Expression of Osteoprotegerin (Osteoclastogenesis Inhibitory Factor) in Cultures of Human Dental Mesenchymal Cells and Epithelial Cells
Abstract
Osteoprotegerin (OPG)/osteoclastogenesis inhibitory factor (OCIF) inhibits osteoclast differentiation, activity, and survival; therefore OPG/OCIF may regulate the resorption of dental hard tissues, such as alveolar bone, cementum, and dentin. To investigate this issue, reverse transcriptase-polymerase chain reaction using specific primers for OPG/OCIF was performed with total RNAs isolated from human gingival keratinocytes (HGKs), human gingival fibroblasts (HGFs), human periodontal ligament cells (HPDLs), and human pulp cells (HPCs) in culture. PCR products were found in HGFs, HPDLs, and HPCs, but not in HGKs, and the DNA sequence of these products was 100% identical to the reported sequence of the OPG gene. Northern blot analyses also showed that HGFs, HPDLs, and HPCs, but not HGKs, expressed OPG/OCIF transcripts of ∼2.5 kb. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) increased OPG/OCIF mRNA levels in a dose-and time-dependent manner in HPDL. After 12 h of treatment, IL-1β at 3 ng/ml and TNF-α at 3 ng/ml increased OPG/OCIF mRNA expression by 190% and 110%, respectively, with a maximal effect. The stimulatory effects of IL-1β and TNF-α were also seen in HPC. However, IL-6 and transforming growth factor-β had little effect on OPG/OCIF mRNA levels in HPDL. These findings suggest that OPG/OCIF synthesized by dental mesenchymal cells locally regulates the resorption of dental hard tissues through cytokines.
INTRODUCTION
OSTEOCLASTS, deriving from hematopoietic cells of the monocyte-macrophage lineage are responsible for bone resorption.1, 2 Osteoblasts or stromal cells in bone are essential for osteoclastogenesis and are involved in osteoclast differentiation, producing soluble factors, and interacting with osteoclast precursors.2-4 Odontoclasts seem to derive from hematopoietic cells and resorb dentin and cementum.5
Osteoprotegerin (OPG) is a novel member of the tumor necrosis factor (TNF) receptor superfamily and is a secreted glycoprotein that suppresses bone resorption by negatively regulating osteoclast maturation, activity, and survival.6-8 It has also been reported that osteoclastogenesis inhibitory factor (OCIF) purified from the conditioned medium of human lung embryonic fibroblasts (IMR-90) inhibits osteoclast development and that OCIF is identical to OPG.9, 10 Various tissues, such as lung, heart, kidney, and placenta, have OPG/OCIF transcripts.6, 10 At the cellular level, bone marrow stromal cells, osteoblastic cells, and osteosarcoma cells as well as IMR-90 express OPG/OCIF mRNA.10-16 These results imply that OPG/OCIF regulates bone resorption locally and systemically.
Dental tissue is uniquely structured with various mineralized tissues, such as alveolar bone, cementum, or dentin. These mineralized tissues are metabolized under physiological or pathological conditions. Therefore, OPG/OCIF released from dental mesenchymal cells may be involved in the resorption of these mineralized tissues by osteoclasts and odontoclasts.
Various cytokines such as interleukin-1β (IL-1β), IL-6, and TNF-α stimulate osteoclast formation and bone resorption.2, 17-20 These cytokines are produced by fibroblasts and inflammatory cells stimulated with lipopolysaccharides of bacteria infecting dental tissues and mediate tissue inflammation and bone resorption in marginal and apical periodontitis.21-28 In addition, transforming growth factor (TGF-β) plays an important role in bone remodeling.29, 30 Previous studies have shown IL-1β, IL-6, TNF-α, and TGF-β modulate the OPG/OCIF expression in bone marrow stromal cells, osteoblastic cells, and osteosarcoma cells.12-15 However, the cytokine regulation of OPG/OCIF expression in dental cells has not yet been examined.
To elucidate the role of dental mesenchymal cells in the metabolism of dental hard tissues, in the present study, we examined the presence of OPG/OCIF transcripts in dental cells as well as the expression manner.
MATERIALS AND METHODS
Cytokines
Human recombinant IL-1β, IL-6, and TNF-α and human platelet-derived TGF-β were purchased from R&D Systems (Minneapolis, MN, U.S.A.).
Preparation and culture of cells
Human periodontal ligament cells (HPDL-1, HPDL-2) were obtained by explant cultures of the periodontal ligament of the midroot of two healthy third molars extracted from two different volunteers and maintained separately. Human gingival fibroblasts (HGF-1, HGF-2) were obtained from two healthy gingival tissue explants from two different volunteers and human pulp cells (HPC-1, HPC-2) were obtained as previously described.31 HPDL-1, HPDL-2, HGF-1, HGF-2, HPC-1, and HPC-2 in cultures at the sixth–eighth passage were harvested, seeded at a density of 35 × 104 cells/60-mm plastic tissue culture plate coated with type I collagen, and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (GIBCO, Buffalo, NY, U.S.A.), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.4 μg/ml amphotericin B, and 50 μg/ml ascorbic acid (medium A) for 7 days. Human gingival keratinocytes (HGK-1, HGK-2) were isolated from two healthy gingiva from two different volunteers by treatment with 0.4% dispase overnight at 37°C. Primary cultures were established in serum-free keratinocyte medium containing 30 μg/ml bovine pituitary extract, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 0.1 ng/ml human epidermal growth factor, 50 μg/ml gentamicin, and 50 ng/ml amphotericin B (medium B) (Clonetics Corp., San Diego, CA, U.S.A.). HGK-1 and HGK-2 in cultures of second or third passages were cultured in medium A or B for 7 days. In addition, HPDL-1 and HPC-1 were exposed to IL-1β, TNF-α, IL-6, or TGF-β for 1, 3, 6, 12, or 24 h before the end of incubation on day 14 in medium A.
RNA preparation
Total RNA from each culture was extracted using ISOGEN® (Wako Junyaku Co., Tokyo, Japan) on day 7 or day 14 and quantitated by spectrometry at 260 nm and 280 nm.
Reverse transcriptase-polymerase chain reaction of total RNA and sequencing of polymerase chain reaction products
To examine whether OPG/OCIF mRNA was expressed in HGK, HGF, HPDL, and HPC, reverse transcriptase-polymerase chain reaction (RT-RCR) was performed using RT-PCR kit (Perkin Elmer, Foster, CA, U.S.A.). To amplify the DNA fragment coding the partial OPG/OCIF, two primers (up primer-1: 5′-GCCCTGACCACTACTACACA-3′, down primer-1: 5′-TCTGCTCCCACTTTCTTTCC-3′) in the internal OPG/OCIF coding region were constructed on the basis of the DNA sequence of OPG/OCIF reported by Simonet et al.6 RT reaction was performed for 20 minutes at 42°C, and this reaction was stopped by heat inactivation for 5 minutes at 95°C. PCR conditions were 95°C for 0.5 minutes, then 30 cycles of 60°C for 1 minutes, and 95°C for 0.5 minutes, followed by 60°C for 20 minutes. Then, the PCR products were cloned into pGEM-T easy vector (Promega, Madison, WI, U.S.A.), and the DNA fragments were sequenced using an automated DNA sequencing system (ALFred; Pharmacia, Uppsala, Sweden). Two primers (up primer-2: 5′-CAATGAACAAGTTGCTGTGCT-3′, down primer-2: 5′-CCAGTTACAAGCAGCTT-3′), which were outside of both ends of OPG/OCIF were constructed to clone the full length of OPG/OCIF cDNA. After the RT-PCR product from total RNA of HPDL was cloned into pGEM T-easy vector, the entire fragment was sequenced and used for the OPG/OCIF cDNA probe.
Northern blot analysis
Total RNA (5 μg) from each cell was denatured with 2.2 M formaldehyde and 50% formamide, resolved by electrophoresis in a 1% agarose gel containing 0.66 M formaldehyde and running buffer in the presence of ethidium bromide and transferred to nylon membranes as described.32 The membranes were irradiated for 5 minutes by UV light to cross-link the RNA, then prehybridized at 68°C for 1 h in 6× SSC (1× SSC: 0.15 M NaCl and 15 mM sodium citrate), 5× Denhardt's solution, 0.5% SDS, 0.01 M EDTA, and 100 μg/ml denatured salmon sperm DNA. Hybridization was carried out at 68°C for 16 h in prehybridization buffer in the presence of the32P-labeled human OPG/OCIF cDNA probe obtained by RT-PCR using up primer-2 and down primer-2 and32P-labeled human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (750 bp) (Cayman Chemical, Ann Arbor, MI, U.S.A.). The membranes were washed three times in 2× SSC/0.5% SDS at room temperature for 1 minute, twice in 0.5× SSC/0.5% SDS at 68°C for 30 minutes, and exposed to X-ray films. Radioactivity was measured using a laser image analyzer (BAS 2000; Fujix, Tokyo, Japan).
RESULTS
We obtained PCR products (736 bp), corresponding to the size estimated from the DNA sequence of OPG/OCIF,6 from HPDL-1, HGF-1, and HPC-1, but not from HGK-1 (Fig. 1). The DNA sequences of these products were 100% identical with that of OPG/OCIF. RT-PCR was then performed with specific primers to amplify the full length of OPG/OCIF cDNA and we found 1212 bp of the PCR product in HPDL-1. The DNA sequence revealed that the OPG/OCIF mRNA in HPDL-1 was identical to that of other tissues, such as heart, placenta, lung, and kidney. Northern blot analysis of human cultured cells identified OPG/OCIF mRNA of ∼2.5 kb and HGF-1, HGF-2, HPDL-1, HPDL-2, and HPC-1, HPC-2 expressed OPG/OCIF transcripts (Fig. 2). However, HGK showed the expression of OPG/OCIF mRNA, cultured neither in medium A (data not shown) nor in medium B (Fig. 2). Figure 3 and Fig. 4 show the effects of the length of exposure of HPDL-1 to cytokines on the levels of OPG/OCIF mRNA. IL-6 at 3 ng/ml and TGF-β at 3 ng/ml had little effect on the levels of OPG/OCIF transcripts at the indicated times (Fig. 3). However, IL-1β at 3 ng/ml and TNF-α at 3 ng/ml increased the level of OPG/OCIF transcripts in a time-dependent manner (Fig. 4). These stimulatory effects were detected at 3 h and became more prominent at 12 h. Figure 5 shows the effect of increasing concentrations of IL-1β and TNF-α on the expression of OPG/OCIF mRNA after 12 h of treatment in cultures of HPDL-1. IL-1β and TNF-α increased the level of OPG/OCIF mRNA in a dose-dependent manner. These effects of IL-1β and TNF-α were induced at 0.01 ng/ml and an increase of OPG/OCIF mRNA expression by 190% and 110% was detected at concentrations of 3 ng/ml IL-1β and 3 ng/ml TNF-α, respectively. These stimulatory effects of both cytokines were detected at 0.5 ng/ml after 12 h treatment of cultured HPC-1 (data not shown).
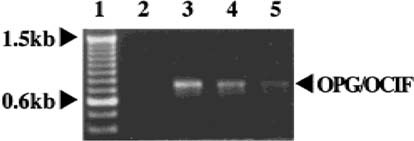
RT-PCR analysis of OPG/OCIF mRNA expression in cultures of HGK, HGF, HPDL, and HPC. PCR products, as described in the Materials and Methods were separated by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. The experiment was repeated once with similar results. Lane 1, 100-bp ladder; lane 2, HGK-1; lane 3, HGF-1; lane 4, HPDL-1; lane 5, HPC-1.
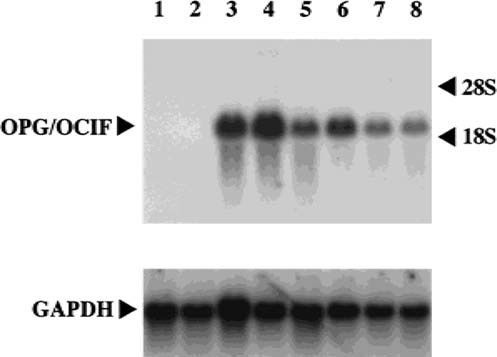
Expression of OPG/OCIF mRNA in cultures of HGK, HGF, HPDL, and HPC. Total RNA (5 μg/lane) was analyzed by Northern blotting using human OPG/OCIF and GAPDH cDNA probes. The mRNA levels of OPG/OCIF (upper panel) and GAPDH (lower panel) are shown. The experiment was repeated once with similar results. Lane 1, HGK-1; lane 2, HGK-2; lane 3, HGF-1; lane 4, HGF-2; lane 5, HPDL-1; lane 6, HPDL-2; lane 7, HPC-1; lane 8, HPC-2.
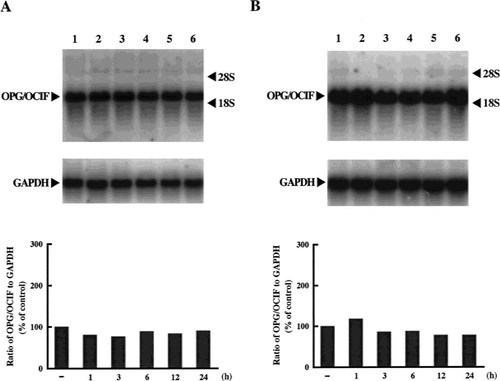
Time-course experiments with IL-6 (A) and TGF-β (B) on the levels of OPG/OCIF mRNA in HPDL-1 cultures. HPDL-1 was exposed to each cytokine (3 ng/ml) for the indicated times before the end of incubation on day 14. Total RNA (5 μg/lane) was analyzed by Northern blotting using human OPG/OCIF and GAPDH cDNA probes. The mRNA levels of OPG/OCIF (upper panel) and GAPDH (lower panel) are shown. Each panel is representative of the results from two experiments. The graphs indicate the ratio of OPG/OCIF to GAPDH as a percentage of the control (normalized to 100%). Bars are averages for two experiments. Lane 1, control; lane 2, 1 h; lane 3, 3 h; lane 4, 6 h; lane 5, 12 h; lane 6, 24 h.
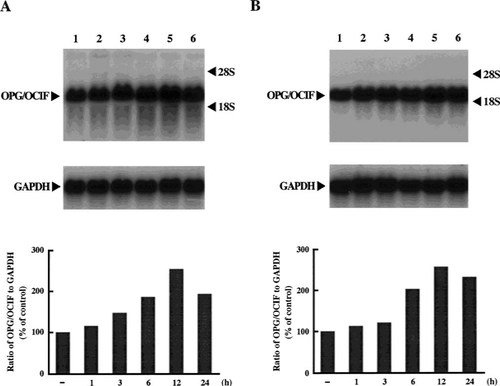
Time-course experiments with IL-1β (A) and TNF-α (B) on the levels of OPG/OCIF mRNA in HPDL-1 cultures. HPDL-1 was exposed to each cytokine (3 ng/ml) for the indicated times before the end of incubation on day 14. Total RNA (5 μg/lane) was analyzed by Northern blotting using human OPG/OCIF and GAPDH cDNA probes. The mRNA levels of OPG/OCIF (upper panel) and GAPDH (lower panel) are shown. Each panel is representative of the results from two experiments. The graphs indicate the ratio of OPG/OCIF to GAPDH as a percentage of the control (normalized to 100%). Bars are averages for two experiments. Lane 1, control; lane 2, 1 h; lane 3, 3 h; lane 4, 6 h; lane 5, 12 h; lane 6, 24 h.
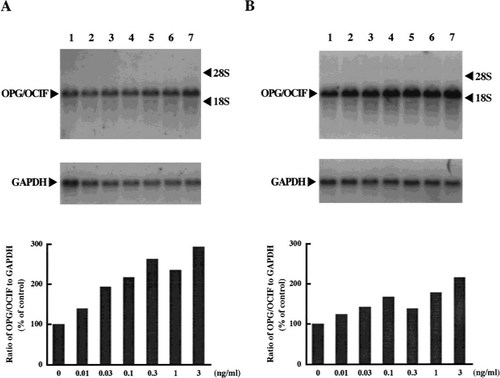
Dose-dependent effects of IL-1β (A) and TNF-α (B) on the levels of OPG/OCIF mRNA in HPDL-1 cultures. HPDL-1 was exposed to various concentrations of IL-1β and TNF-α for 12 h before the end of incubation on day 14. Total RNA (5 μg/lane) was analyzed by Northern blotting using human OPG/OCIF and GAPDH cDNA probes. The mRNA levels of OPG/OCIF (upper panel) and GAPDH (lower panel) are shown. Each panel is representative of the results from two experiments. The graphs indicate the ratio of OPG/OCIF to GAPDH as a percentage of the control (normalized to 100%). Bars are averages for two experiments. Lane 1, control; lane 2, 0.01 ng/ml; lane 3, 0.03 ng/ml; lane 4, 0.1 ng/ml; lane 5, 0.3 ng/ml; lane 6, 1 ng/ml; lane 7, 3 ng/ml.
DISCUSSION
Previous studies have shown OPG/OCIF is expressed in cultures of lung fibroblasts and osteoblastic cells.10-16 In this study, we demonstrated OPG/OCIF transcripts were expressed in cultured HPDL, HGF, and HPC, but not in cultured HGK. Thus, dental mesenchymal cells, but not dental epithelial cells express OPG/OCIF mRNA.
The formation and the function of osteoclasts are promoted in the inflammatory bone resorption observed in periodontitis. This promotion seems to be caused by cytokines, such as IL-1β, IL-6, and TNF-α, synthesized by cells in dental tissue, such as dental mesenchymal cells and inflammatory cells.21-28 However, little is known about the integrated regulatory mechanism of the formation and function of osteoclasts in periodontitis. It has recently been suggested that OPG/OCIF, secreted locally by osteoblasts or stromal cells in bone marrow and carried from nonosseous tissue in an endocrine fashion, suppresses osteoclast maturation and activity by binding the osteoclast differentiation factor/OPG ligand (ODF/OPGL) which is a membrane-bound protein present in osteoblastic cells.7, 10, 33 Furthermore, ODF/OPGL can exist in a soluble form.7 ODF/OPGL seems to bind to the receptor of osteoclast progenitors and osteoclasts to promote osteoclast differentiation and activity.2, 7, 10, 33 The present study has shown that OPG/OCIF mRNA is expressed by HGF, HPDL, and HPC. However, we could not detect ODF/OPGL mRNA in HGF, HPDL, and HPC by RT-PCR with the two up primers and two down primers constructed (unpublished data). Therefore, dental mesenchymal cells are unable to support osteoclast maturation through direct contact with osteoclast precursors. However, OPG/OCIF, which is produced by dental mesenchymal cells and is carried in a paracrine fashion, may bind to ODF/OPGL on osteoblasts in bone around teeth and block the interaction between osteoblasts and osteoclast precursors essential for osteoclast maturation.
We have shown that HPC as well as HPDL and HGF express OPG/OCIF transcripts. It is not clear how OPG/OCIF from HPC works on the metabolism of mineralized tissue in pulp.
Although IL-1β and TNF-α are potent stimulators of bone resorption, they up-regulate the levels of OPG mRNA in cultures of human osteoblastic cells and osteosarcoma MG-63 cells.12, 14 In the present study, IL-1β and TNF-α also increased OPG/OCIF mRNA expression in cultures of HPDL and HPC. These results suggest that IL-1β and TNF-α in dental tissue participate in not only the stimulation but also the inhibition of the resorption of bone around teeth. Furthermore, our results support the speculation that OPG/OCIF constitute a negative feed-back mechanism for the reduction of osteolytic activity.13, 14
It has been reported by Emery et al.34 that a TNF-related ligand called TRAIL-induced apoptosis is blocked when OPG/OCIF binds the TRAIL. Therefore, OPG/OCIF produced by dental mesenchymal cells may bind to TRAIL to inhibit the TRAIL-induced apoptosis during dental inflammation and bone resorption when the level of TNF-α is high.
Previous studies have shown that IL-6 does not affect the OPG/OCIF mRNA levels in osteoblasts and MG-63.13, 14 In the present study, IL-6 had little effect on OPG/OCIF mRNA levels in HPDL. Thus, IL-6 may not be involved in the inhibition of bone resorption, although it acts on osteoblasts to induce osteoclast formation.2
TGF-β increased the levels of OPG/OCIF expression in a mouse bone marrow-derived stromal cell line.15 We showed here that TGF-β had little effect on OPG/OCIF mRNA in cultures of HPDL. Thus, the action of TGF-β on OPG/OCIF expression differs among cells.
In conclusion, the present observations suggest OPG/OCIF is a new local suppressor of osteoclast maturation and activity in dental mineralized tissue metabolism. In addition, the study of the regulatory role of OPG/OCIF in dental tissue might provide insight into the mechanism by which dental mineralized tissue is remodeled.
Acknowledgements
We wish to thank the Research Center for Molecular Medicine, Hiroshima University School of Medicine, for the use of their facilities. This work was in part supported by a Grant-in-Aid for Scientific Research (A) (No. 09307045), Grant-in-Aid for Scientific Research (A) (No. 09307042), and Grant-in-Aid for Encouragement of Young Scientists (No. 09771618) from the Ministry of Education, Science, Sports, and Culture of Japan.




