Interaction Between Nitric Oxide Synthase and Cyclooxygenase Pathways in Osteoblastic MC3T3-E1 Cells
Abstract
Interleukin 1 (IL-1) and tumor necrosis factor α (TNF-α) have been implicated in the pathogenesis of osteoporosis. These proinflammatory cytokines induce both cyclooxygenase (COX) and nitric oxide synthase (NOS) with the release of prostaglandin (PG) and NO, respectively. The present study was undertaken to examine the interaction between COX and NOS pathways and their role in the regulation of osteoblastic function in MC3T3-E1 cells. Addition of IL-1α and TNF-α induced a marked increase in the production of both NO and PGE2. Reverse transcription–polymerase chain reaction analysis showed that the increase in NO production was preceded by the expression of inducible NOS mRNA. The temporal profile of PGE2 production revealed a biphasic pattern: the first small peak at 3 h was caused by de novo synthesis of PGE2 through inducible COX (COX-2) mRNA, while the subsequent progressive accumulation of PGE2 was mediated through the activation of COX pathway by NO since (1) aminoguanidine (AG), a selective inhibitor of inducible NOS, significantly suppressed the PGE2 production by IL-1α and TNF-α, (2) NOC-18, an NO donor, reversed this suppression, and (3) NOC-18 increased PGE2 production by itself. The increase in NO production in response to IL-1α and TNF-α was further stimulated by aspirin and inhibited by exogenous addition of PGE2, suggesting that PGE2 produced by the cytokines, in turn, negatively modulates NO production. IL-1α and TNF-α inhibited alkaline phosphatase (ALP) activity, which was significantly reversed by AG. NOC-18 not only suppressed ALP activity by itself but also blocked the effect of AG, suggesting the role of NO in the inhibition of ALP activity. PGE2 decreased ALP activity, and the inhibitory effect of NOC-18 was attenuated in the presence of aspirin, suggesting the involvement of PGE2 in the negative modulation of ALP activity by NO. These results suggest that NO produced in response to proinflammatory cytokines participates in the modulation of ALP activity via the activation of COX pathway. The interaction between NO and the COX pathways may play an important role in the regulation of osteoblastic functions under physiologic as well as pathologic conditions.
INTRODUCTION
NITRIC OXIDE (NO), a short-lived reactive radical, has been implicated as an important mediator in a variety of biological processes including vasodilation, inflammation, and neurotransmission.1, 2 NO is synthesized from the guanidino nitrogen of L-arginine by NO synthase (NOS). Three distinct isoforms of NOS have been identified: the neural and the endothelial NOS, which exist as constitutive forms (cNOS), and an inducible form (iNOS), which is markedly induced in response to cytokines and/or endotoxin stimulation.2
Accumulating evidence suggests that both cNOS and iNOS are expressed by bone cells and that NO modulates bone metabolism.3, 4 It was initially reported that NO produced retraction of isolated rat osteoclasts and inhibited their bone-resorbing activity.5 Kasten et al.6 demonstrated that chicken osteoclasts exhibited NOS activity and that aminoguanidine, an iNOS inhibitor, potentiated bone loss in ovariectomized rats, suggesting a protective role of NO in postmenopausal osteoporosis through modulation of osteoclastic bone resorption. In a recent study using mouse calvarial organ culture, it has been suggested that NO has biphasic effects on bone resorption, with inhibitory and stimulatory effects being observed at high and low concentrations, respectively.7
Several studies have demonstrated that osteoblasts produce NO in response to proinflammatory cytokines.8-12 MC3T3-E1, an osteoblastic cell line derived from mouse calvaria that retains many osteoblastic characteristics,13 has been shown to produce NO in response to interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ).8 However, the mechanism by which NO produced by osteoblasts modulates their own function remains to be clarified.
IL-1 and TNF-α are also known to induce cyclooxygenase (COX) in osteoblastic cells with the release of prostaglandins (PGs).14 COX exists as constitutive (COX-1) and inducible (COX-2) forms,15, 16 and COX-2 can be induced by proinflammatory stimuli. COX converts arachidonic acid to PGs, which are known to exert a wide variety of effects on bone metabolism.14 Although the expression of COX-1 and COX-2 in osteoblastic cells has been shown to be tightly regulated by cytokines, such as IL-1 and TNF-α,17, 18 cross-talk between NOS and COX pathways in the regulation of bone metabolism remains to be elucidated. The present investigation was undertaken to examine the role of NO in the regulation of osteoblastic functions and to determine the interaction between NOS and COX pathways in osteoblastic MC3T3-E1 cells.
MATERIALS AND METHODS
Chemicals
Recombinant human IL-1α, transforming growth factor β1 (TGF-β1), and mouse TNF-α were purchased from Boehringer Mannheim (Mannheim, Germany). Alkaline phosphatase assay kit and NOC-18, an NO generator,19 were from Wako Pure Chemicals (Osaka, Japan). Other reagents were obtained from Sigma Chemical (St. Louis, MO, U.S.A.).
Cell culture
MC3T3-E1 cells20 were cultured in α-modified essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and 60 μg/ml ascorbic acid. For experiments, cells were seeded at a density of 1.2 × 105 cells/cm2, and after incubation for 24 h, the medium was replaced with α-MEM (1 ml/5 × 105 cells) containing 0.1% bovine serum albumin (BSA) with or without test agents. PGE2 and aspirin were dissolved in 99.5% ethanol and diluted with α-MEM. The final concentration of ethanol did not exceed 0.1%.
Assessment of NO production
The release of NO into the culture medium was assessed by the accumulation of nitrite, a stable metabolite of NO, which was measured by Griess reaction.21 In brief, culture supernatant (50 μl) was mixed with 75 μl of 0.2 M acetate buffer (pH 5.0) and 75 μl of Griess reagent containing 0.05% naphthyl-ethylene diamine, 0.5% sulfanilamide, and 2.5% phosphoric acid. After the sample was incubated for 20 minutes at room temperature, absorbance was measured at 540 nm, and nitrite concentration was determined from a standard curve of serial dilutions of sodium nitrite dissolved in α-MEM.
PGE2 assay
PGE2 production by MC3T3-E1 cells was determined using an enzyme immunoassay kit (Amersham, Buckinghamshire, U.K.). After cells were treated with test agents, the conditioned medium was collected and stored at −30°C until assay. PGE2 concentrations were determined from a standard curve of serial dilutions of PGE2.
RNA isolation and reverse transcription-polymerase chain reaction
Total RNA was prepared by acid guanidinium phenol chloroform method,22 and quantitated by a spectrophotometer. Total RNA (10 μg) was reverse transcribed using an reverse transcription–polymerase chain reaction (RT-PCR) kit (Stratagene, La Jolla, CA, U.S.A.). PCR was carried out using a thermal cycler (GeneAmp PCR System 9600, Perkin Elmer, Norwalk, CT, U.S.A.). The amplification protocol comprised 28 cycles for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and iNOS, 32 cycles for COX-2, and 35 cycles for COX-1 at 95°C for 1 minute, 55°C for 1 minute, 72°C for 2 minutes. The final extension was performed at 72°C for 10 minutes. Sense and antisense primers for iNOS,23 COX-1,24 COX-2,24 and GAPDH25 were as follows:
 GAPDH was used as an internal control. The expected sizes of PCR products were 456 bp for iNOS, 276 bp for COX-1, 581 bp for COX-2, and 988 bp for GAPDH. PCR products were visualized by electrophoresis through agarose gel containing 1 μg/ml of ethidium bromide.
GAPDH was used as an internal control. The expected sizes of PCR products were 456 bp for iNOS, 276 bp for COX-1, 581 bp for COX-2, and 988 bp for GAPDH. PCR products were visualized by electrophoresis through agarose gel containing 1 μg/ml of ethidium bromide.
Determination of alkaline phosphatase activity
Cells were harvested in cell lysis buffer (0.05% Triton X-100 and 2 mM MgCl2), and lysed through two cycles of freeze and thawing and sonication for 1 minute (UltraS Homogenizer VP30S, Taitec, Koshigaya, Japan). After centrifugation at 15,000 rpm for 1 minute at 4°C, alkaline phosphatase (ALP) activity in the supernatant was determined using an assay kit (Alkaline Phospha B-test, Wako). Total cellular protein content was measured with a protein assay system (Bio-Rad, Hercules, CA, U.S.A.), and ALP activity was corrected for protein content.
Statistics
Data were expressed as the mean ± SD, and statistical significance was determined by one-way analysis of variance (ANOVA) and Scheffe's multiple comparison test using StatView software (Abacas Concepts, Berkley, CA, U.S.A.). A difference with a p value of <0.05 was considered statistically significant.
RESULTS
Induction of NO production by iNOS
As shown in Fig. 1, treatment of MC3T3-E1 cells with both IL-1α and TNF-α induced a marked increase in the production of NO, as assessed by nitrite concentration in the culture medium. Either IL-1α or TNF-α slightly increased NO production, although statistical significance was not reached. The stimulation of NO production by IL-1α plus TNF-α was completely abolished by TGF-β1 as well as aminoguanidine (AG), a selective inhibitor of iNOS,26 suggesting that the increase in NO production was mediated through iNOS. The accumulation of NO in response to IL-1α plus TNF-α was progressively increased up to 48 h with a significant response being observed at as early as 9 h (Fig. 2A).
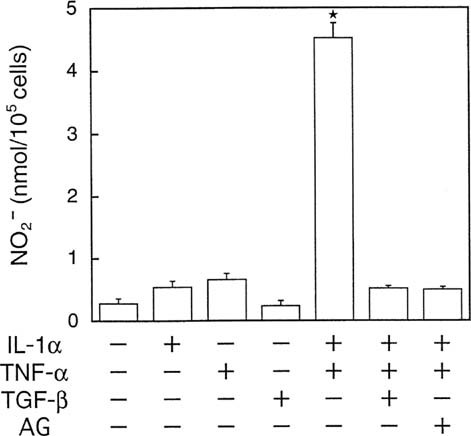
NO production in MC3T3-E1 cells. Cells were incubated with various combinations of cytokines (10 ng/ml each) and aminoguanidine (AG, 1 mM) for 48 h, and nitrite concentration in the conditioned medium was determined as described in the Materials and Methods. Data are corrected for cell number and expressed as the mean ± SD in triplicate. *Significant difference from control (p < 0.05).
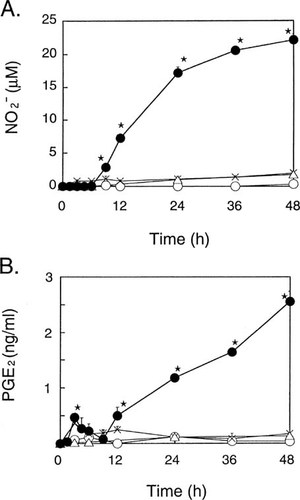
Time course of (A) NO and (B) PGE2 production in MC3T3-E1 cells. Cells were incubated with IL-1α (10 ng/ml) and/or TNF-α (10 ng/ml). Conditioned medium was harvested at the indicated times and assayed for (A) nitrite and (B) PGE2 concentrations. Data represent the mean ± SD in quadruplet. ○, ×, ▵, and • indicate control, IL-1α, TNF-α, and IL-1α plus TNF-α group, respectively. *Significant difference from control (p < 0.05). In IL-1α and IL-1α plus TNF-α groups PGE2 production was significantly increased at 3 h.
RT-PCR analysis revealed that iNOS mRNA was slightly induced by either IL-1α or TNF-α and that a marked induction occurred in the presence of both cytokines at 6 h (Fig. 3A), which correlated well with the amount of NO produced in response to either one or both of these cytokines (Fig. 1).
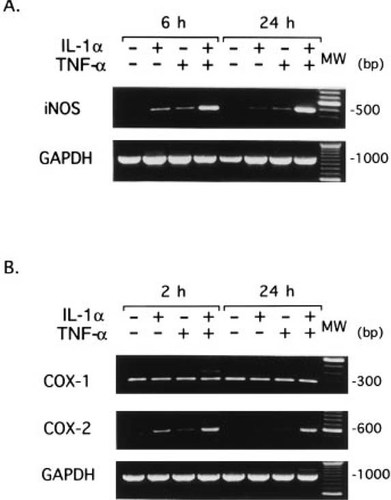
Expression of (A) iNOS, (B) COX-1, and COX-2 mRNA in MC3T3-E1 cells. Cells were incubated with IL-1α (10 ng/ml) and/or TNF-α (10 ng/ml) for the indicated time. Total RNA prepared by AGPC method was reverse transcribed, and PCR was carried out with specific primer sets for (A) iNOS, (B) COX-1, and COX-2 as described in Materials and Methods. PCR products were visualized by electrophoresis through 1% agarose gel containing 1 μg/ml of ethidium bromide. The one hundred base pair interval molecular weight marker is shown on the right (MW).
Correlation between PGE2 and NO production
IL-1α and TNF-α also induced the production of PGE2 in MC3T3-E1 cells. A detailed time course experiment indicated that the effect of these cytokines on PGE2 production was biphasic: the initial response peaked at 3 h, followed by a progressive, much greater increase with time thereafter (Fig. 2B). RT-PCR analysis showed that COX-1 mRNA was expressed constitutively and that COX-2 mRNA was markedly induced as early as 2 h by IL-1α plus TNF-α and remained at the same level at 24 h (Fig. 3B). Thus, it is conceivable that the first peak of PGE2 production is due to the induction of COX-2 gene expression and that the marked increase in PGE2 production after 12 h (Fig. 2B) coincides with the increase in NO production (Fig. 2A).
The increase in PGE2 production by IL-1α plus TNF-α during 48 h was significantly suppressed by AG as well as aspirin (Fig. 4), suggesting that NO produced by these cytokines stimulated the synthesis of PGE2. This concept was also supported by the findings that NOC-18, which releases NO in solution with a half-life of 21 h at pH 7.4 at 37°C,19 not only increased the production of PGE2 by itself but also restored PGE2 production in the presence of AG (Fig. 4).
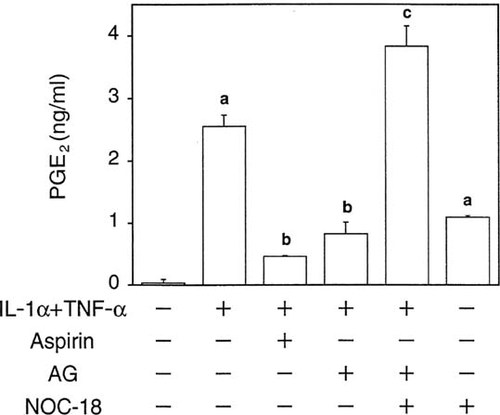
Effect of NOC-18, an NO donor, and aminoguanidine (AG), an iNOS inhibitor, on PGE2 production in MC3T3-E1 cells. Cells were incubated with various combinations of IL-1α (10 ng/ml), TNF-α (10 ng/ml), NOC-18 (30 μM), aminoguanidine (AG, 1 mM), and aspirin (1 mM) for 48 h, and PGE2 concentrations in the conditioned medium were determined by enzyme immunoassay. Data represent the mean ± SD in quadruplet. (a) p < 0.05 versus control, (b) p < 0.05 versus IL-1α + TNF-α, and (c) p < 0.05 versus IL-1α + TNF-α + AG.
Effects of PGE2 and aspirin on NO production
Figure 5 shows the effects of PGE2 and aspirin on NO production. Aspirin stimulated the NO production in MC3T3-E1 cells treated with both IL-1α and TNF-α. The significant effect of aspirin was observed at 1 mM, a dose that inhibits COX-2 almost completely, but not at 0.01 mM, a dose that inhibits only COX-1.16 Conversely, addition of PGE2 suppressed the production of NO in a dose-dependent manner in MC3T3-E1 cells treated with both IL-1α and TNF-α (Fig. 5). These data suggest that PGE2 produced via induction of COX-2 in response to the cytokines negatively regulates NO production.
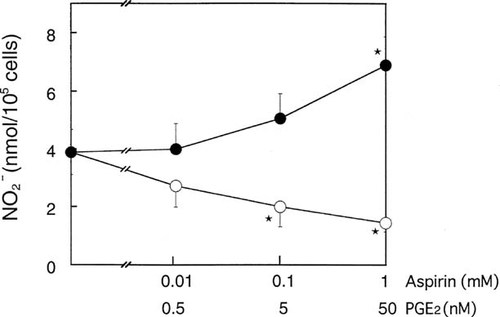
Effects of aspirin and PGE2 on NO production in MC3T3-E1 cells. Cells were incubated with various concentrations of aspirin or PGE2 for 48 h, and the nitrite concentration in the conditioned medium was determined as described in Materials and Methods. Data represent the mean ± SD in triplicate. *Significant difference from control (p < 0.05).
Modulation of ALP activity
Finally, we examined the effects of NO and PGE2 on ALP activity, which plays a key role in bone mineralization and is widely used as an index of bone formation. As shown in Fig. 6, treatment with IL-1α plus TNF-α markedly suppressed ALP activity, and AG significantly reversed this suppression, suggesting that NO produced in response to these cytokines was, at least in part, responsible for the suppression of ALP activity. This concept is also supported by the observations that exogenous NO released from NOC-18 not only suppressed ALP activity by itself but blocked the effect of AG (Fig. 6). 8-bromo-cyclic guanidine monophosphate (cGMP) did not affect ALP activity up to 1 mM (data not shown), suggesting that cGMP does not mediate the regulation of ALP activity by NO in MC3T3-E1 cells.
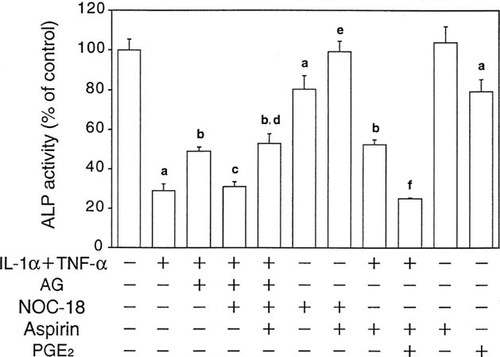
Effects of cytokines and aminoguanidine, an iNOS inhibitor, on ALP activity in MC3T3-E1 cells. Cells were incubated with various combinations of IL-1α (10 ng/ml), TNF-α (10 ng/ml), NOC-18 (30 μM), aminoguanidine (AG, 1 mM), aspirin (1 mM), and PGE2 (50 nM). After 48 h, cells were harvested and ALP activity was measured as described in Materials and Methods. Data represent the mean ± SD in quadruplet. (a) p < 0.05 versus control, (b) p < 0.05 versus IL-1α + TNF-α, (c) p < 0.05 versus IL-1α + TNF-α + AG, (d) p < 0.05 versus IL-1α + TNF-α + AG + NOC-18, (e) p < 0.05 versus NOC-18, and (f) p < 0.05 versus IL-1α + TNF-α + aspirin.
Since these suppressive effects of NOC-18 were abolished in the presence of aspirin (Fig. 6), it seemed likely that the effect of NO on ALP activity was mediated through PGE2 produced via NO production. In fact, aspirin significantly reversed the inhibition of ALP activity by IL-1α plus TNF-α, and PGE2 significantly decreased ALP activity by itself or in the presence of aspirin (Fig. 6).
DISCUSSION
IL-127 and TNF-α28 stimulate bone resorption and inhibit bone formation, and accumulating evidence suggests that these proinflammatory cytokines are involved in the pathogenesis of osteoporosis.29, 30 However, the cellular and molecular mechanisms by which these cytokines act in the bone microenvironment remains elusive. IL-1 and TNF-α are known to induce iNOS8-12 as well as COX-2,14 which release NO and PGs, respectively, in osteoblastic cells. It has been shown that NO can activate COX pathway in pancreas islet cells,31 mesangial cells,32 and endothelial cells33 in vitro as well as in vivo34, 35 and that an enhanced production of PGs may play an important role in the mechanism of NO actions. Ralston and Grabowski36 have demonstrated that IL-1α and TNF-α stimulate NO and PGE2 production in murine calvarial organ cultures and that the effect of these cytokines on bone resorption is determined by a balance between NO and PGE2 levels. However, cross-talk between NO and COX pathways in osteoblastic cells and its role in the regulation of osteoblastic functions are not fully understood.
We have demonstrated in the present study that NO produced by iNOS in response to IL-1 and TNF-α activates the COX pathway, leading to an increased production of PGE2 in osteoblastic MC3T3-E1 cells. Furthermore, our data indicate that NO inhibits ALP activity via PGE2 and that PGE2, in turn, negatively modulates NO production. One NOC-18 molecule releases two NO molecules slowly in a similar manner to the release of endogenous NO by iNOS.19 Based on our analysis that approximately 40% of NO released from NOC-18 can be detected as nitrite (data not shown), the dose of NOC-18 used in the current study is close to the amount of NO produced by MC3T3-E1 cells. In MC3T3-E1 cells, iNOS mRNA was induced and nitrite accumulation increased gradually in response to IL-1 and TNF-α, which was inhibited by AG26 as well as TGF-β37 almost completely. These results indicate that iNOS induced by these cytokines mediates the production of NO.
In contrast, PGE2 production in response to the cytokines exhibited a biphasic pattern. The initial peak at 3 h was preceded by a marked induction of COX-2 mRNA at 2 h. However, the progressive increase in PGE2 production after 12 h was not associated with a further increase in COX-2 mRNA level. Our data indicate that NO is involved in the increased production of PGE2 through the stimulation of COX pathway since (1) the increase in PGE2 production was markedly inhibited by AG, and (2) NOC-18, an NO generator, reversed this inhibition of PGE2 production by AG. With respect to the mechanism of the activation of COX pathway by NO, it was initially proposed that the binding of NO to the heme moiety of COX plays an important role.38 However, it has recently been reported that superoxide is involved in the induction of COX39 and that peroxynitrite, the coupling product of NO and superoxide, activates both COX-1 and COX-2, serving as a link between NO and PG pathways.40
NO production induced by IL-1α and TNF-α was inhibited dose-dependently by exogenous PGE2 and increased by aspirin, suggesting that PGE2 produced by the cytokines, in turn, negatively modulates NOS activity. This negative feedback regulation of NO production by PGE2 may explain the attenuation of the production rate of NO after 24 h in response to IL-1 and TNF-α (Fig. 2A). The mechanism of PGE2-mediated down-regulation of NO production in osteoblastic cells remains to be determined, although previous reports have suggested the involvement of Ca2+/inositol phospholipid pathways via an EP1 receptor.32
IL-1α and TNF-α inhibited ALP activity, which was partially reversed by AG, suggesting that the cytokines may inhibit the osteoblastic function by both an NO-dependent and an independent mechanism. However, there exists an alternative possibility that the NOS inhibitor was unable to alleviate fully the biological effects of NO and that NO induction was largely responsible for the decrease in ALP activity in MC3T3-E1 cells. Recently, Damoulis and Hauschka41 reported that NO induced by inflammatory cytokine stimulation caused loss of osteoblast cell viability due to apoptosis. In our study, the concentrations of NO produced by cytokine stimulation were lower than those of the previous study.41 In addition, we did not observe any change in the viability of cells, as assessed by trypan blue exclusion, during the 48 h period when NO production was induced by cotreatment with IL-1α and TNF-α or when cells were treated with NOC-18 up to 30 μM (data not shown). It is conceivable, therefore, that the decrease in ALP activity following cytokine stimulation in the current study is due to a down-regulated response of viable cells rather than apoptosis.
Our data suggest that NO suppresses ALP activity in MC3T3-E1 cells via PGE2 produced by an NO-activated COX pathway. It has been reported that PGE2 exerts an inhibitory42-44 or a biphasic effect45, 46 on ALP activity in MC3T3-E1 cells. Receptors for PGE2 (EPs) are divided into four subtypes, EP1 through EP4,47 and it has been shown that MC3T3-E1 cells express EP1 and EP446, 48 and that signaling through EP1 inhibits ALP activity, while EP4 is involved in the stimulation of ALP activity.46 Based on the findings that the dissociation constants for EP1 and EP4 are 21 nM and 2 nM, respectively,48 it is tempting to assume that the biphasic effect of PGE2 on ALP activity may be explained by a stimulatory effect through EP4 at low concentrations and an inhibitory effect through EP1 at high concentrations of PGE2.
It has been reported recently that NO protects against bone loss caused by ovariectomy.44 NO produced by osteoblasts in response to proinflammatory cytokines not only inhibits osteoblast functions, as determined by ALP activity, but also may activate COX in osteoclasts, leading to the release of PGE2, which is thought to suppress osteoblastic function and stimulate osteoclastic bone resorption.14 Thus, these in vitro data seem to contradict the protective role of NO in osteoporosis in vivo.49 NO may exhibit biphasic effects on osteoclastic bone resorption in some in vitro studies, being stimulatory at low and inhibitory at high concentrations.7 It is tempting to speculate that when a large amount of NO is produced, the inhibitory effect on osteoclasts outweighs the effects on osteoblasts, resulting in a relative increase in bone formation.
The conclusion of our study is schematically illustrated in Fig. 7. IL-1 and TNF-α activate both NOS and COX pathways, leading to the production of NO and PGE2, respectively, which are involved in the regulation of ALP activity in osteoblastic MC3T3-E1 cells. NO stimulates the COX pathway, and the inhibitory effect of NO on ALP activity is mediated through PGE2. PGE2, in turn, negatively modulates the production of NO in response to IL-1 and TNF-α. Thus, cross-talk between the NOS and COX pathways may play an important role in the regulation of osteoblastic functions by proinflammatory cytokines.
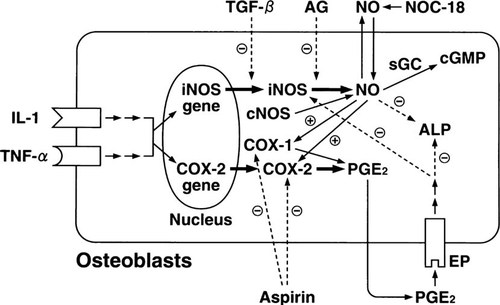
Schematic representation illustrating the cross-talk between NOS and COX pathways in osteoblastic MC3T3-E1 cells. Refer to text for details.
Acknowledgements
We thank Drs. Ken Watanabe and Makoto Nakanishi (Department of Geriatric Research, National Institute for Longevity Sciences) for critical reading of the manuscript and valuable suggestions. This work was supported in part by the Research Grant for Longevity Sciences (8A-02 to K.I.) from the Ministry of Health and Welfare of Japan.




