On the Estrogen–Bone Relationship and Postmenopausal Bone Loss: A New Model
Abstract
In this model of estrogen effects on bone, a postulated mediator mechanism in marrow would affect modeling and remodeling only of bone next to or close to it. That mediator mechanism could sense estrogen. In response to that hormone, it would let remodeling of bone next to marrow proceed in its conservation mode. This would minimize losses of that bone and tend to prevent an osteopenia. But acute estrogen deficiency would make that mechanism switch remodeling of bone next to marrow to its disuse mode. Meanwhile, conservation-mode remodeling would continue for haversian and subperiosteal bone. The resulting losses of bone next to marrow would expand marrow cavities, thin cortices, and reduce trabecular bone “mass,” but would not reduce outside bone diameters. That scheme could explain the osteopenia that follows natural or experimental estrogen deficiency in mammalian females. If so, as estrogen secretion rises in girls at puberty they should begin accumulating more bone next to marrow. They do. Also if so, at menopause women should begin to lose that bone. They do. Those effects would exist in addition to known effects of estrogen on existing osteoclasts and osteoblasts.
INTRODUCTION
THIS ARTICLE SUGGESTS a new model for estrogen effects on bone strength and “mass” in women (when in quotes below, “mass” has its meaning in absorptiometry). By 1950 it was known that osteoblasts make bone and osteoclasts resorb it, and decreased estrogen secretion during and after menopause leads to significant bone losses.1 That and other evidence led to the ideas that osteoblasts and osteoclasts functioned and were controlled independently, that estrogen unilaterally inhibits osteoclastic activity, and that reduced estrogen at menopause removed that inhibition and let osteoclastic activity increase and cause postmenopausal bone loss. Research did find many biochemical, cell- and molecular-biologic effects of estrogen on osteoclasts (and osteoblasts).2
Yet those ideas could not easily explain some anatomical, biological, biomechanical, and clinical evidence summarized next.
SOME EVIDENCE FOUND AFTER 1960
The tissue-level modeling and remodeling mechanisms
Osteoblasts alone do not control postnatal additions in bone strength and “mass,” and osteoclasts alone do not control postnatal losses in bone strength and “mass.” Instead the modeling and remodeling mechanisms do that, and each needs both osteoblasts and osteoclasts to do its work.3-5
Global modeling by formation and resorption drifts can increase but not decrease bone strength and “mass,” while remodeling by BMUs (basic multicellular units) can turn bone over in two modes. In its “conservation mode” it turns bone over without causing appreciable gains or losses of bone. But in its “disuse mode” completed BMUs make less bone than they resorb only for bone next to or close to marrow (endocortical and trabecular bone).6 The resulting bone losses expand the marrow cavity, reduce the amounts of spongiosa in that cavity, and reduce cortical thickness. Yet meanwhile, the outside bone diameter does not decrease and cortical porosity changes little.7
The holes and excavations created by working BMUs temporarily remove some bone. That temporary bone loss defines the remodeling space,3, 5 which increased BMU creations enlarge and decreased creations reduce. Normally it equals about 4% of our bone “mass” but can exceed 20% of it. Remodeling space effects excepted, remodeling seldom if ever increases bone strength and “mass.”3
While modeling and remodeling each creates and uses what seem to be the same kinds of osteoblasts and osteoclasts to do its work, the activities of those cells can decrease in modeling but increase in remodeling, or vice versa, in the same bone at the same time.4, 8, 9 Thus, these are biologically different mechanisms.
A role of BMU creations in modeling and remodeling activities and postmenopausal bone loss
Individual modeling drifts and remodeling BMUs only function for 3 or so months in humans, and they do not exist at all times on all parts of all bone “envelopes” (i.e, periosteal, haversian, endocortical, and trabecular surfaces). Instead, new ones are created if, when, and where they are needed and as many and as long as needed.
The bone losses that follow acute estrogen deficiency in women (and acute disuse in both genders) usually take over 5 years to slow down and plateau,10 so they would depend on over 20 generations of new BMUs, each with its even shorter-lived osteoblasts and osteoclasts. Those generations did not exist when the deficiency or disuse began, so controlling their long-term effects on bone strength and “mass” should require continually creating new ones. The kinds and numbers of cells involved in those processes, including precursor and supporting cells and the new blood vessels that nourish them, are currently uncertain.
Mechanical effects
Mechanical forces on bones deform or strain them. Directly or indirectly those strains help to control modeling and remodeling effects on bone strength and “mass.”3, 5 Where dynamic strains exceed a modeling threshold range, modeling increases bone strength and “mass” by combinations of changes in bone architecture and increases in bone “mass.” Where strains stay below this threshold, mechanically controlled modeling stays off. When strains stay below a lower remodeling threshold range, modeling still stays off but, remodeling space effects excepted, now disuse-mode remodeling permanently removes bone next to or close to marrow. When strains exceed this threshold range, remodeling tends to switch to its conservation mode to retain bone next to marrow. Raising those thresholds should have the same effects on bone strength and “mass” as acute mechanical disuse. It should make disuse-mode remodeling begin to remove bone next to marrow and turn modeling off. When continued mechanical usage of steadily reducing amounts of bone let bone strains rise to the higher remodeling threshold range, conservation mode remodeling would resume and make further bone losses tend to plateau at the new, lower level of bone strength and “mass.”
Figure 1 suggests some combined effects of modeling and remodeling on bone strength and “mass,” and the hierarchy of strain thresholds that apparently help to control them.
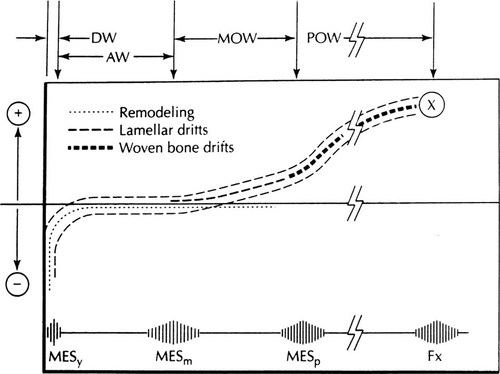
Combined modeling and remodeling effects on bone strength and “mass.” The lower horizontal line suggests typical peak bone strains from zero on the left, to the fracture strain on the right (Fx), plus the locations of the remodeling, modeling and microdamage thresholds (MESr, MESm, MESp, respectively; this text does not discuss microdamage). The horizontal axis represents no net gains or losses of bone strength or “mass.” The lower dotted line curve suggests how disuse-mode remodeling removes bone and reduces bone strength and “mass” where strains stay below the MESr range, but otherwise keeps that bone. The upper dashed line curve suggests how modeling would increase bone strength where strains reach or exceed the MESm range. The dashed outlines suggest the combined modeling and remodeling effects on bone strength and “mass” (D.H. Carter originally suggested such a curve18). Fx = the fracture strain near 25,000 microstrain. At the top, DW = disuse window; AW = adapted window as in normally adapted adults; MOW = mild overload window as in healthy growing mammals; POW = pathologic overload window. (Reproduced by permission: Frost HM 1997 Strain and other mechanical influences on bone strength and maintenance. Curr Opin Orthopaed 8:60–70).
Bone loss after menopause and acute disuse: Where does it come from?
First, during and after menopause in women, and in acute disuse in males and females, permanent bone losses come mainly from bone next to marrow (endocortical and trabecular bone), not from intracortical or subperiosteal bone.3, 10 Indeed, while bone next to marrow is being removed the outside diameters of affected bones can increase slightly,2, 4 so in the same bone at the same time periosteal bone gains can accompany increased losses of endocortical and trabecular bone. Upon adding estrogen after acute estrogen loss, the losses of bone next to marrow decline or stop.4, 10
Effects of estrogen deficiency and excess on bone tissue dynamics
Transient remodeling2, 4, 10-14 space effects excepted, first in acute estrogen deficiency BMU creations increase on all envelopes. Yet disuse-mode remodeling only affects bone next to or close to marrow; conservation-mode remodeling continues on the other envelopes. Second, upon giving estrogen to such females, BMU creations, and thus bone turnover and the remodeling space, decrease on all envelopes, remodeling returns to its conservation mode in bone next to marrow, and it continues in that mode on the other bone envelopes.
Such things suggest that somewhat different mechanisms may control BMU creations on the one hand, and on the other the switching between conservation- and disuse-mode remodeling.
The plateau phenomenon
When acute estrogen deficiency makes a woman's bone strength and “mass” begin to decrease, they do not decrease without limit2 but always tend to plateau at some new lower level, even though the hormonal deficiency persists.10
The marrow mediator mechanism and estrogen
Acute estrogen deficiency and acute mechanical disuse each turns disuse-mode remodeling on for bone next to or close to marrow, but not for haversian or subperiosteal bone. That suggests some mechanism in marrow can sense both that hormone and mechanical disuse. In response, this mechanism could modify modeling and remodeling of bone next to or close to it, and only of that bone.6 This could explain why acute loss of estrogen turns disuse-mode remodeling on only for bone next to or close to marrow. If so, increased estrogen secretion in girls at puberty should conserve that bone better than before to let their bone “mass” increase relative to their muscle mass. That does occur (Fig. 2).15, 16 Also if so, loss of estrogen at menopause should make this mediator mechanism cause removal of the same bone. That occurs too.10
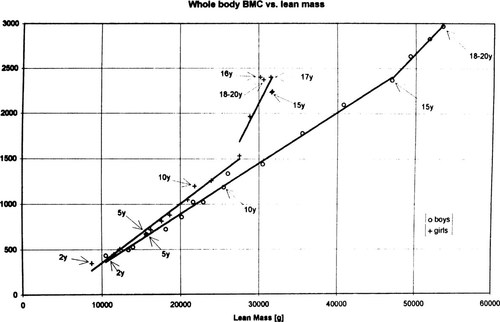
A bone-muscle mass comparison. From an Argentine study of 345 boys and 443 girls between two and 20 years of age,19 this graph plots the grams of total body bone mineral content (TBMC, an index of bone strength) on the vertical axis that correspond to the grams of lean body mass (LBM, an index of muscle strength) on the horizontal axis, as determined by a Norland dual-energy X-ray absorptiometry machine using dynamic filtration. Crosses, girls; open circles, boys. From left to right, each data point stands for an age 1 year older than the data point to its left, and it shows the mean bone and muscle indices for all subjects in that 1-year age group. For similar LBMs, around 12 years of age bone “mass” increased faster than before in girls, and also than in boys with similar LBMs. By ∼15 years of age, TBMC and LBM both plateaued in girls, as shown by the closely grouped data points for their 15–20 years age groups on the far right side of their curve. The 18–20 years age groups combine as a single data point for girls, and another for boys. Since both indices kept increasing in 20-year-old males, they ended up with more muscle and bone than the 20-year-old girls. The data points for 14- and 15-year-old girls overlap. (Reproduced by permission15).
A NEW HYPOTHESIS
To account for the above evidence this model suggests the following features. First, the model accepts known effects of estrogen and other nonmechanical agents on osteoblasts and osteoclasts, as summarized by Riggs et al.2 Second, but the hormone would affect the mediator mechanism in marrow too. Third, the hormone would tend to decrease BMU creations, bone turnover, and the remodeling space on all bone envelopes. Fourth, separately, it would make the marrow mediator mechanism turn conservation-mode remodeling on for bone next to marrow. The resulting decrease in permanent bone losses there would tend to prevent an osteopenia. Fifth, acute loss of estrogen would increase BMU creations on all envelopes, and thus bone turnover and the remodeling space there too. Sixth, but separately, acute estrogen loss would make the marrow mediator mechanism turn disuse-mode remodeling on for bone next to marrow. The resulting increased losses of that bone would cause an osteopenia. Seventh, estrogen's effects on BMU creations, and on the switching between conservation- and disuse-mode remodeling, should act by somewhat different mechanisms. Eighth, contrary to my original idea,17 estrogen would have little or no direct effect on periosteal modeling or its strain threshold range.
COMMENTS
Some predictions of the two models
The older ideas and the present model predict different effects of estrogen deficiency in women.
First, if estrogen deficiency increased osteoclastic activity but had no other large effect on other bone cells: a permanent deficiency should eventually cause loss of all bone (for example, in aged postmenopausal females) without an earlier plateau in bone “mass”; bone losses should affect all bones including those without a marrow cavity; bone losses should occur on all envelopes of those bones, since functionally competent osteoclasts can arise on all envelopes throughout life.
Second, but if a signal-strength threshold in a marrow mediator mechanism controls the switching between conservation- and disuse-mode remodeling of bone next to marrow, and if estrogen lowers that threshold and turns conservation-mode remodeling on for that bone, then acute estrogen deficiency should raise that threshold. At least four effects should follow: at first bone losses should increase; transient remodeling space effects excepted, permanent bone losses should come from bone next to or close to marrow; when progressively diminishing amounts of bone let continuing mechanical usage raise bone strains to the new threshold, conservation-mode remodeling of bone next to marrow would resume and make bone strength and “mass” tend to plateau even though estrogen deficiency continues; and those bone losses should not occur in bones without a marrow cavity, since they would lack this mediator mechanism. Examples include the nasal bones, turbinates, ethmoids, vomer, inner ear ossicles, and the wing of the sphenoid.
While the new model's predictions seem to be better, systematic studies of bone “mass” and tissue dynamics in bones without a marrow cavity are not yet available. Still, no clinical problems with such bones are known to associate with any currently recognized kind of osteoporosis.
Some implications
The newer model suggests the needs to: find and study the “back-up” cells, mechanisms and signals that help to control the creations, work intensities and locations of modeling drifts and remodeling BMUs; find and study the marrow mediator mechanism; and learn how to modify for medical needs the modeling and remodeling thresholds and the difference between the amounts of bone resorbed and made by the “typical” BMU.6 The similar effects of acute disuse and acute estrogen deficiency on bone strength, “mass,” bone-tissue dynamics, modeling, remodeling, and bone architecture suggest both challenges may act via a common pathway.
CONCLUSION
I do not suggest that this article resolves the puzzle of the mechanisms of estrogen effects on bone; instead I suspect we only found a few more parts of a complex mechanism and problem. The matter does seem to need resolution since it could influence future research and the management of postmenopausal bone loss and related problems. Help from others in resolving it is invited and welcome.
Acknowledgements
The author is grateful to colleagues who provided helpful comments and advice in the past on matters discussed in this article. They include Profs. D.B. Burr, J.L. Ferretti, W.B. High, W.S.S. Jee, K. Kuettner, L. Garetto, A.M. Parfitt, E.L. Radin, and S. Stanisaljevic. The author is also deeply indebted to orthopaedic surgeons trained at Henry Ford Hospital between 1957 and 1973 inclusive for their spontaneous and generous aid in a time of great troubles.




