Differentiation of Human Marrow Stromal Precursor Cells: Bone Morphogenetic Protein-2 Increases OSF2/CBFA1, Enhances Osteoblast Commitment, and Inhibits Late Adipocyte Maturation
Abstract
Because regulation of the differentiation to osteoblasts and adipocytes from a common progenitor in bone marrow stroma is poorly understood, we assessed effects of bone morphogenetic protein-2 (BMP-2) on a conditionally immortalized human marrow stromal cell line, hMS(2–6), which is capable of differentiation to either lineage. BMP-2 did not affect hMS(2–6) cell proliferation but enhanced osteoblast differentiation as assessed by a 1.8-fold increase in expression of OSF2/CBFA1 (a gene involved in commitment to the osteoblast pathway), by increased mRNA expression and protein secretion for alkaline phosphatase (ALP), type I procollagen and osteocalcin (OC) (except for OC protein), and by increased mineralized nodule formation. Transient transfection with Osf2/Cbfa1 antisense oligonucleotide substantially reduced BMP-2–stimulated expression of ALP mRNA and protein. The effects of BMP-2 on adipocyte differentiation varied: expression of peroxisome proliferator-activated receptor γ2 (a gene involved in commitment to the adipocyte pathway) was unchanged, mRNA expression of the early differentiation marker, lipoprotein lipase, was increased, and mRNA and protein levels of the late differentiation marker, leptin, and the formation of cytoplasmic lipid droplets were decreased. Thus, by enhancing osteoblast commitment and by inhibiting late adipocyte maturation, BMP-2 acts to shunt uncommitted marrow stromal precursor cells from the adipocyte to the osteoblast differentiation pathway.
INTRODUCTION
BONE MARROW STROMA contains pluripotential cells that can differentiate into myocytes, chondrocytes, osteoblasts, and adipocytes.1-3 Of these pathways, those for the osteoblasts and adipocytes are most closely related. Marrow fat represents 50% of marrow cavity, and marrow adipocytes may supply energy for the differentiation and function of other marrow cell phenotypes.4 Clinical studies have shown an inverse relationship between the amount of trabecular bone and adipose tissue in bone marrow.5, 6 Further, Beresford et al.7 demonstrated a reciprocal relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Our laboratory demonstrated that single early colonies of stromal cells, obtained after gradient separation of bone marrow cells followed by negative immunoselection and plastic adherence, could differentiate into either osteoblasts, or adipocytes depending on culture conditions.8 Thus, these findings strongly support the hypothesis that osteoblasts and adipocytes share a common precursor in the bone marrow and that commitment and maturation to the osteoblast or adipocyte phenotypes is a regulated, reciprocal process.
The factors regulating differentiation of marrow stromal precursors into one or the other of these pathways are not well understood and almost nothing is known about this regulation in humans. Although regulation has been studied in vitro using rodent marrow stromal cells and preosteoblastic9-13 or preadipocytic14-16 cell lines, it is difficult to generalize from these data because of species differences with human cells and because most of these cell lines were already committed to either the osteoblast or the adipocyte differentiation pathways. Thus, the lack of a model system for uncommitted human marrow stromal (hMS) cells that is capable of differentiating into either the osteoblast or the adipocyte pathway has been a major barrier to defining regulation of this reciprocal process.
The bone morphogenetic proteins (BMPs) are important regulators of osteoblast differentiation. These proteins were identified as the active factors that induced ectopic bone formation when bone extracts were injected into soft tissues.17-20 The BMPs are members of the transforming growth factor-β superfamily21 and over 20 of them have been identified and at least seven BMPs have been cloned.22-25 In fetal life, BMPs serve as morphogens for the development of a wide variety of organs and are essential for normal skeletal development, whereas in adult life they act as signaling molecules.26, 27 BMP-2 appears to be the most important BMP affecting the human adult skeleton. Although in rodent28-33 and human34 in vitro systems, BMP-2 stimulates the differentiation of previously committed preosteoblastic cells35, 36 and inhibits the differentiation of previously committed preadipocytic cell lines,37 its simultaneous effects on both differentiation pathways and the mechanisms by which these effects are induced are unclear, particularly in humans.
We have developed conditionally immortalized cell lines from hMS progenitor cells that can differentiate into either the mature osteoblast or adipocyte phenotype.38 Recently, Houghton et al.39 developed a human cell line and Thompson et al.40 developed a murine cell line that also have the capability of potential differentiation. We have studied one of these cell lines, hMS(2–6), to define the effects of BMP-2 on sequential molecular and cellular events in osteoblast and adipocyte commitment and differentiation. The commitment to one or the other differentiation pathway was assessed by measuring the expression of OSF2/CBFA1, a recently discovered transcription factor, which may be required for initiation of osteoblast differentiation41-49 and peroxisome proliferator-activated receptor-γ2 (PPARγ2), an analogous transcription factor required for adipocyte differentiation.50-52
Subsequent maturation along the osteoblast and adipocyte pathways was assessed by measuring mRNA and protein for phenotypic markers and by assessing morphologic changes, such as mineralized nodules and lipid droplets.
MATERIALS AND METHODS
Reagents
Tissue culture media, fetal bovine serum (FBS), trypsin-EDTA, and penicillin-streptomycin were obtained from GIBCO BRL (Grand Island, NY, U.S.A.). Unless otherwise indicated, reagents were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Tissue culture plastic wares were purchased from Corning (Corning, NY, U.S.A.). Molecular biology reagents and enzymes were purchased from Boehringer Mannheim (Indianapolis, IN, U.S.A.). The RNA STAT-60 kit for the RNA isolation was obtained from TEL-TEST, Inc. (Friendwoods, TX, U.S.A.). The random primer labeling kit (Decaprime II) was from Ambion (Austin, TX, U.S.A.). 1,25-dihydroxyvitamin D (1,25(OH)2D) and radioisotopes were purchased from DuPont-NEN (Boston, MA, U.S.A.). L-Ascorbate phosphate (Asc-P) was obtained from WAKO Chemicals, Inc. (Richmond, VA, U.S.A.). Kits for the measurement of procollagen protein and osteocalcin (OC) were generous gifts of Metra Biosystems (Mountain View, CA, U.S.A.). Electroporation cuvettes were purchased from Bio-Rad Laboratories (Hercules, CA, U.S.A.). Fluorescein-ONTM Phosphoramidite was purchased from Clontech (Palo Alto, CA, U.S.A.). Human recombinant BMP-2 (BMP-2) was a generous gift of Dr. G.E. Reidel, Genetics Institute (Cambridge, MA, U.S.A.). Polytract mRNA isolation system was purchased from Promega (Norwalk, CT, U.S.A.). Osf2/Cbfa1 probe was kindly provided by Dr. Gerard Karsenty (Department of Molecular Genetics, The University of Texas, Houston, TX, U.S.A.).
Cell culture
The conditionally immortalized hMS cell line was established in our laboratory by transfecting the hMS cells with a gene coding for a temperature-sensitive mutant TsA58 of SV40 large T-antigen (SV40 LTA).38 Incubation of the cells at the permissive temperature (34°C), when the SV40 LTA is active, results in an increased rate of cell proliferation, whereas incubation at the restrictive temperature (39.5°C), when the SV40 LTA is inactive, results in little or no cell division. Thus, the hMS cell lines express an immortalized state at one temperature and a nonimmortalized state at another temperature.53 Because the six hMS cell lines that we characterized in our laboratories had similar phenotypes and were stable until at least passage 12, for these studies we utilized the hMS(2–6) cell line from passages 9–12. The hMS(2–6) cells were cultured in a humidified atmosphere 5% CO2/95% in α-minimum essential medium (α-MEM) containing 10% (v/v) heat-inactivated (HI)-FBS, 0.2 μg/ml geneticin (G418), and 1% (v/v) penicillin 10,000 U/ml:streptomycin 10,000 μg/ml (hereafter termed standard growth medium). Medium was changed twice a week. The cells were maintained in the standard growth medium at 34°C.
Differentiation media
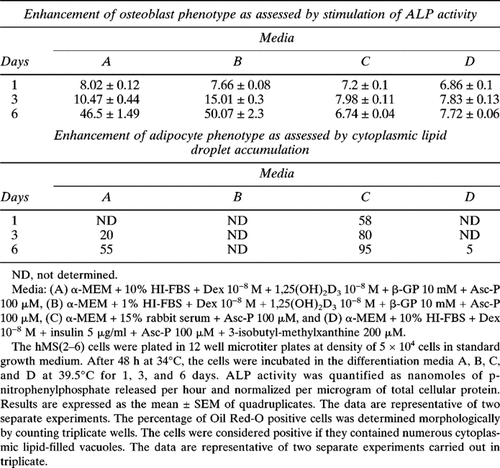
Because a primary objective was to assess the concomitant effects of BMP-2 on the osteoblast and adipocyte differentiation pathways, we performed preliminary studies to define culture conditions that would provide an equal opportunity for the hMS(2–6) cells to enter either pathway (see Results). As assessed by alkaline phosphatase (ALP) activity and cytoplasmic lipid accumulation for the osteoblast or the adipocyte phenotype, respectively, only the medium containing 10% (v/v) HI-FBS + Dexamethasone (Dex) 10−8 M + 1,25(OH)2D 10−8 M + β-glycerol-phosphate (β-GP) 10 mM + Asc-P 100 μM, provided a balanced coexpression of either phenotypes. Thus, all subsequent experiments were performed using this medium (hereafter termed standard differentiation medium) with or without the addition of BMP-2 at doses and time indicated for each experiments.
Cell proliferation
The hMS(2–6) cell proliferation was assessed by cell counting and by [3H]thymidine incorporation. Cells were plated in 48-well microtiter plates at a density of 2 × 104 cells/well in standard growth medium. After 48 h at 34°C, the cells were washed twice in phosphate-buffered saline (PBS) and incubated at 34°C in the differentiation medium in the absence or presence of 0.1, 10, and 100 ng/ml BMP-2 for 2, 4, and 6 days. Cells were harvested by trypsinization and counted with a Coulter counter (Counter Electronics, Ltd., Luton, U.K.). To assess the synthesis of DNA, 1 μCi of [3H]thymidine was added for the last 24 h of incubation. Cells were harvested by trypsinization, and [3H]thymidine was extracted by trichloracetic precipitation and detected by scintillation counting.54
mRNA expression
This was assessed by reverse transcriptase polymerase chain reaction (RT-PCR) as previously described.8 This method has been validated as providing semiquantitative data and has correlation coefficients of ∼0.97.55 Moreover, it provides semiquantitative data on relative changes of a given mRNA when amplified for a fixed number of PCR cycles.56, 57 Cells were plated in 12-well microtiter plates at a density of 5 × 104 cells in the growth medium and cultured for 48 h at 34°C. The cells were then washed twice in PBS and cultured at 39.5°C for various time intervals in the standard differentiation medium in the absence or presence of graded doses of 0.1–1000 ng/ml of BMP-2. Total cellular RNA was isolated using the RNA-STAT kit. cDNA was synthesized from 1 μg of total RNA in a 20 μl reaction mix containing 1× incubation buffer for AMV RT 5×, 2.5 μM of poly·dT, 1 mM each of dATP, dCTP, dGTP, and dTTP, 20 U of RNAse inhibitor, and 20 U of AMV RT for 2 h at 42°C. Aliquots of cDNA were amplified in a 25 μl PCR reaction mixture which contained 0.2 μM of 5′ and 3′ oligo-primers, 1× of expanded high-fidelity PCR buffer 10× with 15 mM MgCl2, 0.1 nM each of dATP, dCTP, dGTP, and dTTP, 0.25 μl of [α-32P]dCTP (10 μCi/μl) and 0.35 U of expanded high-fidelity Taq DNA polymerase. For each assay performed, each cDNA sample was run in duplicate. Amplification reactions specific for the following cDNAs were carried out: adipsin, bone/liver/kidney ALP, core-binding factor a1 (OSF2/CBFA1), leptin, lipoprotein lipase (LPL), OC, PPARγ2, type I collagen (Col I), and the housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH). Amplifications were performed in a GeneAmp 9600 thermal cycler (Perkin-Elmer, Norwalk, CT, U.S.A.). Primer sequences and amplification profiles used for all of these genes were reported previously38 except for OSF2/CBFA1, adipsin, leptin, and PPARγ2 PCR products. Osf2/Cbfa1 primer sequences, reported by Komori et al.41 amplified a PCR product from 136 to 403 location of the human cDNA sequence with a 98% homology. Adipsin, leptin, and PPARγ2 PCR product identity was confirmed by sequence analysis in an automated DNA sequencer (Perkin-Elmer, Norwalk, CT, U.S.A.).
-
adipsin-U 5′-GGT CAC CCA AGC AAC AAA GT
-
adipsin-D 5′-CCT CCT GCG TTC AAG TCA TC
-
leptin-U 5′-GCT TTG GCC CTA TCT TTT CT-3′
-
leptin-D 5′-CAC GTT TCT GGA AGG CAT AC-3′
-
PPARγ2-U 5′-CAG TGG GGA TGT CTC ATA A-3′
-
PPARγ2-D 5′-CTT TTG GCA TAC TCT GTG AT-3′.
The PCR products size were 251 bp for adipsin, 227 bp for leptin, and 390 bp for PPARγ2. The same amplification profiles of the other genes was used for adipsin, leptin, and PPARγ2,38 whereas amplification of OSF2/CBFA1 was performed using 28 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. PCR products were analyzed by electrophoresis of 9 μl samples in 1.5% (w/v) agarose gels. The amplified DNA fragments were visualized by ethidium bromide staining and quantitated by counting the radioactivity in gels slices. The quantitative differences between cDNA samples were normalized to the radioactivity present in the GAPDH PCR products.
Northern blot hybridization
The results obtained by semiquantitative RT-PCR were further confirmed by Northern blot analysis. Poly(A)+ RNA was isolated from hMS(2–6) cells treated for 1 h at 39.5°C with and without 100 ng/ml BMP-2, using the Polytract mRNA isolation system. Poly(A)+ RNA (0.5–1 μg) were resolved on a 1% (w/v) agarose gel containing 6 M glyoxal. mRNA was then transferred to a nylon membrane (Hybond N+; Amersham, Arlington Heights, IL, U.S.A.) by capillary blotting. Fifty nanograms of the probe of Osf2/Cbfa1 5′ untranslated and coding sequence (0.3 kb)47 were radiolabeled with 5 μl of [α-32P]dCTP to a specific activity of >109 cpm/μg DNA using a random primer DNA labeling kit.58 Hybridization was carried out overnight at 42°C. After stringent washing (twice for 10 minutes at room temperature in 3× standard SSC and 0.1% [w/v] SDS; twice for 15 minutes at 43°C in 1× SSC and 0.1% [w/v] SDS) membranes were subjected to autoradiography at –80°C. Band intensity was quantitated by densitometry (Pharmacia LKB Biotechnology, Piscataway, NJ, U.S.A.). All experiments were carried out twice, and representative blots are shown. Control hybridization with GAPDH cDNA verified that equal amounts of RNA were loaded.
Protein assays
The hMS(2–6) cells were plated in 48-well microtiter plates at a density of 2 × 104 cells in growth medium. After 48 h at 34°C, the cells were washed twice in PBS and incubated in standard differentiation medium at 39.5°C in the absence or presence of graded dosages of 0.1–1000 ng/ml BMP-2 for 2–19 days. For the last 24 h, the cells were treated with and without BMP-2 in the standard differentiation medium (without HI-FBS) containing 0.1% (w/v) of BSA. The ALP activity in cell lysates was quantitated at 37°C in assay buffer containing 0.75 M 2-amino-2-methyl-1-propanol, pH 10.3, for 1 h using p-nitrophenylphosphate as a substrate. The release of p-nitrophenol was monitored by measuring absorbance at 410 nm.59 The media were collected, centrifuged to remove cell debris, and then utilized for procallagen I carboxypeptide and for OC measurement by enzyme-linked immunosorbent assay and for leptin measurement by radioimmunoassay. Results of ALP activity, type I procollagen, OC, and leptin secretion were normalized to total cell protein, as measured by the Bradford protein assay method.
Transient transfection with Osf2/Cbfa1 oligonucleotides
The hMS(2–6) cell line was cultured in the growth medium to subconfluence at the permissive temperature of 34°C and then switched to the restrictive temperature of 39.5°C. After 24 h, the cells were transiently transfected by electroporation in α-MEM with a field strength of 600 V/cm and a capacitor of 960 μFD using a Bio-Rad electroporation device, in the presence of 0.1 μM of Osf2/Cbfa1 antisense or nonsense oligonucleotides.47 Following the electroporation, hMS(2–6) cells were maintained at 39.5°C in standard differentiation medium with and without 100 ng/ml of BMP-2. The ALP mRNA levels were analyzed by semiquantitative RT-PCR after 3 days, and the ALP activity was measured, as reported above, after 4 days. The effect of transient transfection of Osf2/Cbfa1 antisense and nonsense oligonucleotides on OSF2/CBFA1 mRNA steady-state level was assessed by semiquantitative RT-PCR 24 h after electroporation. The efficiency of transfection was assessed by using 0.1 μM fluorescein-labeled Osf2/Cbfa1 antisense and nonsense oligonucleotides synthesized with flourescein-oligonucleotide phosphoramidite at the 5′ terminal. The unlabeled Osf2/Cbfa1 antisense and nonsense oligonucleotides were used as control. After electroporation, hMS(2–6) cells were resuspended to ∼106 cells/ml of PBS before being sorted using a fluorescence-activated cell sorting system (FACS) (FACScan; Becton Dickinson, Mountain View, CA, U.S.A.).
Measurement of mineralized matrix formation
Cells were plated at 5 × 104 cells/well in 12-well microtiter plates in growth medium at 34°C and allowed to proliferate for 48 h. The medium was then changed to standard differentiation medium at 39.5°C in the presence or absence of 100 ng/ml of BMP-2 for 12, 15, 19, and 21 days. During the course of the experiments, the medium was changed and fresh BMP-2 was added every 3 days. The formation of mineralized matrix nodules was determined by alizarin red-S staining.60 Briefly, the cells were fixed in 70% ethanol for 1 h at room temperature. The fixed cells were washed with PBS and stained with 40 mM alizarin red-S, pH 4.2, for 10 minutes at room temperature. The cells were washed with distilled water five times and rinsed with PBS for 15 minutes. For quantitative assessment, the alizarin red-S dye was eluted and measured spectrophotometrically as described by Bodine et al.61
Assessment of cytoplasmic lipid droplet formation
The hMS(2–6) cells were plated in 12-well microtiter plates at density of 5 × 104 cells in standard growth medium. After 48 h at 34°C, the cells were washed twice with PBS and incubated at 39.5°C in standard differentiation medium in the absence or presence of 100 ng/ml BMP-2 for 6, 9, and 12 days. Cytoplasmic inclusions of neutral lipid were assessed by Oil Red-O staining. Briefly, the cells were fixed for 3 h at room temperature in 10% (w/v) neutral buffered formalin. Cells were then rinsed with PBS and stained for 15 minutes with Oil Red-O solution, rinsed again with 60% (v/v) isopropanol, washed with distilled water, and counterstained with hematoxylin. After rinsing with tap water, the nuclei were counterstained with 0.05% of lithium chloride. The percentage of Oil Red-O positive cells was determined by counting the number of cells having numerous cytoplasmic lipid-filled vacuoles in 30 contiguous fields. These measurements were made without knowledge of whether the plates were from the BMP-2 treatment or control groups.
Statistical analysis
All values are expressed as mean ± SEM. Student's paired t-test was used to evaluate differences between the stimulated samples and their respective controls. The significance of dose or time responses were assessed by multiple measures analysis of variance (ANOVA). A p value < 0.05 was considered statistically significant.
RESULTS
Development of differentiation media
A series of experiments was undertaken to determine empirically what was the optimal medium to test the concomitant effects of BMP-2 on modulating hMS(2–6) cell differentiation to either of the osteoblast and adipocyte pathways. The four media conditions (Table 1) were selected based on their potential for both osteoblastic and adipocytic differentiation. Rabbit serum was used because previous studies have shown that it is a strong inducer of lipid accumulation in preadipocytic cell lines.39, 62, 63 As indices of differentiation, we used the expression of ALP activity and cytoplasmic lipid accumulation for the osteoblast or adipocyte phenotype, respectively. As shown in Table 1, ALP activity and cytoplasmic lipid accumulation were assessed after 1, 3, and 6 days. The hMS(2–6) cells, incubated with Medium A (α-MEM, 10% [v/v] HI-FBS, Dex 10−8 M, 1,25(OH)2D3 10−8 M, β-GP mM, and Asc-P 100 μM) and Medium B (α-MEM, 1% [v/v] HI-FBS, Dex 10−8 M, 1,25(OH)2D3 10−8 M, β-GP mM, and Asc-P 100 μM), were able to increase ALP activity expression with time in culture, whereas cells incubated with Medium C (α-MEM, 15% [v/v] rabbit serum, and Asc-P 100 μM) or Medium D (α-MEM, 10% [v/v] HI-FBS, Dex 10−8 M, insulin 5 μg/ml, Asc-P 100 μM, 3-isobutyl-methylxanthine 200 μM) did not increase ALP activity expression. Cells incubated with Medium A or Medium C showed an increase in lipid droplet accumulation, but those incubated with Medium B or Medium D failed to accumulate cytoplasmic lipid with time in culture. Thus, Medium A, named standard differentiation medium, allowed a balanced coexpression of either phenotypes and was used in all studies assessing hMS(2–6) cell proliferation and differentiation.
Effect of BMP-2 effect on cell proliferation
The BMP-2 at doses between 0.1 ng/ml and 100 ng/ml had no statistically significant effect on [3H]thymidine incorporation (Fig. 1A) or on cell number (Fig. 1B).
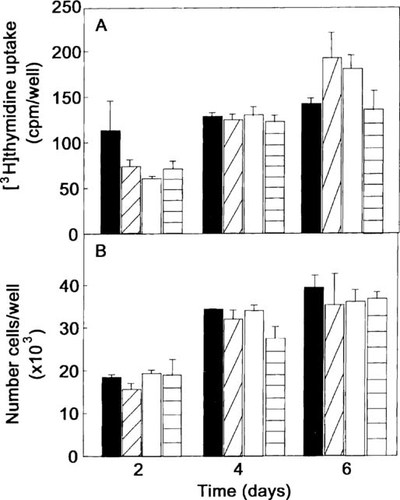
Effect of BMP-2 on cell proliferation. Cells were plated in 48-well microtiter plates at a density of 2 × 104 cells in standard growth medium. After 48 h at 34°C, the cells were incubated at 34°C in the differentiation medium in the absence (control, solid bar) or presence BMP-2 at doses of 0.1 ng/ml (cross-hatched bar), 10 ng/ml (open bar), and 100 ng/ml (horizontal bar) for 2 days, 4 days, and 6 days. (A) To assess the synthesis of DNA, 1 μCi of [3H]thymidine was added for the last 24 h of incubation. [3H]thymidine was extracted by trichloracetic precipitation and detected by scintillation counting. (B) Cells were counted with a Coulter counter. The data shown are representative of three experiments. Results are expressed as mean ± SEM of quadruplicates. No significant differences were observed among control and BMP-2 treatment.
Effect of BMP-2 on osteoblast differentiation
Steady-state mRNA levels:
To determine the role of BMP-2 on commitment of hMS(2–6) cells to the osteoblast pathway, we performed semiquantitative RT-PCR to assess OSF2/CBFA1 expression in the hMS(2–6) cell line. The hMS(2–6) cell line expressed constitutive mRNA levels of OSF2/CBFA1. As shown in Figs. 2A and 2B, treatment with100 ng/ml of BMP-2 induced an early, significant increase of 80% over the control in the OSF2/CBFA1 expression that was maximal at 1 h. The induction of OSF2/CBFA1 expression by BMP-2 at 1 h was consistent and occurred in each of four different experiments. To confirm the effect of BMP-2 on OSF2/CBFA1 expression, we further performed Northern blot analysis with a probe of Osf2/Cbfa1 untranslated and coding sequences. As shown in Fig. 3A, hMS(2–6) cells expressed one major transcript of 6.0 kb, and BMP-2 at a dose of 100 ng/ml for 1 h increased its expression 1.9-fold (Fig. 3B). Additional bands that may represent different isoforms of the CBFA1 were also observed, as previously reported.44
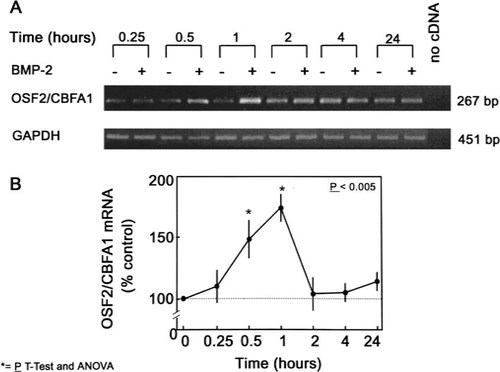
Effect on BMP-2 on OSF2/CBFA1 expression in hMS(2–6) cell lines. The cells were plated in 12-well microtiter plates at a density of 5 × 104 cells in the growth medium and cultured for 48 h at 34°C. The cells were then cultured at 39.5°C for various time intervals in the standard differentiation medium in the absence or presence of 100 ng/ml BMP-2. Aliquots of cDNA, synthesized from 1 μg of total RNA, were amplified in a 25 μl PCR reaction mixture with 0.25 μl of [α-32P]dCTP (10 μCi/μl), and the expression of OSF2/CBFA1 was analyzed by RT-PCR and corrected for GAPDH expression. (A) Ethidium bromide–stained agarose gels of PCR products performed in duplicate. A reaction from which cDNA was omitted was used as a negative control. The size of the PCR product is given in base pairs. (B) Relative mRNA production determined from the amount of radioactivity incorporated into each PCR product and normalized to the GAPDH mRNA in the same cDNA samples. Values are means for four experiments ± SEM carried out in triplicate, *p < 0.005 compared with the corresponding percentage of control values by ANOVA followed by Student's t-test.
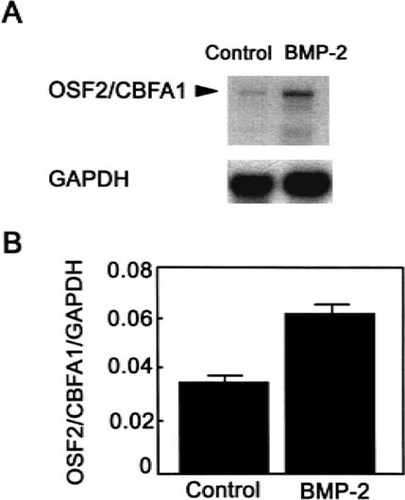
Northern blot analysis of OSF2/CBFA1 mRNA. Poly(A)+ RNA was isolated from hMS(2–6) cells treated for 1 h at 39.5°C with and without 100 ng/ml BMP-2, using the Polytract mRNA isolation system. Poly(A)+ RNA was transferred to nylon membranes and hybridized to32P-labeled cDNA probe. (A) Autoradiogram of hybridization with Osf2/Cbfa1 cDNA probe. The experiments were carried out twice, and a representative blot is shown. Control hybridization with GAPDH cDNA probe verified the amounts of mRNA loaded. (B) Densitometric analysis. The numbers represent ratios of OSF2/CBFA1 and GAPDH in treated and untreated cells ± SD.
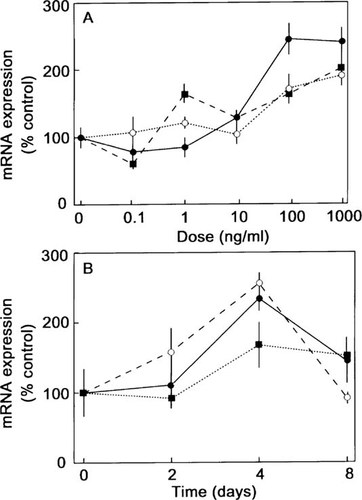
Effect of BMP-2 on osteoblastic marker mRNA expression. hMS(2–6) cells were plated in 12-well microtiter plates at a density of 5 × 104 cells in the growth medium and cultured for 48 h at 34°C. (A) Dose response. The cells were cultured at 39.5°C for 4 days in the standard differentiation medium in the absence or presence of graded dosages of 0.1–1000 ng/ml of BMP-2. (B) Time course. The cells were cultured at 39.5°C for various 2, 4, and 8 days in the standard differentiation medium in the absence or presence of 100 ng/ml of BMP-2. Aliquots of cDNA, synthesized from 1 μg of total RNA, were amplified in a 25 μl PCR reaction mixture with 0.25 μl of [α-32P]dCTP (10 μCi/μl). The expression of ALP (filled circle), Col I (filled square), or OC (open circle) mRNAs was analyzed by RT-PCR and corrected for GAPDH expression. Results are expressed as the percentage of the mean of the control values ± SEM. The data are representative of four separate experiments carried out in quadruplicate. p < 0.005 for dose effect for ALP, Col I, or OC as deviations from control values, p < 0.005 for time effect for ALP and Col I, and p < 0.05 for OC as assessed by multiple measures ANOVA.
To determine the role of BMP-2 on the maturation along the osteoblast differentiation pathway mRNA levels for the osteoblastic markers67 were measured by semiquantitative RT-PCR. The mRNA levels for ALP, Col I, and OC were examined at the restrictive temperature in the presence and absence of BMP-2. As shown in Fig. 4A, concentration of BMP-2 (0.1–1000 ng/ml) enhanced mRNA expression for ALP, Col I, and OC in a dose-dependent manner with a maximal effect within 4 days, of 145, 101, and 90% over their controls, respectively. Treatment of the hMS(2–6) cell line with 100 ng/ml of BMP-2 increased steady-state levels of ALP, Col I, and OC mRNA in a time-dependent fashion with a maximal effect of 133, 70, and 130%, respectively, compared with control by 4 days post-treatment (Fig. 4B).
Protein secretion:
To assess osteoblast differentiation, we measured ALP activity, type I procollagen synthesis, and OC secretion. Consistent with the increased ALP mRNA expression, 6 days of treatment with BMP-2 induced ALP activity in a dose-dependent manner by 107% over the control at doses of 100 ng/ml (Fig. 5A). BMP-2 (100 ng/ml) increased the enzyme activity in a time-dependent manner with a maximum of 106% over the control at 8 days (Fig. 5B). Type I procollagen synthesis was measured in the media of hMS(2–6) cells cultured at 39.5°C for 6 days in the absence and presence of BMP-2 (0.1–1000 ng/ml). The BMP-2 increased type I procollagen synthesis in a dose-dependent manner, with the maximum increase of 130% over control values at 100 ng/ml of BMP-2 (Fig. 6A). BMP-2 (100 ng/ml) increased the type I procollagen synthesis in a time-dependent fashion with a maximal effect of 125% over the control at 6 days (Fig. 6B). OC protein levels were decreased in a dose-dependent manner, with a maximum decrease of 40% over control values at 1000 ng/ml of BMP-2 (Fig. 7A) and over time in culture with a maximal effect of 70% at 10 days over the control values (Fig. 7B).
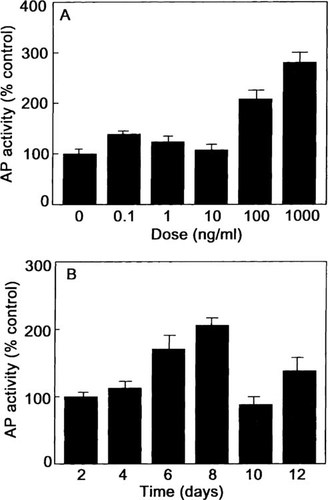
Effect of BMP-2 on ALP activity. The hMS(2–6) cells were plated in 48-well microtiter plates at a density of 2 × 104 cells in growth medium for 48 h at 34°C. (A) For effect of dose, the cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of graded dosages of 0.1–1000 ng/ml BMP-2 for 6 days. The ALP activity was quantitated as nanomoles of p-nitrophenylphosphate released per hour per microgram of total cellular protein. (B) For effect of time, the cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of 100 ng/ml BMP-2 for 0, 2, 4, 6, 8, 10, and 12 days. For both dose and time response experiments, the ALP activity was quantitated as nanomoles of p-nitrophenylphosphate released per hour per microgram of total cellular protein. The data are representative of three separate experiments carried out in quadruplicate. Results are expressed as percentage of the mean control values ± SEM. p < 0.001 as assessed by multiple measures ANOVA.
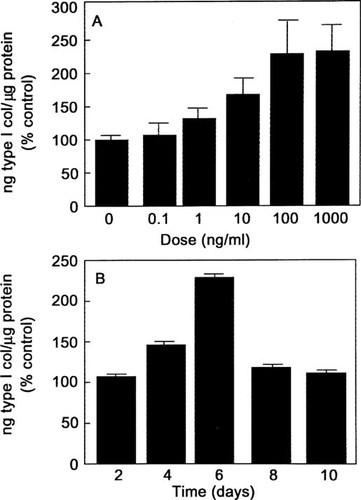
Effect of BMP-2 on COL I secretion. The hMS(2–6) cells were plated in 48-well microtiter plates at a density of 2 × 104 cells in growth medium for 48 h at 34°C. (A) The hMS(2–6) cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of graded dosages of 0.1–1000 ng/ml BMP-2 for 6 days. (B) For effect of time, the cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of 100 ng/ml BMP-2 for 0, 2, 4, 6, 8, and 10 days. The results were normalized to total cellular proteins, as measured by Bradford protein assay method. The data shown are representative of three separate experiments ± SEM carried out in quadruplicate. p < 0.005 for effect of dose and time by multiple measures ANOVA.
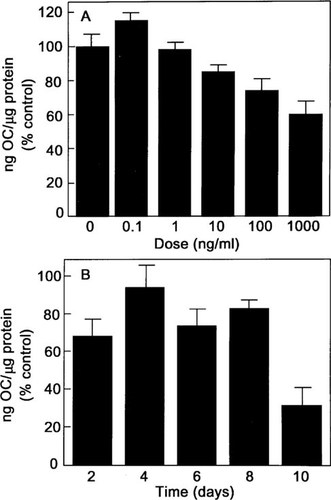
Effect of BMP-2 on OC secretion. The hMS(2–6) cells were plated in 48-well microtiter plates at a density of 2 × 104 cells in growth medium for 48 h at 34°C. (A) The hMS(2–6) cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of graded dosages of 0.1–1000 ng/ml BMP-2 for 6 days. (B) For effect of time, the cells were incubated in the standard differentiation medium at 39.5°C in the absence or presence of 100 ng/ml BMP-2 for 0, 2, 4, 6, 8, and 10 days. The results were normalized to total cellular proteins, as measured by Bradford protein assay method. The data shown are representative of three separate experiments carried out in quadruplicate ± SEM. p < 0.0005 for effect of dose and p < 0.05 for time by multiple measures ANOVA.
Effect of Osf2/Cbfa1 antisense oligonucleotide:
Since the hMS(2–6) cell line expressed mRNA for OSF2/CBFA1 and BMP-2 rapidly enhanced OSF2/CBFA1 expression before the expression of any osteoblast-specific gene, we investigated whether OSF2/CBFA1 gene expression was essential for the BMP-2–induced osteoblast differentiation. We transiently transfected hMS(2–6) cells utilizing an Osf2/Cbfa1 antisense oligonucleotide previously reported to block the expression of specific osteoblastic genes.47 Electroporation conditions were first optimized by FACS analysis, to give the highest possible transfer efficiency, which was 35% of cells after electroporation of fluorescein-labeled Osf2/Cbfa1 antisense oligonucleotide (data not shown). The same results were obtained with fluorescein-labeled Osf2/Cbfa1 nonsense oligonucleotide (data not shown). When Osf2/Cbfa1 antisense oligonucleotide was transiently transfected by electroporation in the hMS(2–6) cell line, the OSF2/CBFA1 mRNA levels were decreased by 35% as compared with cells electroporated without exposure to Osf2/Cbfa1 antisense oligonucleotide, whereas the Osf2/Cbfa1 nonsense oligonucleotide did not affect OSF2/CBFA1 expression (Fig. 8A). The Osf2/Cbfa1 antisense oligonucleotide selectively inhibited the increase in ALP mRNA expression and ALP activity observed with BMP-2 treatment, by 34% and 27%, respectively (Figs. 8B and 8C). The Osf2/Cbfa1 nonsense oligonucleotide did not affect ALP mRNA expression and ALP activity (Figs. 8B and 8C).
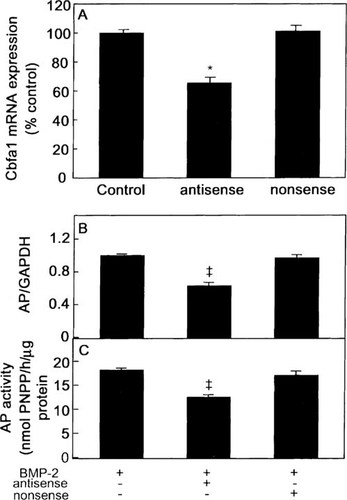
Effects of Osf2/Cbfa1 antisense and nonsense oligonucleotides on constitutive OSF2/CBFA1 mRNA expression and on BMP-2–induced increases in ALP mRNA and protein levels. The hMS(2–6) cell line was transiently transfected with or without 0.1 μM Osf2/Cbfa1 antisense or nonsense oligonucleotides. (A) The cells were cultured at 39.5°C in standard differentiation medium for 1 day. Aliquots of cDNA synthesized from 1 μg of total RNA were amplified in a 25-μl PCR reaction mixture with 0.25 μl of [α-32P]dCTP (10 μCi/μl), and the expression of OSF2/CBFA1 was analyzed by RT-PCR and corrected for GAPDH expression. (B) The hMS(2–6) cells were cultured at 39.5°C in standard differentiation medium for 3 days with 100 ng/ml of BMP-2. Aliquots of cDNA were amplified and analyzed as above. (C) The hMS(2–6) cells were cultured with 100 ng/ml of BMP-2 at 39.5°C in standard differentiation medium for 4 days. ALP activity was expressed as nanomoles of p-nitrophenylphosphate released per hour per microgram of total cellular proteins. Values are means of quadruplicates ± SEM. The data are representative of two experiments. *p < 0.001, ‡p < 0.005 compared with BMP-2 treatment as assessed by ANOVA followed by Student's paired t-test.
Mineralized nodule formation:
Because we have previously demonstrated that hMS cells are able to form mineralized nodules after 21 days in culture,38 we next investigated whether BMP-2 treatment enhanced and accelerated this process. The cells treated with 100 ng/ml of BMP-2 for 19 days and 21 days showed more intense staining nodules compared with the control (data not shown). When mineralization was quantitated by elution of alizarin red-S from stained mineral deposits at 21 days, there was a 2.7-fold increase in dye content in the BMP-2–treated cells compared with controls (Fig. 9).
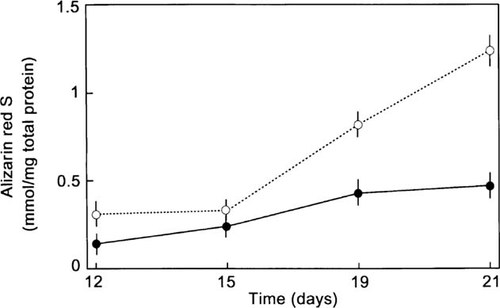
Time course for effect of BMP-2 on mineralized nodule formation assessed by quantitative Alizarin Red-S histochemical staining. hMS(2–6) cell line was seeded with regular growth medium at 5 × 104 cells/well into 12-microtiter plates and allowed to proliferate at 34°C for 48 h. The cells were then incubated in the differentiation medium in the absence (filled circle, control) or presence (open circle) of 100 ng/ml BMP-2 for 12, 15, 19, and 21 days at with 39.5°C. To quantitate the formation of mineralized nodules, Alizarin Red-S histochemical staining was performed. The alizarin red-S dye was eluted and measured spectrophotometrically. Results are expressed as millimoles of alizarin red S per milligram of total cellular protein. The results are the means ± SEM of quadruplicate. The data are representative of three separate experiments. p < 0.001 compared with the corresponding control values by multiple measures ANOVA.
Effect on adipocyte differentiation
mRNA expression:
We next investigated whether BMP-2 regulates the commitment of hMS(2–6) cells to the adipocytic pathway. Semiquantitative RT-PCR was performed to examine expression for PPARγ2, a transcription factor required for adipocyte differentiation. While the hMS(2–6) cells express constitutive levels of PPARγ2 mRNA, treatment with 100 ng/ml of BMP-2 had no effect on PPARγ2 expression (data not shown). To assess the effect of BMP-2 on adipocyte maturation, we measured mRNA levels for LPL and adipsin, early and midmarker of adipogenic differentiation, respectively, and mRNA levels of leptin, a late marker of adipocyte differentiation. Following treatment with BMP-2, there was a biphasic peak of LPL mRNA expression at 1 ng/ml and 1000 ng/ml of BMP-2 treatment (Fig. 10A). In addition, LPL mRNA expression increased after 4 days of BMP-2 treatment (Fig. 10B). Treatment with BMP-2 decreased leptin mRNA in a dose-dependent manner with a maximal effect of 44% at doses of 100 ng/ml compared with control (Fig. 9A). In addition, 100 ng/ml of BMP-2 decreased in a time-dependent fashion the leptin mRNA levels. The effect was maximal after 4 days of treatment compared with control (Fig. 10B). BMP-2 treatment did not affect adipsin mRNA expression (data not shown).
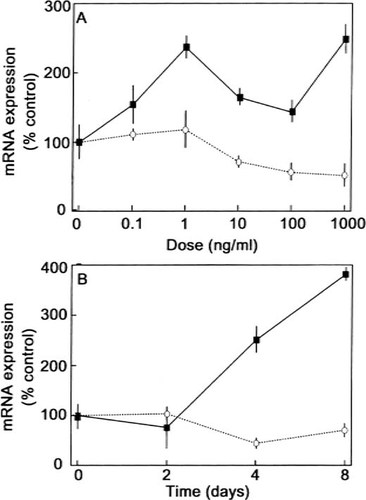
Effect of BMP-2 on adipocytic marker mRNA expression. The hMS(2–6) cells were plated in 12-well microtiter plates at a density of 5 × 104 cells in the growth medium and cultured for 48 h at 34°C. (A) Dose response. The cells were cultured at 39.5°C for 4 days in the standard differentiation medium in the absence (control) or presence of 0.1–1000 ng/ml of BMP-2. Aliquots of cDNA, synthesized from 1 μg of total RNA, were amplified in a 25-μl PCR reaction mixture with 0.25 μl of [α-32P]dCTP (10 μCi/μl). (B) Time course. The cells were cultured at 39.5°C for various time intervals in the standard differentiation medium in the absence (control) or presence of 100 ng/ml of BMP-2. The expression of LPL (filled square) and leptin (open circle) was analyzed by RT-PCR and corrected for GAPDH expression. Data are representative of four separate experiments carried out in quadruplicate. Results are reported as the percentage of the mean control values ± SEM. p < 0.001 for effect of dose as multiple measures ANOVA for both LPL and leptin. p < 0.001 for LPL and p < 0.05 for leptin for effect of time as assessed by multiple measures ANOVA.
Synthesis of leptin protein:
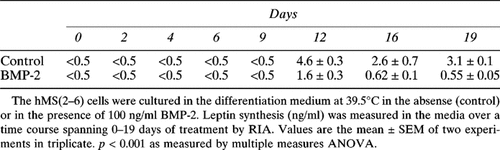
In the hMS(2–6) cells, leptin protein, a late adipocyte differentiation marker, has a time-dependent secretion pattern, appearing only after 12 days in culture at 39.5°C. Interestingly, BMP-2 treatment resulted in a significant decrease in leptin synthesis by 66% at 12 days, by 77% at 16 days, and by 83% at 19 days of treatment compared with untreated control cells (Table 2).
Accumulation of cytoplasmic lipid droplets:
BMP-2 effect on cytoplasmatic lipid accumulation was assessed by Oil Red-O staining. The hMS(2–6) cells were incubated in the differentiation medium at 39.5°C in the absence or presence of 100 ng/ml BMP-2 for 6, 9, and 12 days. BMP-2 treatment resulted in a decrease in the number of Oil Red-O–stained lipid droplets. The inhibition of lipid droplet formation is shown in Table 3. BMP-2, at a dose of 100 ng/ml, decreased the number of positive cells compared with control by 83% after 9 days of treatment and by 63% after 12 days.
DISCUSSION
The hMS(2–6) cell line is an excellent model system to study the concurrent molecular and cellular effects of BMP-2 treatment on the osteoblast and adipocyte differentiation pathways. The cell line was created by conditional immortalization of a precursor cell from hMS by transfection with a construct containing the temperature-sensitive mutant of the SV40 LTA.38 At the permissive temperature of 34°C, the SV40 LTA is functional and cell numbers can be expanded without increasing differentiation. At the restrictive temperature of 39.5°C, SV40 LTA is nonfunctional and differentiation can be studied using what is essentially a clonal population of normal stromal precursor cells. The cells are of human origin and have the phenotype of normal marrow stromal cell phenotype, thereby avoiding the problems of species differences between rodent and human cells and the incomplete, fixed phenotype that is present in spontaneously transformed cell lines.38 The hMS(2–6) cells are bipotential and, under appropriate experimental conditions, can differentiate into either the mature osteoblastic or adipocytic phenotypes including the formation of mineralized nodules and cytoplasmic lipid droplets. Thus, the effect of regulatory factors on either the osteoblast and adipocyte differentiation pathways can be stimulated concurrently.
We developed empirically a standard differentiation medium that maintains a balanced bipotential state and provides the hMS(2–6) cells with a comparable opportunity to differentiate into either osteoblasts or adipocytes. In addition to serum and standard tissue culture reagents, Dex, 1,25(OH)2D3, and ascorbate were essential for both the osteoblast and the adipocyte differentiation. Glucocorticoids stimulate the differentiation of many types of cells,9, 12, 13, 28, 65 so their requirement for both phenotypes was expected. Although the effect of 1,25(OH)2D3 on osteoblast differentiation is well established,66 we were surprised to find that it was also required for adipocytic differentiation. Consistent with this finding, however, was the report that 1,25(OH)2D3 treatment increases adipogenesis in cultures of fetal rat calvarial cells.67, 68 Ascorbic acid is a requirement for formation of a collagenous extracellular matrix before expressing specific genes that permit the differentiation of marrow stromal cells to adipocytes, myoblasts, and chondroblasts.69 In addition, as previously reported,70 β-GP was required for mineralization of newly formed matrix. Interestingly, insulin and the phosphodiesterase inhibitor 3-isobutyl-methylxanthine, which have previously been shown to enhance differentiation of committed preadipocytic cell lines,14 had no effect on adipocytic differentiation in the hMS(2–6) cells. This is probably because the hMS(2–6) cells represent an early, uncommitted stage of differentiation, whereas the preadipocytic cell lines used in the previous studies were already committed to the adipocyte pathway.
Treatment with BMP-2 enhanced overall osteoblast differentiation by 2-fold or more as assessed by increased steady-state levels of mRNA and protein secretion of osteoblast markers as ALP, type I procollagen, and by the increased formation of mineralized nodules by these cells. Since BMP-2 increased, in a time- and dose-dependent fashion, the OC mRNA steady-state levels, an unexpected finding was the decrease in protein secretion following BMP-2 treatment. The explanation for this anomalous result is unclear. Using gel electrophoresis, we found no evidence that125I-OC added to the conditioned medium was degraded, and the effect of BMP-2 on decreasing OC secretion was present during both early (day 6) and late (day 21) stages in osteoblast differentiation (data not shown).
In contrast with its consistent stimulation of osteoblast differentiation, BMP-2 appears to have a more complex effect on inhibiting adipocyte differentiation. mRNA expression was increased for the early differentiation marker gene, LPL. Although it is one of the first genes induced during adipocyte differentiation,71 LPL codes for an enzyme that is also found in other cell types that utilize fatty acids for metabolism.72-75 Furthermore, both human fetal osteoblastic (hFOB) cells and normal mature osteoblastic cells expressed constitutive levels of LPL mRNA (data not shown). The increased expression of LPL mRNA observed in these studies, may have occurred in a subpopulation of the uncommitted state of stromal cells and increases as the cells are activated to enter either the osteoblast or the adipocyte differentiation pathway. Furthermore, we found that the middle phase differentiation marker gene, adipsin, was unchanged by BMP-2 treatment (data not shown). In contrast, leptin, which has been shown to be expressed late in the differentiation of white adipose tissue,76 was decreased by BMP-2 treatment at both the mRNA and protein levels. Moreover, leptin was not secreted by hMS(2–6) cells until late in culture. Furthermore, after BMP-2 treatment, there was a substantial decrease in reduction of Oil Red-O staining of cytoplasmic droplets, a characteristic feature of the mature adipocytic phenotype. Thus, BMP-2 appears to inhibit late adipocyte maturation.
Cell commitment involves the activation of genes for specific transcription factors that initiate the differentiation process. These products of the so called “master genes” then induce the expression of genes characteristic of the phenotype. In contrast to most of the other differentiation pathways of marrow stromal cells for which the master genes governing phenotype commitment have been identified, a gene (regulating commitment) acting as a differentiation switch for the osteoblast lineage has only recently been elucidated when Osf2/Cbfa1 was shown to be essential for osteoblast differentiation from early precursors.42, 45 Osf2/Cbfa1 codes for a transcription factor that is a member of the core binding factor family and binds to the osteoblast-specific cis-acting element in the OC gene and, presumably, other genes mainly expressed in osteoblasts.46, 47 Moreover, the overexpression of Osf2/Cbfa1 induces the markers for osteoblast in fibroblast cell lines and in primary skin fibroblasts.47 Targeted disruption of this gene completely blocks intramembranous and endochondral ossification, resulting in formation of a cartilaginous skeleton that is not replaced by bone.41, 42, 48, 49
After BMP-2 treatment, the expression of OSF2/CBFA1 mRNA was increased by 80%, as shown by semiquantitative RT-PCR. As with most of early genes that code for transcription factors, the regulated expression of OSF2/CBFA1 occurred very early after treatment and was maximal at 1 h, well before the increases in expression of ALP, COL I, and OC mRNA, which did not occur until 48 h post-treatment. Moreover, using the Osf2/Cbfa1 cDNA probe, we report here that the OSF2/CBFA1 transcript is present in hMS(2–6) cells, and is up-regulated by BMP-2 treatment. As previously reported,44, 45 we observed the presence of different bands that may represent the other isoforms of the CBFA1 gene.
Transient transfection with the Osf2/Cbfa1 antisense oligonucleotide, but not with Osf2/Cbfa1 nonsense oligonucleotide, decreased the constitutive mRNA expression of OSF2/CBFA1. Furthermore, cotreatment of the hMS(2–6) cells with BMP-2 and the Osf2/Cbfa1 antisense oligonucleotide blunted the increase in ALP mRNA and protein by 34% and 27%, respectively, following BMP-2 treatment. Because only 35% of electroporated cells were permeable to fluorescein-labeled Osf2/Cbfa1 antisense oligonucleotide, the block of osteoblast differentiation induced by Osf2/Cbfa1 antisense oligonucleotide appears to be nearly complete. Moreover, these results are consistent with the data reported by Ducy et al.47 showing that BMPs regulate the Osf2/Cbfa1 expression in C3H10T1/2 fibroblast cells, which do not normally express osteoblast-specific genes, and that ROS 17/2.8 osteoblastic cells transiently transfected with Osf2/Cbfa1 antisense oligonucleotide fail to express specific osteoblastic genes. Our results show that hMS precursor cells express OSF2/CBFA1 as the initial change in the BMP-2 signaling cascade regulating osteoblast differentiation.
Although BMP-2 treatment strikingly inhibited late adipocyte phenotype development, it had no effect on the expression of PPARγ2 mRNA levels, the corresponding master gene involved in commitment to the adipocyte differentiation pathway.50-52 These findings suggest that BMP-2 may not affect commitment to the adipocyte lineage but rather acts late in the adipocyte pathway to inhibit maturation to the mature phenotype.
Whatever the molecular mechanisms, the net effect of BMP-2 on stromal precursor cells is to enhance their differentiation to osteoblasts and to inhibit their differentiation to mature adipocytes. Thus, BMP-2 may stimulate shunting of uncommitted precursor cells from the adipocyte differentiation pathway into the osteoblast differentiation pathway. Elderly women have increased yellow (fatty) marrow and decreased red (cellular) marrow,77 a feature that is further exaggerated in elderly women with osteoporosis.5, 6 Also, elderly women78 and women with osteoporosis79 have a relative decrease in the number of osteoblasts as compared with the number of osteoclasts. Given the findings reported here, studies should be performed to determine if there is a deficiency of BMP-2 production or action in osteoblast lineage cells of aging women with and without osteoporosis.
In conclusion, our results indicate that BMP-2 acts on uncommitted stromal cell precursors in human bone marrow to modulate their differentiation but has no effect on their proliferation. The BMP-2 enhances the osteoblast differentiation pathway apparently by acting mainly at the level of commitment, through increased expression of OSF2/CBFA1. Concomitantly, it inhibits adipocyte differentiation, apparently by acting at some point late in the adipocyte differentiation pathway to decrease subsequent maturation. Thus, BMP-2 may be the major cytokine regulating the balance between osteoblast and adipocyte formation in the bone marrow microenvironment.
Acknowledgements
We are greatly indebted to Dr. Theresa Hefferan for helpful assistance in the Osf2/Cbfa1 antisense oligonucleotide studies and to Drs. Sundeep Khosla and Lorenz C. Hofbauer for their helpful discussions and advice. We thank Ms. Susan K. Bonde for her excellent technical assistance. We also thank Nurit Geller for assistance in analysis of the data. This work was supported by Grant AG-04875 from the National Institutes of Health.