Relationship of baseline and percentage change marker quartiles to changes in BMD
In the placebo group, when baseline BTM results were stratified by quartile, differences in 12-month changes in BMD showed only nonsignificant trends (data not shown). In the active treatment group, only baseline NTX showed a significant relationship between baseline quartile and change in spine BMD (Fig. 3), but did not reach significance at the hip (hip data not shown). Quartile trends for other resorption markers did not reach significance at either site. For formation markers, only the baseline osteocalcin quartile was significantly related to change in spine BMD (Fig. 4), but this did not reach significance at the hip. No other formation marker quartile trend approached significance.
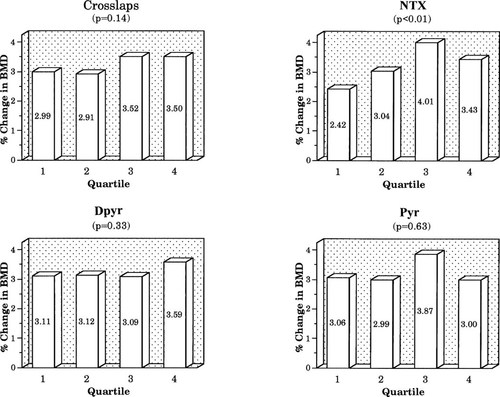
Relationship of percentage change in spine BMD to quartile of baseline resorption markers.
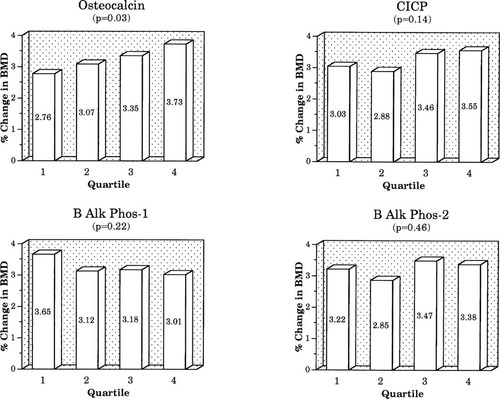
Relationship of percentage change in spine BMD to quartile of baseline formation markers.
We also stratified women in the active treatment group into quartiles based on %Δ in marker. No significant effect on BMD response was seen for the percentage change quartile for resorption markers (Fig. 5), but the BMD rise was significantly greater for women in the highest quartiles for change in BAP-1 and BAP-2 (Fig. 6).
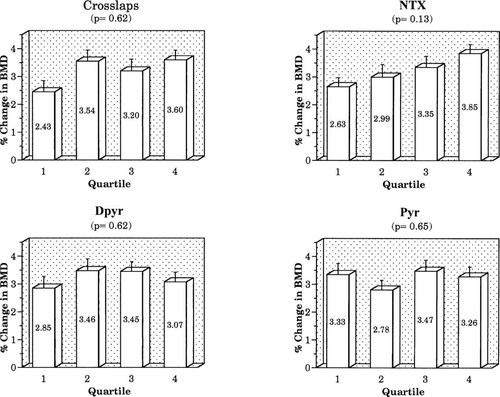
Relationship of percentage change in spine BMD to quartile of percentage change in resorption markers.
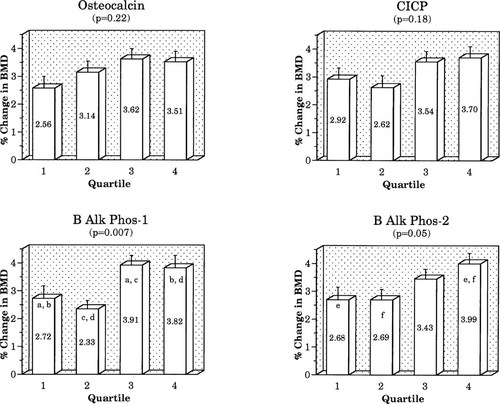
Relationship of percentage change in spine BMD to quartile of percentage change in formation markers. Columns showing the same letter differ significantly from each other: (a, b, e, f) p < 0.05; (c, d) p < 0.01.
DISCUSSION
The results of this study confirm that administration of replacement estrogen to menopausal women suppresses mean values for markers of bone turnover for up to 3 years, an effect that is attended by increases in BMD at both the lumbar spine and hip. By contrast, women given placebo demonstrate bone loss as well as persistence of average marker concentrations at baseline values. In addition, the results indicate considerable variation in the average magnitude of suppression of different markers, the most robust responses (∼60%) occurring with the two telopeptide resorption assays and osteocalcin. One limitation of our study is the inability to assess the tempo at which marker suppression occurred, but Prestwood et al.7 reported that older women treated with conjugated estrogens substantially decreased marker activity within 6 weeks. Similar to that study, the suppression of resorption markers we observed at 12 months exceeded in magnitude that of the formation markers, although unlike Prestwood et al.7 we observed greater suppression of the telopeptides than of the free Pyr cross-links at 1 year (Fig. 1).
To address the clinical utility of bone markers as predictors of BMD change we assumed that a physician has available a basic package of information, including patient age, BMI, and baseline spine and hip BMD. We asked what additional increment in the variance in bone mass or its change over time could be predicted by adding the values from one or several different bone markers, either at baseline or over time. We considered it of practical relevance to examine the effect of a single best marker as well as the single best resorption and formation markers in combination. Certainly, no physician in a clinical setting would likely request more than one resorption and one formation marker. We also carried out multiple regressions by forcing all available marker data into the analysis to determine the maximum amount of additional variance in bone density change that could be accounted for by turnover markers.
In our correlation analysis, the combined baseline values for age and BMI accounted for the greatest portion of variance in baseline BMD (16% at the spine, 25% at the hip). Only NTX, Crosslaps, and BAP-2 singly “explained” more than 5% of total variance at either site. Adding the single most robust resorption and formation markers to age and BMI increased R2 to no more than 23% of the variance at the spine and 28% at the hip. Thus, bone turnover markers, even when combined with anthropometric measures, offered little practical information for estimating BMD in individual women, and certainly did not qualify as a surrogate measure to identify patients with low bone mass.
We considered women in the placebo group to provide a reasonable model for menopausal women who are not prescribed estrogen. Baseline BMI, age, and spine BMD accounted for 7% of the variance in subsequent 1-year change at the spine, and, substituting hip BMD, accounted for 0.3% of change at the hip. Adding either of the telopeptide resorption markers or C1CP modestly increased the R2 to 19% (spine) and 5.5% (hip). Adding a combination of one resorption and one formation marker did not lead to further improvement in R2 although inclusion of all eight baseline and all 8% change markers increased R2 to 44% (spine) and 33% (hip). Thus, even when the maximum available marker information is utilized, 60% of the variance in bone mass change remained unexplained. Consequently, use of markers to predict the magnitude of change in bone mass for an individual woman who does not receive estrogen also appears to be of limited practical value.
For women receiving active treatment, change in BMD over 1 year was best predicted by a combination of variables, including baseline age, BMI, and BMD, plus both the baseline and change in two markers, NTX and skeletal ALP-2. Although this result was plausible, since it is based not only on baseline data but also the response to therapy, the achieved R2 values were still quite low, ∼20% at the spine and 8% at the hip. Entering all baseline and change variables into the regression resulted in R2 values of only 28% (spine) and 12% (hip). Once again, therefore, addition of bone marker data contributed very little to the prediction of BMD change in women treated with estrogen. We emphasize that most of the results presented here represent the prediction of 1 year changes in BMD. Associations determined for 3-year BMD changes proved to be of even lesser magnitude.
Although significant relationships between marker activity and bone loss have been shown in longitudinal population studies,8 relatively few clinical trials have related bone turnover markers to changes in BMD following intervention. Chesnut et al.9 described their experience with NTX in monitoring the response to therapy and predicting the change in BMD for a large group of recently postmenopausal women. Estrogen therapy markedly decreased NTX excretion, and women in the highest quartiles for baseline NTX, or whose excretion values decreased the most, showed the greatest gain in BMD. A significant relationship was observed between the baseline NTX value and subsequent change in bone mass for women who received placebo, and between the degree of NTX suppression and change in bone mass for women who received active treatment. That report has been interpreted to indicate a good correspondence of NTX change with the response to estrogen in postmenopausal women. Although the results of that study and ours differ, a component of this difference is related to the fact that Chesnut et al.9 reported correlation coefficients (r) rather than r2 (or R2 values). Thus, their r values of ∼0.35 between marker and changes in BMD, would correspond to an R2 of 11%.
In another study, Greenspan et al.10 published the results of a clinical trial using the bisphosphonate Alendronate in elderly women. In that study, baseline activity of a few, but not all, markers were reported to be associated with subsequent changes in BMD. The correlation coefficients of baseline marker with change in BMD were not reported, but baseline NTX was moderately correlated (r = 0.28) with spine BMD at 12 months. In that same report, changes in several markers, particularly NTX and osteocalcin, were associated with increases in BMD at both the spine (r = –0.41) and hip (r = –0.35) at 2.5 years. The putative R2 values from these results were of greater magnitude than those observed in the present study. The basis for this difference is not immediately clear, since baseline values and the degree of suppression of marker activity were reasonably comparable for the two studies. However, it is plausibly related to differences in subject characteristics (age, initial BMD) or in treatment interventions (alendronate vs. hormone replacement therapy).
Chesnut et al.9 found that menopausal women in the highest quartile of baseline NTX excretion were most likely to experience a beneficial BMD response to estrogen. In addition, Gonnelli et al.11 reported a greater BMD response to transdermal estrogen for postmenopausal women with “high” bone turnover based on whole body99Tc-methylene diphosphonate uptake than for those with “low” turnover. Our experience confirms the general principle that initial turnover level predicts subsequent response, but no single marker quartile was related to BMD change at more than one site, and quartile stratification by most of the markers tested did not significantly predict BMD response for group means at 1 year.
An alternative interpretive approach would be to assign a specific magnitude of marker suppression (a “cutoff” level) to identify women who would be predicted to respond positively to treatment. We assessed the relationship of baseline and change in markers to the 1 year change in lumbar spine BMD for women who had sustained a reduction in markers exceeding that needed to be significant (twice the compounded precision error for changes). Rather than improve the regressions, this restricted analysis actually gave lower values for R2. This predictable finding relates to the fact that some of the strength of these regressions was attributable to the contribution of a few subjects whose marker activity increased over time and who lost bone. Thus, we find no support for the utility of a bone marker “cutoff” for accurate prediction of BMD response.
Adding complexity to this set of analyses is the fact that both the marker and BMD variables are subject to substantial degrees of measurement error. Bone turnover markers characteristically show day-to-day variation of ∼15–35%,12, 13 while the CV based upon replicate measurements of spine and hip BMD in PEPI was 1.1%.4 Such random measurement errors tend to attenuate estimates of regression and correlation coefficients in both simple and multiple linear regression models.14 Assuming the CVs for the error of measurement in each marker variable were 20%, observed simple regression coefficients squared, R2 were underestimated from 64% to 93% of their true level when measuring the strength of relationships between 1 year marker changes and one year spine BMD changes. Corresponding underestimates for the logarithm of marker variables in baseline cross-sectional models ranged from 46% to 100%. The R2 for baseline spine BMD and its 1 year change were estimated at 45% of their true level. In clinical practice, compromise of fastidious attention to timing of collections and sample processing would magnify the errors of measurement, limiting further the applicability of bone marker results to predict changes in BMD.
This analysis of the PEPI experience addressed the utility of bone turnover markers as predictors of BMD change in recently menopausal individual women. The results do not confirm an important clinical role for markers in this regard. The possibility that markers can be usefully applied for other purposes in individual patients, such as documenting states of high bone turnover, as surrogate measures for bone histomorphometry, as independent estimates of fracture risk,15 or as documentation of treatment compliance, requires further prospective evaluation.
Acknowledgements
The authors wish to express their gratitude to the following individuals for their cooperation and generosity in providing reagents necessary to conduct this study: Mr. Gopal Savjani and Dr. Paul Walton (Diagnostics Systems Laboratories), Ms. Karen Shepard ( Metra Biosystems, Inc.), Ms. Karen Armour and Dr. Isaac Mizrahi (Hybritech, Inc.).