Further Observations on Programmed Cell Death in the Epiphyseal Growth Plate: Comparison of Normal and Dyschondroplastic Epiphyses
Abstract
The objective of the investigation was to provide information on apoptosis in the normal epiphysis and to assess apoptosis in the plate of the dyschondroplastic chick. Apoptosis was evaluated using two terminal deoxynucleotide transferase end-labeling procedures, DNA fragmentation and nuclear morphology. We found that there was a minimal level of apoptosis in the dyschondroplastic cartilage. In the tibial dyschondroplastic (TD) lesion itself, only about 3% of cells are positive in the articular and proliferative regions; 11% of prehypertrophic chondrocytes are stained by the end-labeling procedure, and most of the cells are localized around vascular channels at the calcifying front. This finding suggests that dyschondroplasia is linked to impairment of apoptosis, and as a result the tissue contains immature cells that have outlived their normal life span. In contrast, in the normal plate, we noted that when the proliferative period was complete, the cells became terminal transferase positive; in addition, chondrocytes in the normal plate exhibited DNA fragmentation. Semiquantitative analysis of stained chondrocytes in the growth plate indicate that in the proliferative zone 15.5% of cells are terminal deoxynucleotidyl transferase (TUNEL) positive; in contrast, 44% of postmitotic chondrocytes are stained by the TUNEL procedure. The presence of a sharp border between the pre- and postmitotic zones suggests that the stimulus for apoptosis is maturation dependent and reflects local metabolic control. We also examined apoptosis in metaphyseal osteoblasts. We found that adjacent to the epiphysis, many osteoblasts were undergoing apoptosis. In more mature sites in the metaphysis, there was less cell death, indicating that osteoblast apoptosis was delayed and cells were completing their normal life cycle. Although terminal transferase end-labeled cells were not seen in articular cartilage, we noted that fibroblasts, in the perichondrial ligament surrounding the articular as well as the epiphyseal regions of the plate, were undergoing apoptosis. Apoptosis at this site may be related to lateral expansion of the cartilages, reflect a high cell turnover rate at the junction between the tissues, and result from paracrine signals received from the underlying cartilage.
INTRODUCTION
THE PROCESS OF BONE elongation is mediated by cells contained within the epiphyseal growth cartilage. In this tissue, prior to development of a terminally differentiated state, cells undergo a series of well characterized changes in phenotype. These changes include increased activity of plasma membrane alkaline phosphatase, synthesis of type X collagen and down-regulation of type II collagen, secretion of osteonectin, expression of vitamin D receptors, and release and mineralization of matrix vesicles.1-5 Accompanying these phenotypic changes, there is a concomitant shift in energy metabolism such that the hypertrophic cells generate almost all their energy through glycolytic processes.6-8 Once hypertrophy is complete, the calcified cartilage is replaced by bone.
While the mechanism for the replacement of cartilage by bone is unknown, the differentiated chondrocyte must be removed to maintain the steady-state thickness of the cartilage. If this process is impaired, the retained tissue interferes with the formation of endochondral bone. Not surprisingly, the ultimate fate of the terminally differentiated chondrocyte is disputed. Some authorities contend that these chondrocytes are converted into bone cells9; recently, it has been proposed that the hypertrophic cell proliferates, and following this event, one daughter chondrocyte dies while the remaining cell becomes an osteoblast.10 A third viewpoint provides direct support for the notion that terminally differentiated chondrocytes undergo programmed cell death.11-14 Indeed, when chondrocyte DNA is end-labeled, using the terminal deoxynucleotidyl transferase (TUNEL) procedure, there is preferential staining of hypertrophic and terminally differentiated chondrocytes.14,15 In concert with these observations, it has been demonstrated that cells in the calcified region of the growth plate undergo nuclear condensation, while preserving the continuity of the plasma membrane.3,11,13,16 All of these latter findings are in accord with the concept that the cells of the growth plate undergo apoptosis, and following their death, elimination from the epiphysis promotes bone formation.
Although the biochemical studies provide powerful support for the argument that chondrocytes in the growth plate undergo programmed cell death, questions remain concerning the timing and extent of the apoptotic process.14,15 The objective of this communication is to provide further details of apoptosis in the normal growth plate and perichondrial tissues. In addition, using two separate cytochemical procedures, we assess apoptosis in the epiphyseal growth plate of the tibial dyschondroplastic (TD) chick. It is noteworthy that a number of investigators have reported that dyschondroplastic cells accumulate in an almost avascular matrix and that lesion chondrocytes are necrotic.17-20 The results of the current study provide new information on chondrocyte function in TD and lend further support to the contention that cells in the growth plate complete their life history through programmed cell death.
MATERIALS AND METHODS
Animals and tissues
Male broiler chicks (Ross 1 strain) were maintained from hatching on a diet that resulted in a high incidence of TD.21 After 3 weeks, proximal tibiotarsi were dissected and examined for the presence of dyschondroplastic lesions by sectioning lengthwise through the joint. For the electrophoretic analyses, spontaneously appearing 6-week-old TD chickens were utilized. To study the morphological characteristics of the tissues, longitudinal pieces (2 × 0.5 cm) of TD growth plate, isolated from the central region of severely affected tissue, and normal plate were fixed in 4% paraformaldehyde; samples were demineralized for 1 week with 15% EDTA, pH 7.4. Specimens were then processed into paraffin wax according to standard procedures, and 5-μm-thick sections were cut, deparaffinized in xylene, rehydrated through graded alcohols, and stained by the end labeling methods described below.
TUNEL procedure
In general, the procedure used was similar to that described earlier.15 Briefly, this method takes advantage of the fact that during apoptosis, between nucleosomes, nuclear endonucleases digest genomic DNA into fragments of multiples of approximately 200 bp. We labeled the fragmented nucleotide ends using ApopTag Plus [In Situ Apoptosis Detection Kit Peroxidase (Oncor, Gaithersberg, MD, U.S.A.)]. Prior to labeling, sections were treated into 1% Triton X-100 (see below). Tissue sections were then treated with 20 μg/ml proteinase K at room temperature for 15 minutes and, to inhibit peroxidase activity, incubated with 3% H2O2 in phosphate-buffered saline (PBS). Prior to labeling, the sections were equilibrated in a transferase buffer for 5–10 minutes and then incubated in a reaction mixture containing biotin-labeled deoxynucleotides and the terminal deoxynucleotidyl transferase at 37°C. After 60 minutes, the reaction was stopped, and the nucleotides were detected using an antidigoxigenin antibody conjugated to peroxidase. This peroxidase label was detected using the chromogenic substrate, diaminobenzidine. To relate the stained section to tissue structure, samples were also examined by phase contrast microscopy. Controls for the study included samples that had not been treated with the antibody or with the terminal transferase, and as a positive control, some sections were treated with DNAse prior to staining.
Klenow-dependent DNA fragmentation end-labeling procedure
We also end-labeled chondrocyte nuclei using the FragEL system [FragEL-Klenow DNA fragmentation detection kit (Oncogene Research Products, Cambridge, MA, U.S.A.)]. This assay is based on the observation that the Klenow enzyme binds to exposed 3′-OH ends of DNA fragments and catalyzes the template-dependent addition of biotin-labeled deoxynucleotides. The protocol for the initial treatment of the sections was the same as indicated above. Thus, the tissue was treated with proteinase K, Triton, and H2O2. The sections were then treated with the Klenow labeling solution containing a mixture of biotin-labeled and unlabeled deoxynucleotides for 1.5 h at 37°C in a humidified atmosphere. The reaction was stopped using 0.5 M EDTA, pH 8.0. After blocking with 4% bovine serum albumin in PBS, for 30 minutes, sections were treated with streptavidin-horse radish peroxidase conjugate. Biotinylated nucleotides were detected with diaminobenzidine, which generated an insoluble colored substrate at the site of DNA fragmentation. Tissue sections were viewed by light microscopy and phase contrast microscopy. Controls sections were the same as those described using the TUNEL procedure.
Triton treatment
We noted that many TUNEL positive cells were stained unevenly, possibly due to macromolecular components of cartilage interfering with the assay. To remove the interfering materials, we treated samples with Triton X-100 prior to performing the TUNEL assay. The Triton concentration was fixed at 1%, while the extraction time was varied from 0–30 minutes. Sections were then subjected to proteinase K digestion and stained by the TUNEL method (see above). We found that as the treatment time was increased, there was a rise in the staining intensity of the TUNEL positive cells. However, Triton treatment did not change the negative staining response of chondrocytes in proliferative or articular cartilage. Since maximum staining with minimum loss of morphological details was seen after 10 minutes of treatment with Triton, this treatment period was used as part of the standard procedure for all of the TUNEL assays described above.
Transmission electron microscopy
Specimens for ultramicroscopy were taken from the central and severely affected region of TD growth plates. They were fixed in Karnovsky's fixative, postfixed in 2% osmium tetroxide, dehydrated in graded series of ethanols, and embedded in araldite. Ultrathin sections were cut on an LKB Ultratome picked up on formvar-coated grids and contrasted with uranyl acetate and lead citrate. Specimens were evaluated in a JEM 100CX II transmission electron microscope (TEM) operated at 80 kV. TEM studies of normal growth plate were reported previously.15
DNA extraction and agarose electrophoresis
Thin slices of cartilage were obtained from fresh growth plate of normal chicks and the lesional area of TD chicks. The tissue was digested for 3 h in collagenase and cells were isolated and collected.15 Chondrocytes were washed and sedimented by low-speed centrifugation. Separation of DNA fragments was performed using the method described by Eastman.22 Briefly, cells were resuspended in a sample buffer containing 10% glycerol, 10 mM Tris, pH 8.0, and 1% bromophenol blue that was mixed with an equal volume of 10 mg/ml RNAse (ribonuclease A) in 10 mM Tris, 0.1 M EDTA, pH 7.4. Cells were inserted into wells of 2% alkaline agarose gel that had been preloaded with 1% agarose, 2% sodium dodecyl sulfide (SDS), and 64 μg/ml proteinase K. Samples were electrophoresed for 14 h at 60 V. The gels were stained with ethidium bromide (0.4 μg/ml). DNA fragments were visualized using a UV (302 nm) transilluminator.
Quantitation of apoptotic cells
To gain some insight into the extent of apoptosis, we determined the percentage of stained cells in selected regions of the growth plate. We counted 200–300 chondrocytes in the proliferating and postmitotitc mature cells zones, in three separate stained sections, of both the normal and TD plates.
RESULTS
Morphology of the growth plates of normal and TD chicks
The histomorphology of the growth plates of normal and TD chicks have been described in detail elsewhere. It is important to note however, that in the normal animal, there are well demarcated zones of resting, proliferative, and hypertrophic cartilage, and a sharp border between the extensive proliferative zone and the mature postmitotic chondrocytes is evident. In addition, an abundance of metaphyseal vascular channels penetrate the hypertrophic region. These channels form the loci for mineral formation; as we have demonstrated previously, apatite is first deposited in the extracellular matrix of hypertrophic cells that surround the vascular channels.23 In contrast, in the TD growth plate there is minimum chondrocyte zonation, and sharp borders between the regions are not seen. As a result there is a characteristic accumulation of enlarged (prehypertrophic) lesion cells, an extensive extracellular matrix and minimum mineral deposition.19 Few metaphyseal or epiphyseal blood vessels are present in the cartilage.
Apoptosis in the normal chick growth plate
TUNEL-stained and phase contrast views of selected regions of the normal growth plate are presented in Fig. 1. Figure 1A shows chondrocytes in the postmitotic maturing region of the epiphysis; hypertrophic chondrocytes are evident and vascular channels can be seen permeating the tissue. A zone of proliferating cells is also present in this section. When the section was treated by the TUNEL procedure, many of the nuclei of the hypertrophic cells were stained (Fig. 1B). The staining is particularly intense at the junction between the proliferative and postmitotic mature chondrocytes and around and in the vascular channels. Figure 1C is a high power phase contrast view of hypertrophic chondrocytes localized around a vascular channel; nuclei of chondrocytes as well as perivascular cells are TUNEL positive (Fig. 1D). Some of the cells that are present in the channel also appear to be stained. Semiquantitative analysis of stained chondrocytes in the growth plate indicate that in the proliferative zone 15.5% of cells are TUNEL positive; in contrast over 44% of postmitotic chondrocytes are stained by the TUNEL procedure.
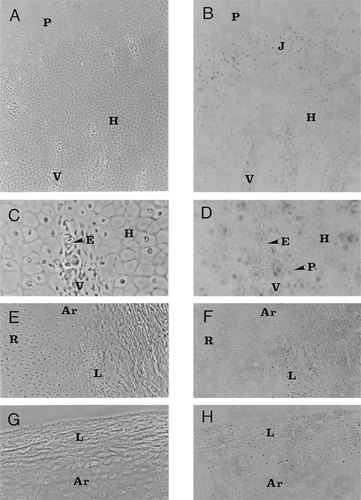
TUNEL-stained and phase contrast views of the normal growth plate. (A) Phase contrast view of a 5 μm thin longitudinal section through the proximal tibial plate. This photomicrograph shows a region of the cartilage containing hypertrophic chondrocytes (H) and vascular channels (V); a small zone of proliferating cells (P) is also evident. Magnification ×100. (B) Same section as (A) stained by the TUNEL procedure. Note that many of the nuclei of the hypertrophic cells are stained. The staining is particularly intense at the junction between the proliferative and postmitotic mature chondrocytes (J) and around and in the vascular channels (V). (C) High-power phase contrast view of hypertrophic chondrocytes (H) localized around a vascular channel (V). Red cells (E) are present within the channel. Magnification ×400. (D) Same section as (C) stained by the TUNEL procedure. Many of the nuclei of the hypertrophic chondrocytes (H) are stained. Perivascular cells (P) and cells that are present in the channel also appear to be TUNEL positive. (E) Phase contrast view of the proximal region of the tibial epiphysis. A small number of articular chondrocytes (Ar) can be seen separated by abundant extracellular matrix. Below the articular cells, resting chondrocytes (R) can be seen. The articular surface is bounded by soft tissue and enclosed by a fibrous articular ligament (L). Magnification ×200. (F) Same section as (E) stained by the TUNEL procedure. Note that none of the cartilage cells appear to be apoptotic. However, the strong positive reaction from cells of the capsular ligament (L) indicates that these cells are undergoing apoptosis. (G) Phase contrast view of the capsular ligament (L) covering the superior surface of the articular cartilage (Ar). Magnification ×200. (H) Same section as shown in (G) stained by the TUNEL procedure. Note that while the cartilage cells are negative, fibroblasts that are present in the ligament are TUNEL positive.
Aside from chondrocytes within the plate, we also evaluated apoptosis in cells of the articular region of the proximal head of tibiotarsi. Figure 1E shows that a small number of articular chondrocytes are present in the section and that there is abundant extracellular matrix. Below the articular region, resting and some proliferating chondrocytes can also be seen. The articular surface is bounded by soft tissue and enclosed in a fibrous articular capsule. When stained by the TUNEL method, none of the cartilage cells appear to be apoptotic (Fig. 1F). However, the strong positive reaction of cells of the capsular tissue surrounding the resting and proliferating regions indicates that these cells are undergoing apoptosis. We further explored the extent of apoptosis in the capsular ligament that covers the superior surface of the articular cartilage (Fig. 1G); fibroblasts that are present in the ligament are TUNEL positive (Fig. 1H).
We examined apoptosis in bone that had formed around the vascular canals of the metaphysis. Figure 2A shows that a thin extended layer of bone has formed on a trabeculum of cartilage; osteoblasts are present both on the superficial and deep surfaces of the bone. Stained by the TUNEL procedure, many of these cells are positive, indicating that they are apoptotic (Fig. 2B).
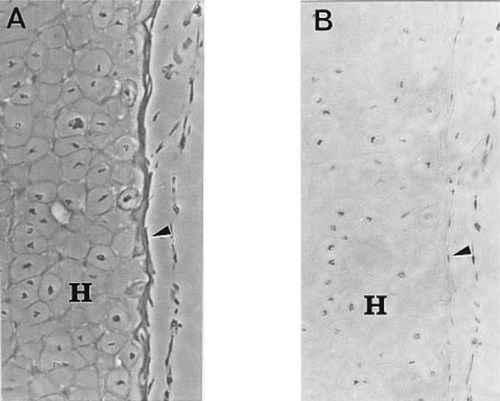
TUNEL stained and phase contrast views of a trabeculum of calcified cartilage. (A) Phase contrast view of a trabeculum of cartilage and a contiguous vascular channel. A thin extended layer of bone has formed on hypertrophic cartilage (H); osteoblasts (arrow) are present both on the superficial and deep aspects of the bone. Magnification ×400. (B) Same section as Fig. 3A stained by the TUNEL procedure. Note the presence of TUNEL positive cells (arrow).
A considerable number of controls were performed to validate the specificity of the TUNEL staining reaction. As might be expected, when stained by the TUNEL procedure, most of the cells in proliferating cartilage are negative (Fig. 3A). Treatment of the proliferating tissue with DNAse, prior to staining, results in a significant increase in the number of TUNEL positive cells (Fig. 3B). In other control studies, the terminal deoxynucleotidyl transferase was omitted from the reaction mixture. In this case, none of the cells in the hypertrophic region are TUNEL positive (data not shown).
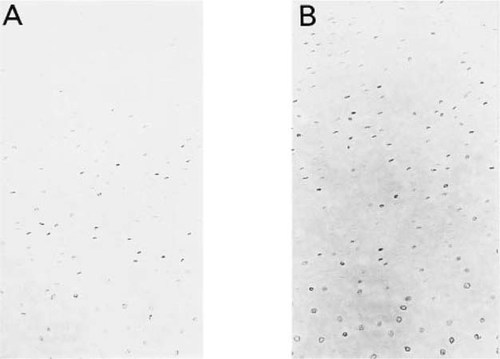
Positive control for the TUNEL assay performed on tissue isolated from the proliferating region of the growth plate. Magnification ×200. (A) Tissue stained by the TUNEL assay prior to treatment with DNAase. Note that very few cells are positive. (B) Tissue stained by the TUNEL procedure after treatment with DNAse. Note, the significant increase in the number of TUNEL positive cells.
Assessment of apoptosis in the TD growth plate
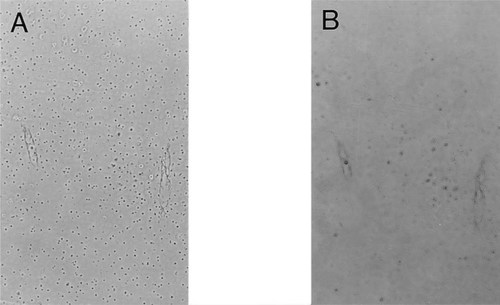
TUNEL stained and phase contrast views of selected regions of the TD growth plate are presented in Fig. 4. Figure 4 shows cells in the articular and early proliferative regions of the epiphysis; these cells are negative when stained by the TUNEL assay (Fig. 4B). However, some staining is seen around the articular and epiphyseal blood vessels (Fig. 4B). Cells in the body of the lesion are shown in Fig. 4C. There is little evidence of apoptosis in the prehypertrophic lesion chondrocytes. Moreover, in the tissue at the chondro-osseous junction itself, few cells are TUNEL positive (Fig. 4D). When sections of the TD plate were stained using the Klenow-dependent end-labeling procedure, about 3% of cells are positive in the articular and proliferative regions (data not shown). In the TD lesion itself, only about 11% of prehypertrophic chondrocytes are stained by the end-labeling procedure, and these chondrocytes are located at the calcifying front (Fig. 5).
Klenow-dependent end-labeling and phase contrast views of the TD growth plate. (A) Phase contrast view of a 5 μm thin longitudinal section through the proximal TD plate. This photomicrograph shows prehypertrophic chondrocytes and epiphyseal blood vessels of the TD plate. Magnification ×100. (B) Same section as (A) stained by the Klenow-dependent end-labeling procedure. Some stained prehypertrophic chondrocytes are evident; cells that border the epiphyseal blood vessels are also stained.
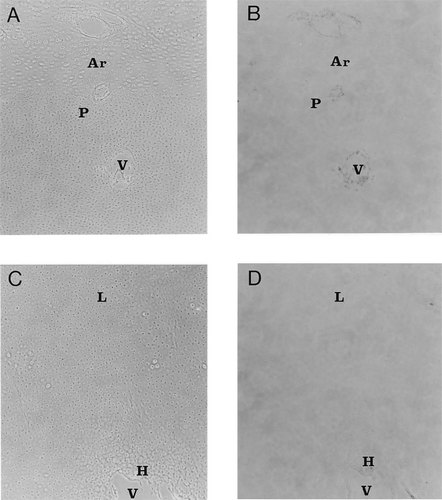
TUNEL-stained and phase contrast views of the TD growth plate. (A) Phase contrast view of a 5 μm thin longitudinal section through the proximal tibial plate. This photomicrograph shows the articular (Ar) and early proliferative regions (P) of the TD epiphysis. Vascular channels (V) can also be discerned. Magnification ×100. (B) Same section as Fig. 5A stained by the TUNEL procedure. Note that the chondrocytes in both zones are TUNEL negative. However, some staining is seen around the articular and epiphyseal blood vessels. (C) Phase contrast view of tissue at the chondro-osseous junction. Immature prehypertrophic lesion cells (L) are present together with some hypertrophic chondrocytes (H) close to the vascular canal (V). Magnification ×100. (D) Same section as Fig. 5E stained by the TUNEL procedure. Note that the lesion and hypertrophic cells are TUNEL negative. There is some positive staining material at the vascular canal.
To ascertain whether the vascular cells and chondrocytes in the TD growth plate exhibit the characteristic morphology of apoptosis, tissue sections were examined by TEM. A prehypertrophic chondrocyte from a dyschondroplastic lesion is shown in Fig. 6A. Although the nuclear membrane appeared to be crenated, there is no evidence of chromatin condensation. In contrast, when the cells bordering the vascular channels were examined by electron microscopy there was considerable evidence of cell death and apoptosis. Figure 6B shows that there is peripheral chromatin condensation. Morphological evaluation of cells present in the channel (some of which were probably dislodged from the cartilage during specimen preparation) indicates that some are apoptotic. We have presented elsewhere detailed apoptotic analysis at the electron microscopic level of normal hypertrophic chondrocytes.15
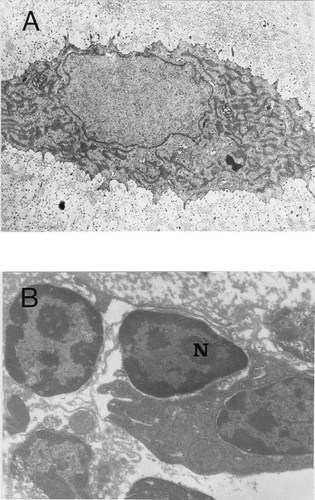
Transmission electron microscopic views of TD chondrocytes and perivascular cells. (A) Photomicrograph of an immature prehypertrophic chondrocyte. Although the nuclear membrane appears to be crenated, there is no evidence of chromatin condensation or apoptotic bodies. Magnification ×9000. (B) Photomicrograph of cells at a vascular channel. Note that the nuclei of a numbers of cells exhibit characteristic apoptotic chromatin condensation (N). Magnification ×14,000.
Since apoptosis is usually accompanied by chromatin degradation, we examined the DNA of both normal and TD chondrocytes for nucleic acid fragmentation by agarose gel electrophoresis. Figure 7 shows that DNA extracted from cells of the normal growth plate forms both a high molecular weight band as well as a number of low molecular weight fragmented DNA bands. In contrast, all of the TD growth plate DNA bands at the origin as high molecular weight species; there is no evidence of low molecular weight DNA in extracts of the TD cartilage.
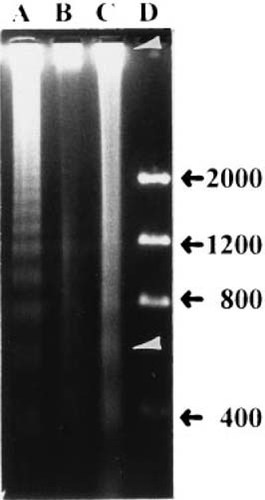
Agarose gel electrophoresis of DNA isolated from TD and normal growth plate. Lane A, DNA fragmentation analysis of apoptotic HL-60 cells. Lane B, DNA fragmentation pattern of cells from TD growth plate. All of the DNA bands at the origin as a high molecular weight species. Lane C, DNA fragmentation pattern of normal growth plate cells. Arrows indicate the presence of high molecular weight DNA and bands of lower molecular weight material. Lane D, DNA molecular weight markers ranging in size from 400–2000 bp.
DISCUSSION
The results of this investigation confirm and extend our earlier study of apoptosis in the normal chick growth plate. Here we demonstrate that apoptosis does occur in maturing epiphyseal chondrocytes, and we demonstrate that this process begins early in the life history of the cell. Thus, following chondrocyte proliferation there is formation of a sharp band of apoptotic cells, and as might be expected from functional and ontological considerations, TUNEL positive cells are not seen in articular cartilage. We show for the first time that fibrous and ligamentous cells of both the articular and epiphyseal capsule undergo extensive apoptosis. We presume that this event is linked to expansion of the cartilage disk as the tibial head grows both in length and in width. Last, the study clearly demonstrates that TD, a condition characterized by the accumulation of prehypertrophic chondrocytes, is linked to failure of the epiphyseal chondrocytes to undergo programmed cell death. This finding is surprising since many of the cells in TD plate have been described to be necrotic.17-20 While our findings do not contradict this assumption, the mode of death is not through apoptosis.
Considering the normal growth plate first, in our earlier report, we indicated that we were surprised by the finding that most of the postmitotic cells exhibit DNA fragmentation.15 Possible explanations for the wide distribution of TUNEL-stained cells include use of an overly sensitive fluorescence tagging system, and the presence of charged intracellular macromolecules that may artifactually bind the digoxigenin probe. In this study, we used a less sensitive assay procedure and we treated sections with Triton to remove matrix components that may confound the immunohistochemical assay. Nonetheless, the results of both studies are very similar. Thus, as soon as the proliferative period is complete and chondrocytes exhibit features consistent with the mature nonmitotic stage, the cells become TUNEL positive. The presence of a sharp border between the pre- and postmitotic zones suggests that the stimulus for apoptosis is maturation-dependent and reflects local metabolic control. Indeed, our earlier studies of the growth plate indicate that when cells become postmitotic, there is a profound drop in intracellular nucleotides, a loss of reductive reserve, and a dramatic decrease in the energy charge ratio.6-8,24 In other systems, these metabolic events are considered to be triggers for apoptosis.25,26
The TUNEL procedure was used to assess apoptosis in the mineralizing metaphysis and in perichondrial tissues of the chick. We noted that fibroblasts in the perichondrial ligament surrounding the articular as well as the epiphyseal regions of the plate are TUNEL positive. Ligament cell apoptosis may be related to lateral expansion of the cartilages and reflect a high cell turnover rate at the junction between the tissues. There is a strong possibility that cell death may also result from paracrine signals received from the underlying cartilage. Relevant to the latter consideration is the recent report that cells of the perichondrium communicate with epiphyseal chondrocytes through agents such as PTHrp and Indian Hedgehog and thereby modulate expression of genes that direct the development of the hypertrophic chondrocyte.27 Thus, signals from ligament cells may serve to control the very short life cycle of the epiphyseal chondrocyte. Immediately below the epiphysis, we noted that many osteoblasts were undergoing apoptosis; in this case, it is likely that the half life of these cells is short. In more mature sites in the metaphysis, there was less cell death, suggesting that osteoblasts were being permitted to complete their normal life history (osteoblast → osteocyte).
We have noted earlier that, in vivo, hypertrophic chondrocytes fail to exhibit well defined DNA bands when evaluated by electrophoresis.15 Instead, a “smear” of low molecular weight DNA is often seen. In the current study, by modifying the electrophoretic procedure and inserting whole cells rather than nuclei into the agarose wells, much clearer bands were visible. In itself, this finding suggests that in situ there is rapid DNA fragmentation, and a considerable portion of the low molecular weight fraction is lost when the nuclei alone are subjected to electrophoresis. In contrast to the normal chick, DNA isolated from TD cells exhibited no evidence of low molecular weight species. This latter observation, in concert with the results of TUNEL and Klenow fragment assays and TEM, indicates that few cells in the TD plate are apoptotic. Since apoptosis serves to eliminate mature cells from the tissue, a disturbance of the apoptotic process would be expected to result in accumulation of both chondrocytes and extracellular matrix. For this reason, we propose that an underlying feature of TD is failure or delay of the normal maturation process due to impairment of apoptosis. As a result, a tissue is formed that is made up of immature cells, many of which have outlived their normal life span. This conclusion is in line with other biochemical and morphological investigations of the TD plate.17-19,27
Previous studies of the dyschondroplastic growth plate indicates that chondrocytes are arrested in the prehypertrophic state17,18 and we have previously speculated that the underlying molecular mechanism may be related to alterations in vitamin D metabolite activity21 and c-myc and TGF-β expression.28 Compared with the normal plate, analysis of TD cells indicates that TGF-β levels are substantially reduced. It has been suggested that a reduction in the cytokine level in transitional cells of the TD plate disturbs chondrocyte terminal differentiation. Interestingly, since TGF-β has been shown to be a potent apoptotic agent,29,30 it would not be unreasonable to assume that defective TGF-β synthesis would limit chondrocyte maturation and decrease death signals in the maturing cartilage. Another possible effect of the TGF-β is regulation of vascularization of the growth plate.31 A decrease in TGF-β synthesis would serve to limit vascular invasion and at the same time decrease the impact of a process that promotes cell death. With respect to c-myc, reduced expression of this gene is regarded as a causative factor for development of the chondrodystrophy.28 Since c-myc expression is associated closely with the regulation of apoptosis, it is plausible that altered expression of this gene would result in a defect in the apoptotic mechanism. The transcription factor may also regulate chondrocyte maturation through modulation of vitamin D receptor expression.32 In this context, in the disease state, receptor expression is down-regulated33; moreover, dietary supplementation with vitamin D metabolites results in increased chondrocyte differentiation34 and a lower incidence of TD.21,35
While all of the agents discussed above impact individually on cell function in the growth plate, together they form an interactive group of powerful modulators of chondrocyte maturation and apoptosis. Clearly, even a small alteration in the level of one of these factors may alter the proliferative response and cause delayed or abnormal maturation. When this occurs, TD-like lesions can develop that are characterized by an accumulation of immature chondrocytes. Ongoing experiments are aimed at exploring the role of each of these agents in promoting chondrocyte terminal differentiation and programmed cell death.
Acknowledgements
This work was supported by National Institutes of Health grants DE-09684, DE-10875, and AR-41525 (I.M.S.) and the Ministry of Agriculture, Fisheries, and Food (C.F., C.C.W.). We would like to thank Mr. G.W. Robertson and Mrs. S. Decker for technical assistance.




