Premenopausal Ovariectomy-Related Bone Loss: A Randomized, Double-Blind, One-Year Trial of Conjugated Estrogen or Medroxyprogesterone Acetate
Abstract
The purpose of this study was to contrast the effects of conventional estrogen treatment with medroxyprogesterone on cancellous and cortical bone change in the first year following premenopausal ovariectomy. This 1-year double-blind randomized therapy trial was stratified by osteoporosis family history and performed in an academic medical center and community hospitals. Premenopausal women 45 ± 5 years old, postovariectomy for benign diseases were provided 600 mg/day of calcium and randomized to daily therapy with conjugated equine estrogen (CEE, 0.6 mg) or medroxyprogesterone (MPA, 10 mg). The primary outcome variable was spinal quantitative computed tomography (QCT) bone density change over 1 year with additional outcomes of dual-energy X-ray absorptiometry (DXA) of proximal femur (FN), whole body (WB), and spine segment (WBS) and N-telopeptide, bone-specific alkaline phosphatase, and other bone marker, hormonal, and weight changes. Results in the 33 women completing the study, whose initial bone densities were normal (QCT 133 mg/cm3, femoral neck 0.94 g/cm2, whole body DXA 1.13 g/cm2), showed annual QCT loss during CEE therapy of −11.5 mg/cm3 (p < 0.0007) and MPA bone loss of −19.7 mg/cm3 (p < 0.0001). Losses were marginally greater on MPA than CEE (p = 0.04). Extremely high postovariectomy (5 days) and pretreatment resorption markers (>3 SD above premenopausal normal levels) were significantly related to bone loss. Across the year, resorption decreased during CEE but increased on MPA treatment. Significant DXA bone losses were prevented by CEE treatment (−1.4% FN, −.4% WB, and −1.5% WBS, all NS). However, DXA bone loss was not prevented by MPA treatment (−5% FN, −2.8% WB, and −6.1% WBS, all p < 0.03). Average weight gain was significant (+3.2 ± 4.0 kg) and greater on CEE than MPA (+4.7 vs. +2.0 kg, p = 0.049). In conclusion, CEE therapy did not prevent significant 8% cancellous spinal bone loss in the first year following premenopausal ovariectomy, despite supplementation with 600 mg/day of calcium, good control of vasomotor symptoms, and nearly 5 kg of gain in weight. Significant DXA bone loss, however, was prevented by CEE, but not by MPA therapy. These unexpected results were statistically related to high bone resorption following ovariectomy, which CEE suppressed but MPA did not. Bone formation markers increased during MPA therapy but were unchanged during CEE therapy.
INTRODUCTION
PROPHYLACTIC PREMENOPAUSAL OVARIECTOMY (OVX) is sometimes performed to eliminate the risk for ovarian cancer in women over 40 years of age when a hysterectomy is indicated for menorrhagia, endometriosis, uterine myomata, or ovarian cysts. Prophylactic OVX assumes that estrogen therapy postoperatively effectively prevents bone loss, controls vasomotor symptoms, and is acceptable for women. Not all women, however, are able to or should take estrogen therapy. For example, if the reason for surgery is extensive endometriosis, early estrogen treatment may be contraindicated. In addition, approximately 10% of women will have severe classical migraine headaches, estrogen-related thrombotic disease, breast cancer, or active liver disease which preclude estrogen therapy. Moreover, there are women who are reluctant to take estrogen because of a fear that they will develop breast cancer or experience adverse effects. For these reasons an alternate therapy was tested in parallel with estrogen.
Post-OVX therapy was required to have reasonable efficacy against vasomotor symptoms because surgical menopause commonly causes severe climacteric symptoms. Poor control of symptoms by one agent could effectively unblind the study. The rationale for choosing medroxyprogesterone as the alternate therapy was reported efficacy in control of menopausal symptoms1 and positive bone density effects.2, 3 The evidence for a bone formation effect for medroxyprogesterone derives from a variety of basic science and clinical investigations including randomized controlled therapy trials in women and animal models. In vitro data suggest that progesterone stimulates receptor-mediated osteoblast proliferation.4, 5 A randomized, placebo-controlled trial of cyclic medroxyprogesterone therapy in active women with abnormal menstrual cycles showed a significant treatment-related increase in spinal bone density.3
Recently, more sensitive markers of bone resorption, such as cross-linked breakdown products of bone collagen (e.g., cross-linked amino-terminal telopeptide [NTx] or pyridinoline [PYD] and deoxypyridinoline [D-PYD]), are improving our understanding of bone turnover.6, 7 More specific biochemical indicators of bone formation are also now available. These include the two-site immunoradiometric assay for the bone-specific isoenzyme of alkaline phosphatase (BAP)8 and osteocalcin assays using immunoradiometric methods and antibodies raised against human rather than bovine osteocalcin (HOC).9 To date, changes in these newer bone markers have not been reported in women immediately following OVX.
Although any therapy trial should ideally be placebo-controlled, a placebo-treated group was considered inappropriate because of dramatic cancellous bone loss in placebo-treated women during the 2 years after OVX.10 In addition, severe vasomotor symptoms are anticipated for a significant portion of these women. Drug regulatory bodies, such as the Federal Drug Administration, furthermore, are now recommending comparison rather than placebo-controlled trials.
This 1-year randomized double-blind study was designed to determine whether the changes in bone density (especially in spinal cancellous bone measured by QCT) immediately following premenopausal hysterectomy and bilateral OVX would differ between women treated with daily conjugated equine estrogen (CEE) and those prescribed medroxyprogesterone acetate (MPA). Secondary aims of this study were to measure bone changes by dual-energy X-ray absorptiometry (DXA) and to interpret any bone changes in light of systematically studied changes in the newer biochemical markers of bone resorption and formation, hormone levels, and body weight.
MATERIALS AND METHODS
Subject selection and study design
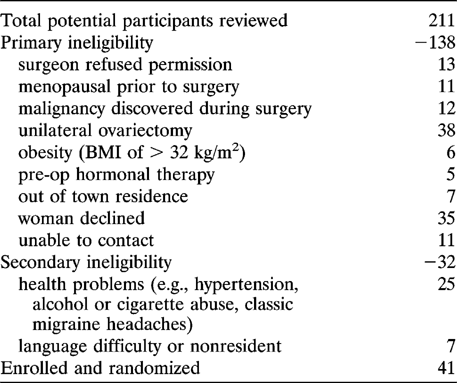
Healthy premenopausal women scheduled for bilateral OVX at the time of hysterectomy were identified by the name of their surgeon on preoperative lists at three hospitals affiliated with a medical school. One of us (J.C.P.) spoke with the surgeon (in person or by telephone) requesting permission to discuss the study with the preoperative patient and to inquire about factors determining her eligibility. If permission was granted, women were provided with written information about the study. (This occurred before or immediately following surgery.) The study nurse subsequently visited those interested in participating and screened for initial eligibility. Some additional participants were also identified by a surgeon operating in community hospitals. The sequence of events leading to randomization is shown in Table 1.
Women were enrolled who provided informed consent, were premenopausal (menstruating and not experiencing vasomotor symptoms), age 30–55 years, had an average body weight as manifest by a body mass index (BMI; weight in kilograms divided by height in meters squared) of 19–32 kg/m2, were ambulatory, and in good general health.
Women were excluded if they had any malignancy including cervical cancer, were taking or had taken any medication in the preceding 6 months with a potential effect on mineral metabolism (including any gonadal steroids, antiandrogens, fluoride, corticosteroids, or high-dose vitamin D or calcium), or had a history of classical migraine headaches, thrombotic disease (thrombophlebitis or pulmonary embolism), or significant hypertension. Randomization was stratified by a family history of osteoporosis (defined as a primary relative who had broken a bone without major trauma or who had become kyphotic and/or significantly shorter).
Subjects
Forty-one eligible women were enrolled. All gave written informed consent. The study was approved by the Clinical Research Ethics Board of the University of British Columbia.
Intervention groups and randomization
Women were randomly assigned to blinded therapy with either CEE 0.6 mg/day or MPA 10 mg/day which was dispensed by the hospital pharmacy in a schedule of two powder-filled identical capsules daily. Randomization table-based assignment was done by the pharmacy. Because a powder was used to fill gelatin capsules, the dose for CEE became 0.6 mg rather than the conventional 0.625 mg. (The study design allowed provision of three capsules per day for control of vasomotor or other symptoms if needed without breaking the code or excluding the participant. This was used by two women on MPA and for one on CEE. The longest use was for 3 weeks in one CEE-treated woman who discontinued participation.) All women were also provided with supplemental elemental calcium in a dose of 600 mg daily as a single tablet taken at bedtime (Caltrate, calcium carbonate, Lederle Pharmaceuticals, Pearl River, NY, U.S.A.).
Enrollment took place over almost 3 years. For this reason, some women were completing the study while others were just beginning. At the end of the study the pharmacy provided the participant a sealed envelope containing a letter which revealed the therapy to her family doctor. The investigators remained blinded to the assignment until all participants had completed the study and all data were entered in a database and confirmed for accuracy.
Time course of monitoring
Women were enrolled prior to hospital discharge on day 3–7 following surgery (an average of day 5). Baseline fasting sera and overnight fasting urine were obtained the morning of discharge, at which time the pharmacy dispensed the study drug and calcium. Within the next 1–3 weeks, the initial bone density investigations and a screening mammogram (if none were available within the preceding year) were obtained.
Women were seen at monthly intervals for 6 months, then every 3 months until the end of the year. At each visit the nurse clinician reviewed the diet, exercise, medication use, and any adverse events or intercurrent illnesses. Exercise and high calcium, low fat diets were systematically encouraged. Height, weight, blood pressure, and a physical examination were performed by a physician at each visit. Pills were counted to ascertain adherence. Additional supplies of the study medications were dispensed each visit. Repeat overnight fasting urine tests were obtained at 3, 6, and 12 months, and subsequent sera were obtained at 6 and 12 months. The final visit included a pelvic examination and a vaginal lateral wall sample for maturation index. From enrollment, a Daily Menopause Diary, an instrument previously developed and validated,11 was completed every evening. This instrument includes self-reports of the number and severity of vasomotor symptoms during the day and night. Repeat mammograms were arranged at the end of the study year. (The vasomotor symptom, vaginal maturation, lipid, and mammographic data have been presented as abstracts12, 13 and will be reported in full.)
Bone density measurements
Cancellous bone mineral density in vertebral centra from the 12th thoracic to the 4th lumbar spinal segments was measured by single-energy quantitative computed tomography (QCT) as previously reported.14 In our hands, QCT has a coefficient of variation (CV) of 0.8% from repeated examinations with repositioning in 78 premenopausal women.14 Measurements were obtained at 11.2 ± 0.7 (SD) month intervals (range 9–13 months). The rate of change was divided by the number of months between examinations, and this monthly change rate was multiplied by 12 to provide a common time frame for comparisons. A QCT-associated computer system change allowed only the first 33 enrolled (of 41) women to have QCT measurements.
Dual-energy X-ray absorptiometry (DXA, Lunar DPX, Lunar Corp., Madison, WI, U.S.A., software version 3.2) of the proximal femur region and whole body bone density were measured at baseline and 6 and 12 months following OVX. In our center, CVs in the proximal femur for eight normal-weight women age 40–60 years studied twice with repositioning were 2.9% for the femoral neck (FN), 2.3% for the Ward's area, and 2.1% for the trochanter bone density. The CVs for whole body determinations with repositioning in healthy adult women were 0.7% for whole body bone density (WB) and 1.6% for the total (thoracic and lumbar) spinal region of the whole body DXA (WBS; n = 8). The total spine segment of the DXA whole body scan was studied for comparison with the spinal QCT measurement.
Hormonal and biochemical analyses
Routine hematology, lipid levels, chemistry, and urinary calcium/creatinine excretions were analyzed immediately in the hospital laboratory. All other specimens were separated, divided into aliquots, and frozen at −70°C until analysis. All stored specimens from a given participant were analyzed in duplicate in the same assay. Initial samples were obtained at hospital discharge following OVX (i.e., 3–7 days post-OVX). Urines were obtained overnight, fasting, after voiding at bedtime, and included the first voiding in the morning, and any urine passed during the night (approximately an 8-h time collection). All sera were obtained fasting in the morning. Women were instructed to move their calcium supplement to an earlier meal (from bedtime) on the night before the urine test. On subsequent urine testing at 3, 6, and 12 months, each participant collected an overnight fasting urine specimen and brought it with her to the laboratory.
Bone resorption markers:
Fasting overnight urine collections for calcium, cross-linked n-telopeptides, and pyridinoline and deoxypyridinoline were reported per millimole of creatinine in the specimen from which they were measured. A reference calcium to creatinine value was provided by 45 normal premenopausal women with regular menstrual cycles who collected a second-voided, 2-h fasting urine in the follicular phase: 0.18 ± 0.14 (mmol calcium/mmol creatinine).15, 16 Cross-linked N-telopeptide (NTx) measurements were performed by D.R.E. as reported6 with a normal premenopausal follicular phase mean value (± SD) of 30.4 ± 16.6 nmol bone collagen equivalents (BCEs) per millimole of creatinine.7 PYD and D-PYD were measured by L.A. and P.R.E. using high performance liquid chromatography following urine hydrolysis and extraction by cellulose chromatography.17 Normal premenopausal mean values for PYD and D-PYD, respectively, are 33.4 ± 8.1 and 5.4 ± 1.8 nmol/mmol creatinine.7
Bone formation markers:
Serum BAP was measured in the laboratory of Vancouver Hospital and Health Sciences Centre by an immunoradiometric assay (IRMA) method using two monoclonal antibodies raised against human bone isoenzyme (Ostase, Hybritech, San Diego, CA, U.S.A.).8 BAP has a normal premenopausal follicular phase mean value of 8.2 ± 2.8 ng/ml.7 Serum for HOC was measured using an IRMA (Immutopics, Inc., San Clemente, CA, U.S.A.). The normal osteocalcin range in women age 19–58 years is 2.4–10 ng/ml but a premenopausal follicular phase mean value was not available (personal communication, Nichols Laboratory).
Fasting blood samples for calcium, phosphate, albumin, alkaline phosphatase (ALP), routine hematology, liver function tests, creatinine, cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides, and thyroid stimulating hormone (TSH) were measured by standard clinical methods in the hospital laboratory. Normal ranges for serum calcium and total ALP are 2.0–2.6 mmol/l and 45–125 U/l, respectively.
Serum cortisol, dehydroepiandrosterone sulphate (DHEAS), testosterone, and sex hormone binding globulin were measured in the Biochemistry Laboratory of the Royal Melbourne Hospital. Cortisol concentrations were measured by radioimmunoassay (RIA), had an intra-assay CV of 7.1% and a normal morning mean value of 375 ± 265 nmol/l. Serum DHEAS was measured by RIA (Diagnostic Products Corporation, Los Angeles, CA, U.S.A.) had an intra-assay CV of 7% and a normal premenopausal mean value of 5.3 ± 4.3 nmol/l. Testosterone was measured by RIA (Diagnostic Products Corp.) and had an intra-assay CV of 13.4% and a normal morning mean value in premenopausal women of 2.35 ± 1.35 nmol/l. Sex-hormone binding globulin was measured by an IRMA method (Orion Diagnostica, Espoo, Finland), had an intra-assay CV of 8% and a normal mean value of 55 ± 35 nmol/l.
Statistical methods
All data were confirmed for accuracy, entered into a computer database, and analyzed for normal distribution. The primary analysis assessed annual bone change for QCT. Number calculations based on preliminary QCT data showing 0–8% increases/year during medroxyprogesterone treatment in OVX women18 and on the six conjugated estrogen-treated women by Genant10 who showed a mean 2-year cumulative change of −3 ± 10% indicated that 25 women/group would be needed to produce a difference at a 0.05 level with a β of 80. Changes in QCT bone density over time within each group were analyzed with a single sample t-test and between the two therapy groups using a two sample t-test.
Changes in bone density by DXA measured at six monthly intervals provided the secondary outcome variable. These bone changes were evaluated by analysis of variance (ANOVA) with repeated measures (therapy and time) using Biomedical Database Program statistical software.19 Multiple regression models were systematically developed to include therapy and one variable with the strongest linear relationship to the annual bone change value at each site from each of the following categories: resorption marker, formation marker, hormonal values, and morphometric values. Regression models were developed with or without including bone resorption marker variables (which bore the strongest bone change relationships), thus revealing other explanatory factors in bone change. Bone resorption marker and bone-specific ALP data, for comparison, were reported as Z scores [(reference premenopausal follicular phase mean − X) divided by the standard deviation (SD) of the measurement in a population of normal women]. Parametric data were expressed as mean ± standard deviation (SD). All tests were two-sided. Significance was assigned to p values of less than 0.05.
RESULTS
Subjects
Forty-one women were enrolled (Table 1); 33 women completed the 1-year study. Immediately following randomization, one woman decided against participation and another discontinued at 1 week because of trouble swallowing the large capsules. Because she developed thyrotoxicosis, one CEE-treated woman was withdrawn at 4 months. Five women dropped out of the study after the first month: three assigned to CEE withdrew because of headache, fatigue, and insomnia and two assigned MPA left the study, one because of trouble swallowing the capsules and one because of inadequate vasomotor symptom control.
Hysterectomy with OVX had been performed for menorrhagia in 10 women, ovarian cysts in 12, endometriosis in 11, uterine myomata in 10, adenomyosis in 2, and chronic salpingitis in 1—some women had more than one indication for surgery. These diagnoses were equally represented in each therapy group (e.g., of the 11 women with the diagnosis of endometriosis, 6 were randomized to CEE and 5 to MPA).
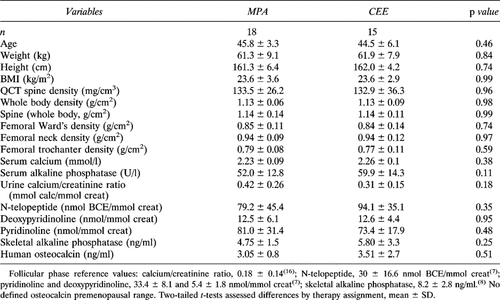
The initial characteristics of all who completed the study, by randomized therapy assignment, are shown in Table 2. Women assigned to the two groups were well matched. Participants were 45.2 ± 4.7 years old with an average BMI of 23.6 ± 3.2 kg/m2. These values are not different from population means for women in the fifth decade. No abnormalities of hematology, liver function, creatinine, chemistry values, nor TSH were documented (data not presented); lipid levels will be reported separately.
Initial bone density values and biochemical markers of bone turnover
The average initial bone density measured by QCT was normal at 133.2 ± 30.6 mg/cm3. This value was not significantly different from normal in our laboratory established in 37 initially ovulatory women whose mean age was 41 years (p = 0.134).20 These control women, however, were younger than the ovariectomy population (p = 0.00220). DXA initial mean values for the femoral neck of 0.94 ± 0.10 g/cm2, Ward's region of 0.85 ± 0.12 g/cm2, trochanter of 0.78 ± 0.10 g/cm2, and whole body of 1.13 ± 0.07 g/cm2 did not differ from reference values for premenopausal women. Initial bone density variables did not differ by therapy assignment (Table 2).
The initial values for biochemical markers of bone resorption (overnight urinary excretion of NTx, PYD, and D-PYD) were significantly elevated (Table 2) and not different by therapy assignment. The NTx initial mean was 85.9 ± 40.6 and ranged from 38.3 to 200.4 nmol of BCE/mmol of creatinine; these values were significantly higher and more variable than the normal of 30 ± 16 nmol of BCE/mmol of creatinine.7 The average Z score for initial NTx excretion was 3.33 ± 2.49. Similar Z score elevations were present in initial D-PYD and PYD excretion values (3.95 ± 2.94 and 5.46 ± 3.23) but not for calcium excretion (1.39 ± 1.60). In contrast, initial values for serum calcium, total and skeletal ALP, osteocalcin, cortisol, testosterone, DHEAS, and sex hormone binding globulin were normal.
Changes in bone density and morphometrics over time
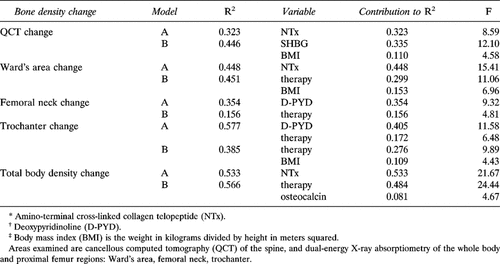
QCT cancellous spinal bone density decreased significantly across the year of therapy in both groups and averaged 12% in the entire cohort of 29 women for whom QCT data were available (Fig. 1). CEE-treated women experienced a highly significant annual QCT loss of −11.5 mg/cm3 (−8%) (t = −4.5, p = 0.0007), while MPA-treated women experienced a loss of −19.7 mg/cm3 (−15%) (t = −7.4, p = 0.0001). The greater loss during MPA treatment just reached significance (p = 0.039).
Changes in single energy quantitative computed tomography (QCT) of cancellous spinal bone density in milligrams per cubic centimeter (in the 12th thoracic through the 4th lumbar vertebrae) across the first year after premenopausal ovariectomy by randomized blinded therapy with conjugated equine estrogen (CEE, 0.6 mg/day) or medroxyprogesterone acetate (MPA, 10 mg/day). Bone loss was significant during CEE treatment (t = −4.5, p = 0.0007). Bone loss was also significant during MPA (t = −7.4, p = 0.0001).
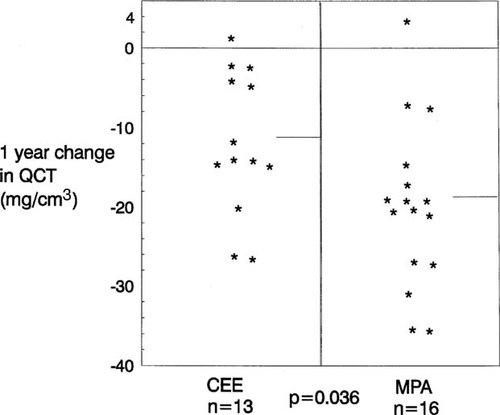
Bone loss was less pronounced at all sites as measured by DXA compared with QCT; however, the total spine segment from the whole body also showed a significant loss of −4.0 ± 4.6% (t = −4.97, p ≤ 0.0001). Again, the loss in the CEE group was less than in the MPA group (−1.5 ± 4.2% vs. −6.1 ± 3.7%/year, respectively, (p = 0.002). By DXA, spinal bone loss in the CEE group was not significant (t = −1.36, p = 0.20). Figure 2 shows that the CEE group experienced mean DXA bone losses in the femoral neck and whole body density; however, no region showed statistically significant loss. At 12 months, the MPA group experienced significant loss in all hip sites, in the whole body, and in the spine region of the whole body bone density by DXA. These losses averaged −2.8% in whole body, −5.2% in the femoral neck, and −8.1% in Ward's area.
Six and 12 month percentage changes (± SD) in bone density following premenopausal OVX by dual-energy X-ray absorptiometry in the whole body and three regions of the hip by randomized blinded therapy with conjugated equine estrogen (CEE, 0.6 mg/day) or medroxyprogesterone acetate (MPA, 10 mg/day). Annual bone loss was not significant at any site during CEE but was significant at all sites during MPA therapy. At 12 months, bone density was different between CEE- and MPA-treated women at every site (p values: 0.002 in whole body, 0.02 in femoral neck, 0.0003 in Ward's area, and 0.001 in the trochanter).
Weight increased significantly in the majority of women and averaged +3.2 ± 4.0 kg. That increase averaged 2.85 and 4.72 kg at 6 and 12 months in the CEE-treated women and 1.33 and 2.85 kg at 6 and 12 months in women treated with MPA. Weight gain was not different by therapy at 6 months, but by 12 months the CEE-treated women had gained significantly more than the MPA-treated women (p = 0.049).
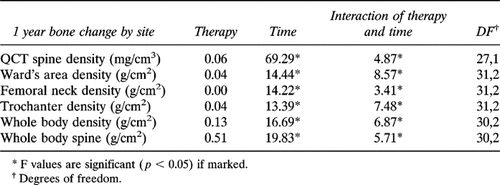
ANOVA with repeated measures for QCT and DXA bone measurements showed no significant effect of therapy (Table 3). Statistically, CEE or MPA had no influence on the 1-year rate of bone change following premenopausal OVX. However, the ANOVA did show significant time effect and an interaction of time and therapy effects.
Hormone values did not change by therapy or time as assessed by ANOVA except for sex hormone binding globulin, which was significantly different by therapy (although this variable did not meet the sphericity test for the ANOVA statistic). Testosterone values were at the lower end of the normal range 0.72 ± 0.43 nmol/l (0.3–3.0 nmol/l) initially, not different by therapy assignment (p = 0.53), and did not change or become different by therapy over the study. DHEAS levels were initially normal (2.4 ± 1.0 μmol/l), and not different by therapy and remained normal and not different by therapy across the study. Sex hormone binding globulin values were initially normal (75.6 ± 35.4, normal range 20–90 nmol/l) and decreased to 42 nmol/l in the MPA-treated women while increasing to 133 nmol/l in the CEE-treated women (p = 0.0001). Mean cortisol levels were in the upper part of the normal range initially (437.2 ± 37.8, normal range 120–650 nmol/l) and remained in this range with no difference by therapy. Serum cortisol levels were higher than the upper limit of normal in four different women at each measurement point. These women were not different in therapy assignment or dose, weight, or other characteristics from those with cortisol values consistently within the normal range.
Changes in biochemical markers of bone metabolism
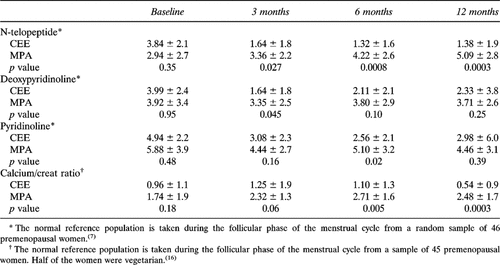
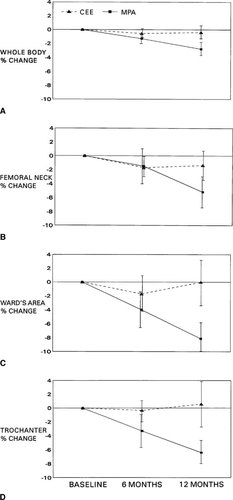
Serum calcium and total ALP values increased by 6 months in both groups. By 12 months, calcium remained stable in both groups but ALP showed a nonsignificant decrease in the CEE-treated women while continuing to increase in the MPA-treated women (−0.4 ± 13.2 vs. +11.7 ± 18.1 U/l, respectively, p = 0.041). Figures 3a and 3b show the NTx and BAP individual values reported as Z scores by therapy assignment across the year on randomized therapy. In Table 4, the bone resorption marker Z scores by therapy group are shown for each measurement point and analyzed by therapy-related differences. Initial NTx values were elevated in the majority of women and decreased toward normal in CEE-treated women but continued to increase in the MPA-treated women. This led to significant differences by therapy at the 3-, 6-, and 12-month assessment times (p = 0.027, 0.002, and 0.0006, respectively). A similar pattern was shown for PYD and D-PYD, but therapy-related differences were significant only at the 3-month assessment for D-PYD and the 6-month assessment for PYD (Table 4). In contrast, the calcium excretion Z scores showed significant differences by therapy at 6 and 12 months, similar to those documented by NTx (p = 0.007 and 0.0006, respectively) and were marginally different at 3 months (p = 0.068) (Table 4).
(A) Individual values for the excretion of N-telopeptide (reported as a Z score in relationship to the standard deviation of a reference premenopausal population [see text]) at four measurement times following premenopausal OVX during randomized blinded therapy with conjugated equine estrogen (CEE 0.6 mg/day, closed triangles) or medroxyprogesterone acetate (MPA 10 mg/day, closed circles). See statistical differences by therapy in Table 4 in the text. (B) Individual values for the fasting serum level of skeletal ALP (reported as Z scores in relationship to the standard deviation of a reference premenopausal population [see text]) at four measurement times following premenopausal OVX during randomized blinded therapy with conjugated equine estrogen (CEE 0.6 mg/day, closed triangles) or medroxyprogesterone acetate (MPA 10 mg/day, closed circles). See statistical differences by therapy in Table 4 in the text.
Biochemical markers of bone formation showed a different pattern than resorption markers. The initial values for both bone-specific ALP and osteocalcin were normal. By 6 months, bone formation markers tended to increase. Figure 3b shows that Z score values of bone ALP did not change in CEE-treated women but increased significantly in MPA-treated women at both 6 and 12 months (+0.87 ± 3.3 vs. +6.89 ± 6.1 ng/ml, respectively, p = 0.002). Serum osteocalcin levels likewise did not change over 12 months in the CEE-treated group but increased significantly in the MPA-treated women (+0.86 ± 2.4 vs. +3.69 ± 2.2 ng/ml, respectively, p = 0.002).
A family history of osteoporosis was reported by 9 of 33 women (27% of this population). This characteristic was not significantly related to any initial demographic, morphometric, bone density, or other characteristic. Randomization to therapy was stratified by this family history; therefore, five women were treated with MPA and four with CEE. No differences were seen in responses to therapy, in bone markers, or other characteristics related to a family history of osteoporosis. This variable also did not enter the multiple regression models of bone change during the year.
Effect of covariates on response to therapy
All of the baseline variables, changes, and mean values (Table 2, Figs. FIG. 1.-FIG. 3.) were assessed as potential modifiers of the effects of CEE or MPA therapy on changes in bone density. Mean serum levels of testosterone, osteocalcin, and skeletal ALP, mean excretions of telopeptide, both pyridinolines, and calcium correlated significantly with the change in QCT (r values ranging from a high of 0.586 for NTx to a low of 0.399 for BAP). Linear regression analysis showed differing related variables at the various bone measurement sites, but in all assessments bone resorption markers held the strongest negative linear correlations with bone changes; r values ranged from −0.52 to −0.75. Mean sex hormone-binding globulin (SHBG) and testosterone, and to a lesser extent BMI, showed positive linear relationships with bone change, especially at the spine by QCT; r values were 0.269 for BMI, 0.464 for testosterone, and 0.579 for SHBG. Whole body DXA density also related positively to BMI (r = 0.251), testosterone (r = 0.396), and SHBG (r = 0.486).
Multiple regression models were developed with the annual bone change at each site as the dependent variable, and included therapy, plus one each of the strongest bone resorption, bone formation, hormonal, and morphometric variables as independent factors. The strongest bone resorption marker was the mean N-telopeptide value for QCT, Ward's area, and total body density changes, while mean deoxypyridinoline was the strongest bone resorption marker for annual changes in the femoral neck and trochanter regions of the proximal femur. Table 5 shows multiple regression models with (Model A) and without (Model B) bone resorption markers. SHBG entered the model for QCT change, but the positive effects appear to reflect therapy; that explained 34% of the variance. Mean BMI entered the model only when bone resorption markers were excluded; BMI related to annual bone changes in the spine by QCT, and the Ward's area and trochanter by DXA. Thirty-two to 57% of the variance could be explained by the best models.
DISCUSSION
This randomized double-blind clinical trial showed that treatment with conjugated equine estrogen did not prevent significant cancellous bone loss during the first year after premenopausal OVX in healthy women. The only previous comparable study found similar results. Genant and colleagues, in a double-blind placebo-controlled dose-finding trial of conjugated equine estrogen (CEE) showed that six women treated with CEE for 2 years in a dose of 0.625 mg/day experienced a nonsignificant mean QCT loss of −3.0 ± 10% (p = 0.488).10 When adjusted to a 1-year change, those data did not differ from the significant −8 ± 6.8% 1-year loss we documented in the 13 CEE-treated women in this study (p = 0.065).
Women randomized to receive medroxyprogesterone acetate treatment in a daily dose of 10 mg experienced even more cancellous spinal bone loss as well as significant losses at every DXA measurement site. In contrast, our initial hypothesis, based on pilot data in women treated many years after OVX, was that QCT bone density would not change or would increase.21 Subsequent evidence suggests that the medroxyprogesterone-related spinal bone change in OVX women relates to how many years following surgery that therapy is begun.22 This study clearly documents that physiological doses of MPA, although they control vasomotor symptoms,12 do not decrease bone resorption or prevent bone loss when bone resorption is high.
This trial was planned to be both larger (25 women per arm) and longer. However, as the results showing nearly universal QCT bone loss became apparent (Fig. 1), we felt it unethical to continue a randomized, blinded trial. We discontinued further enrollment and ended the study after 1 year of observation.
The prevalent concept that estrogen therapy prevents bone loss evolved during an era in which cortical bone was the only bone tissue accessible for study.23, 24 Most previous estrogen treatment studies following OVX are not comparable because they did not measure spinal cancellous bone by QCT.23, 25-28 Other studies differ also because therapy was begun some months or years after surgery28-30 or because other estrogenic hormones and different relative doses were used.30, 31
Estrogen therapy decreases bone resorption as shown by histomorphometry,32-34 and by markers of bone resorption.34, 35 During CEE therapy, significant decreases in the levels of NTx, and the pyridinolines (and nearly significant decreases in the ratio of calcium/creatinine, p = 0.07) occurred. Decreased resorption was associated with prevention of significant 1-year bone loss in areas of predominantly cortical bone such as the proximal femur. However, CEE treatment, despite decreasing bone resorption, did not prevent acute spinal cancellous bone loss, perhaps because bone remodeling is maximum during the year following (OVX)29, 36 and high rates of bone resorption were already present at hospital discharge before therapy was begun. Studies suggest that loss of bone in trabecular envelopes occurs earlier following surgery and is more significant than in cortical sites.36, 37
Bone turnover has previously been documented following OVX.25, 27, 30, 38-41 Our results are consistent with these data. They differ in that none have been obtained as early following surgery and in the use of newer bone resorption markers. Stepan et al. and others25, 27, 30, 38-41 have shown that various markers of bone resorption are significantly increased by 3 months after OVX. Of the bone resorption markers, N-telopeptide appears to be more sensitive to the differing effects of the two hormones than the pyridinolines and is the most strongly related to early bone change in most regions. The excretion of calcium, although not as quick to change nor quite as sensitive as NTx, showed similar results. Bone formation indicators, as also documented in this study, have previously been shown to rise more slowly following premenopausal OVX than the bone resorption markers.38-40
Medroxyprogesterone therapy did not decrease bone resorption following OVX. Therefore, resorption markers continued to increase across the year. This was an unexpected finding because progestins have previously been shown to decrease urinary excretions of hydroxyproline and calcium.2, 30, 42-44 Explanations for this may include lack of specificity of hydroxyproline, therapy that started later after OVX, and cortical bone outcome measurements. This study indicates that medroxyprogesterone in “luteal phase replacement” doses does not decrease bone resorption.
That MPA therapy did not prevent significant cancellous and cortical bone loss was unexpected. Several previous controlled clinical studies have shown prevention of spinal2, 3, 21, 42, 44 or cortical45, 46 bone loss during progestin therapy. With the exception of studies of higher dose medroxyprogesterone,47-49 however, the progestins tested have been androgenic or 19-nortestosterone derived compounds.30, 42-44 Cundy and colleagues50 also recently showed by DXA that premenopausal women treated with 50 mg/day of MPA lost spinal bone.
A possible explanation for bone losses during both CEE and MPA therapies is that the doses used were inadequate. The 0.6 mg/day dose of CEE is only 4% less than 0.625, which is understood to prevent bone loss51 and which did prevent significant DXA proximal femur and whole body bone losses in this study. In addition, the doses of both hormones were effective in control of vasomotor symptoms and vaginal dryness.12 Furthermore, pill counts showed over 90% adherence to therapy in both groups. An MPA dose of 10 mg/day produced a secretory endometrium when given cyclically with estrogen52 and caused an elevation of basal temperature in menopausal women53 suggesting that this dose is physiological. Also, MPA in a dose of 10 mg/day for only 10 days/month increased DXA spinal bone density in a randomized placebo-controlled trial in premenopausal women with amenorrhea or abnormal cycles.3
The cancellous bone loss in the CEE-treated women raises questions about the accuracy of the QCT measurements. Technical error appears unlikely to account for the documented QCT changes because the same CT scanner, software, phantom, table height, and technologists were used throughout the study. Rates of loss during CEE therapy reported here, as discussed above, are not different from those documented by Genant and colleagues.10 That single rather than dual-energy QCT accounts for these findings is an unlikely speculation because Genant and colleagues showed that rates of bone loss did not differ between dual- and single-energy QCT (n = 7).10 Additional evidence that these QCT data are accurate is suggested by a study performed on the same instrument during the same time period in women with similar initial densities that showed a mean annual loss rate of less than 1%/year.20 Finally, the pattern of loss in the total spine segment of the DXA whole body was similar to (but less than) the QCT change (WBS, −1.5%/year on CEE vs. −6.1%/year on MPA therapy, p = 0.002).
This study provides evidence that estrogen and medroxyprogesterone fundamentally differ in their actions in bone. Estrogen decreased bone resorption toward a premenopausal normal level. Formation markers were essentially unchanged probably because the CEE-related decrease in resorption, through coupling, limited the expected post-OVX increased formation. Perhaps because rapid remodeling was present before therapy started; however, CEE did not prevent bone loss in the cancellous compartment as measured by QCT. Medroxyprogesterone, by contrast, did not decrease bone resorption. Bone formation markers increased significantly and this is consistent with progesterone's known osteoblast effects.2, 4, 21, 54 Alternatively, the increased formation markers during MPA therapy may simply reflect a persistently high rate of bone resorption.
The present study showed that many women on both therapies lost bone at 6 months after OVX. Other investigators have shown similar 6-month bone loss following either hysterectomy or OVX.55, 56 The similarity in rates of bone loss make it likely that surgical trauma, anesthesia, pain, and decreases in nutrition and activity contributed to this early bone loss. That OVX is not the only cause for increased bone resorption and loss does not negate its potential importance. Further studies appear necessary to understand bone remodeling changes during the perioperative periods of common surgical procedures.
This study was limited by both the lack of presurgical control data and by its short duration. Because surgical lists are confidential, potential subjects for the study were unable to be identified prior to admission for surgery. This made a true presurgical baseline impossible to obtain. Ideally, therapy studies of bone need to be continued for at least six bone remodeling cycles (approximately 2 years) before change can be ascribed to therapy rather than to bone remodeling transients. This study was terminated before the full complement of 50 women were enrolled and after only 1 year of therapy because of near-universal significant QCT bone loss. We recommended to the family physicians who directed each patient's subsequent therapy that estrogen be used in the highest tolerable dose and perhaps in combination with progestin. This suggestion was based on the limited but controlled animal57, 58 and human59 therapy data showing synergistic bone effects of estrogen and progestin.
In summary, CEE therapy did not prevent cancellous spinal bone loss in the first year following premenopausal OVX. This loss occurred despite decreasing bone resorption markers, weight gain, and calcium supplementation. Further research is necessary in premenopausal women having OVX for benign disease, especially studies in which bone is monitored during controlled therapy study over 2 or 3 years. Strategies may need to be developed and systematically tested that would control the early, massive increases in bone resorption following OVX. In the presence of high rates of bone resorption, any possible formation effects of medroxyprogesterone appear to be obscured.22
Acknowledgements
This work was supported by grants from British Columbia Medical Services Foundation and Upjohn Company of Canada. Lederle Pharmaceuticals provided 600 mg/day of calcium as Caltrate for each participant daily for 1 year, Upjohn provided the active conjugated estrogen and medroxyprogesterone in identical capsules, Hybritech made Ostase kits available at a reduced price, and Merck Frosst (through Pat Lauzon) contributed to the purchase of kits for human osteocalcin. We are grateful to Nenita Alojado, R.N. for her work on this study. We especially thank the women who participated, kept careful records, and provided their observations. We are especially grateful to Dr. Doug Yackel (as well as the other gynecologists) for referral of patients that made this study possible. Drs. Susan Barr, Carol Mase, E.C. Cameron, and Sheila Pride provided early encouragement, practical support, and subsequent review. We thank Drs. Morris Pudek and Joan Trepanier of the Department of Clinical Chemistry at the Vancouver Hospital and Health Sciences Centre (VHHSC) for excellent technical support and the Pharmacy Department for management and dispensing of blinded medications. The technologists, especially Cori Rexworthy for the DXA measurements and the CT technologists at VHHSC, Koerner Pavilion, provided reproducible data that are the basis of this study.