Skeletal Changes in Transgenic Male Mice Expressing Human Cytochrome P450 Aromatase†
The authors have no conflict of interest
Abstract
In this study, we showed that overexpressing P450 aromatase in male mice can increase bone mass and strengthen the tibia. Probably as a result of the action of products of local estrogen biosynthesis at different stages of life, the increased bone mass in young mice was induced by decreased bone turnover, but in aged animals, it was induced by increased bone formation.
Introduction: To understand the skeletal responses to the testosterone/estrogen balance, especially to excess estrogen produced by extragonadal biosynthesis, we investigated the bone changes in transgenic mice overexpressing human aromatase.
Materials and Methods: Sixty-one young (40 days) and 25 aged (9 months) transgenic and wildtype (WT) mice were used. Bone samples were analyzed using pQCT, histomorphometry, and mechanical testing. Concentrations of testosterone (T) and estradiol (E2) were measured in serum and testicular interstitial fluid.
Results and Conclusions: Young P450 aromatase-positive (AROM+) mice had much higher trabecular BMD in the proximal tibia than WT mice, and the tissue area was significantly smaller in the former. Histomorphometric data further showed that the longitudinal growth rate of the tibia was decreased in AROM+ mice, and the bone formation rate (BFR) was decreased in trabecular bone and periosteum. All the changes were more striking in males than in females. Aged male AROM+ mice showed similar changes in trabecular bone as young animals, but their BFR was obviously increased. Another dramatic change was in the tibias of aged AROM+ mice: length was shorter (−23.2%), whereas ash weight was much heavier (+24.0%), and bending strength was markedly higher (+21.2%) compared with WT mice. The concentration of T was decreased in both serum and testicular interstitial fluid in young AROM+ mice versus WT animals; E2 levels were increased only in the testes of young AROM+ mice. However, in aged AROM+ mice, the levels of T and E2 were highly increased in both serum and testis versus WT animals. These results are in agreement with the suggestion that enhanced production of estrogen from testosterone in the peripheral tissues as a result of aromatase action can affect skeletal growth and strengthen bone in males. The results also suggest a marked difference in response between femur and tibia.
INTRODUCTION
ESTROGEN IS AN important hormone in maintaining normal bone metabolism. Estrogen deficiency is known to induce osteoporosis in women1-3 and lead to abnormal bone development and osteoporosis in men with mutations in genes encoding either the estrogen receptor4 or aromatase enzyme.5, 6 Accordingly, treating osteoporotic patients with estrogen can improve their bone quality by increasing bone mass and decreasing the fracture rate both in females and males.1,6-9
In addition to the gonads, the biosynthesis of estrogen in other tissues could be an important source of local and circulating estrogen. Aromatization of androgens into estrogens is catalyzed by the enzyme P450 aromatase (P450arom),10-12 and this process has been shown in several tissues including adipose tissue,13 skin,14 muscle,15 and bone.16, 17 Although the presence of aromatase in bone suggests that local estrogen production may play an important role in the maintenance of bone tissue, postmenopausal bone loss indicates that it is clearly not enough to prevent bone loss in older women.
Experimental treatment of rats with aromatase inhibitors induces a decrease in BMD and impaired skeletal modeling in growing and aged male animals.18, 19 Comparable results were obtained by others when they studied bone changes in mice with targeted disruption of the aromatase gene (ArKO mice).20-22 The results showed that ArKO mice had osteopenia in the lumber spine, significant decreases in trabecular bone volume and thickness, and increased bone turnover compared with their wildtype (WT) controls. The bone changes in ArKO mice can be reversed by estradiol (E2) treatment and markedly higher concentrations of testosterone (T), but similar levels of estrogen in the sera of ArKO mice versus WT animals suggest that decreased local estrogen biosynthesis induces bone loss in these animals. It also suggests that, probably, upregulation of local estrogen levels by aromatase could be an effective way to maintain or improve the bone tissues.
We have recently produced transgenic P450 aromatase-positive (AROM+) mice expressing human P450 aromatase under the human ubiquitin C promoter.23 The mice show low universal expression of P450arom, and the male mice have increased concentrations of E2 and reduced concentrations of T in their blood circulation. The imbalanced androgen to estrogen ratio induces severe changes in structure and function of the male reproductive system.23, 24 In this study, we investigated bone changes in AROM+ mice to obtain more understanding of the importance of a prolonged imbalance of the E2 to T ratio regarding bone structure.
MATERIALS AND METHODS
Animals
The generation of AROM+ mice and initial characterization of the phenotype of the reproductive tissues have been reported previously.23, 24 In this study, AROM+ mice were used to study the bone phenotype in young (40 days old) and aged (9 month old) mice. For studying the young mice, 15 AROM+ and 8 AROM− males and 11 AROM+ and 8 AROM− females were analyzed, together with 10 WT males and 9 WT females, used as controls. The genotypes of the mice were analyzed by PCR analysis using DNA extracted from tail biopsy specimens. Because AROM+ males are infertile, only AROM+ females were used for breeding; they were mated with WT FVB/N males. The resulting transgenic negative pups (AROM−) are potentially affected by the extra P450 aromatase expressed in the pregnant and lactating females. Hence, the AROM− mice were not used as controls, but were partially analyzed and compared with WT mice at 40 days of age.
All mice were given intraperitoneal injections of oxytetracycline (20 mg/kg; Pfizer, Sandwich, UK) and calcein (20 mg/kg; Sigma, St Louis, MO, USA) 10 and 4 days, respectively, before death. Animals were housed one to six per cage with commercial mouse chow and tap water ad libitum in controlled conditions of light and temperature. For phenotypic analysis of aged mice, 16 AROM+ and 9 WT male mice were killed at the age of 9 months. Fluorescent double labeling was performed 11 and 3 days before death. The animals were handled in accordance with the institutional animal care policies of the University of Turku (Turku, Finland).
Tissue samples and hormone measurements
The mice were anesthetized by intraperitoneal injection of 300-600 μl 2.5% avertin, and blood was collecting by cardiac puncture. Serum samples were separated by centrifugation and stored at −20°C until used for T and E2 measurements. The concentrations of T were measured by DELFIA after diethyl ether extraction, and the concentrations of E2 were measured using commercial RIA kits (Wallac Delfia; Perkin Elmer), as described previously.25 For measuring the intratesticular concentrations of E2 and T, testes were homogenized in PBS, and T and E2 concentrations were measured using the methods described for the serum samples. Left tibias and femora were stored in 40% ethanol at 4°C for histomorphometric study, and the right tibias and femora were stored at −20°C for BMD measurement.
pQCT measurements
Right tibias of the young mice were thawed at room temperature for 1 h. After removing the soft tissues, the bone was fixed in plastic tube (8 mm diameter) with a spring and scanned with pQCT equipment (XCT 540; Stratec). For the measurement levels in tibia, the reference line was placed at the proximal end of the bone. Three cross-sections, at 0.3-mm intervals, were analyzed 1.8 mm from the reference line. Measurements were also taken from two sections separated by 1 mm, starting 2.5 mm above a reference line at the tibiofibular junction. Special Software version 5.40 (Stratec) was used to analyze the images of each section, with a voxel size of 0.10 mm. Standardized analysis (peel mode 2, cort mode 1, contour mode 1, threshold 0.25 mg/cm3 for trabecular bone and 0.71 mg/cm3 for cortical bone) was applied. After measuring the BMD and length, the tibias were burned at 500°C for 18 h to obtain individual bone ash weights. The right tibias and femora from the aged mice were also analyzed. Because the thickness of the epiphysis of the proximal tibia was thinner than that of young animals, three sections were measured, starting 1.5 mm from the proximal end of the tibia. As regards the femur, three sections were measured 1.8 mm above the distal end, and another two sections were measured 6 mm from the distal end. All section levels are presented in Fig. 1. Before the right tibias were burned, the bending strength was tested, following our earlier mechanical testing method.26 The span of the supporter was 10 mm.
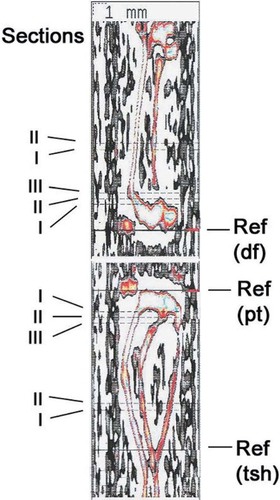
Positions of different measured sections of femur and tibia in pQCT measurement. Ref, reference line; df, distal femur; pt, proximal tibia; tsh, tibial shaft.
Bone histomorphometry
Left tibias and both femora of young and aged mice were embedded in methylmethacrylate as described previously.26, 27 Four- and 8-μm-thick undecalcified longitudinal sections were prepared from the left tibia and femur. Cross-sections of 60 μm were cut 6 mm above the distal end of the right femur using a low-speed saw. Four-micrometer sections were stained using the Masson-Goldner-Trichrome method, and histomorphometric data on trabecular bone in proximal tibias of young mice were collected in a field of 0.75 mm2 located at the middle of the section and 0.6 mm below the growth plate. For aged animals, the measured fields were near the growth plate of the proximal tibia or distal femur. Eight- and 60-μm unstained sections were used to measure the dynamic parameters of bone formation. All sections were measured and analyzed using an Olympus microscope connected to a computer and the OsteoMeasure program (version 3.21; OsteoMetrics, Atlanta, GA, USA).
Statistical analysis
The data were analyzed by one-way ANOVA, and if this revealed a significant difference, further comparison between two groups was performed. All values were expressed as mean ± SD, and p < 0.05 was considered significant.
RESULTS
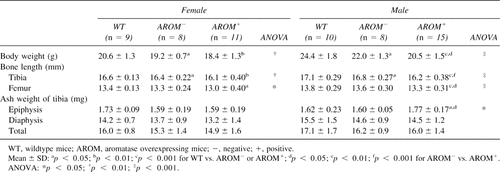
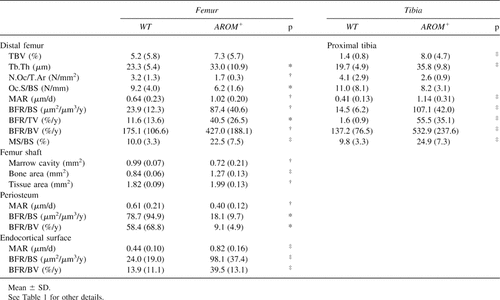
Table 1 shows body weight, bone length, and ash weight of tibias of the animals at the age of 40 days. Both body weight gain and long bone growth were retarded in AROM+ mice. AROM− animals were lighter than WT animals but clearly heavier than AROM+ animals. AROM+ mice had significantly shorter femora and tibias in both sexes compared with WT animals, but there were no differences in total ash weight. In contrast, the ash weight of tibial epiphysis was higher in male AROM+ mice than in other mouse groups. Bone ash weight showed no difference between WT and AROM− animals. All results including pQCT measurement and histomorphometric analysis showed that the differences were more marked in the males.
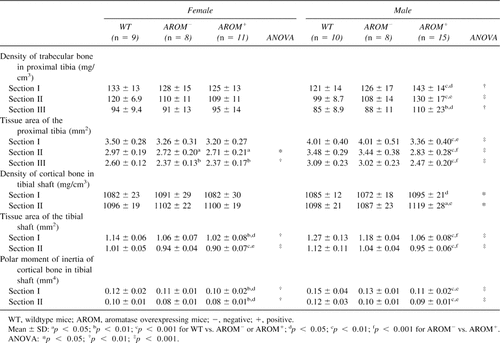
BMDs in the young mice are presented in Table 2. As expected based on ash weight data, AROM+ male mice showed increased density of both trabecular and cortical bone. The tissue area of cortical bone was markedly reduced, leading to a decreased polar moment of inertia at the tibial shaft. In female mice, there was no difference in BMD between control and AROM+ mice. However, in females also, there was a smaller tissue cross-sectional area in tibias of AROM+ mice versus the controls. In both AROM− males and females, most of pQCT measurements fell between the values in WT and AROM+ animals.
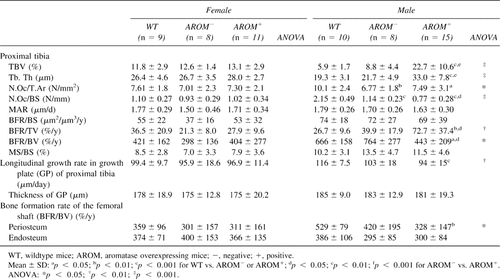
Results from bone histomorphometric analyses of young animals are presented in Table 3. Similarly to the BMD analysis, the data showed that there were significant changes in histomorphometric parameters in males but not in females. Owing to increased trabecular thickness, trabecular bone volume in the proximal tibias of AROM+ males was much higher than in the WT mice (Fig. 2A). The number of osteoclasts on the trabecular bone surface was decreased in AROM+ mice. Bone formation rate per trabecular bonevolume (BFR/BV) was significantly decreased, but it was significantly increased when calculated as the bone formation rate per tissue volume (BFR/TV). The longitudinal growth rate of the proximal tibial growth plate was decreased in AROM+ males, while there was no difference in the thickness of the growth plate between groups. The reduced bone formation rate in cortical bone was also evident after measuring the tetracycline and calcein labels in the cross-sections of the femoral shafts (Fig. 2B). BFR at the endocortical surface was equal in all animal groups. All other histomorphometric values, except number of osteoclasts, were similar between WT and AROM− animals.
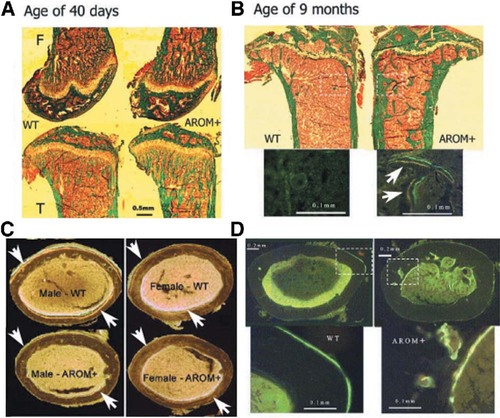
Micrographs stained using the Masson-Goldner-Trichrome method or fluorochrome labeling are from the distal femur, proximal tibia, and femoral shaft of AROM+ male mice. (A) Compared with the WT mice, trabecular bone volume was significantly increased in femora and tibias of young AROM+ mice. The thicknesses of the growth plate and calcified cartilage layer in the growth plate were not different in these two mouse groups. (B) The fluorochrome labeling patterns (white arrows; yellow: tetracycline; gray or green: calcein) in the femoral shafts of young male and female mice showed that bone formation was inhibited in the AROM+ mice both at the periosteum and at the endocortical surface. Transverse bone tissue areas were much smaller in male AROM+ mice than in WT mice. (C) In aged male mice, trabecular bone volume and thickness of the cortical bone in the proximal tibia were increased in the AROM+ animals (green area). Fluorochrome-labeled micrographs are from the white rectangles in the above pictures (green: calcein; yellow: tetracycline [white arrows]). (D) Cross-sections of the femoral shaft were taken at the same level as in young animals. Compared with WT mice, AROM+ males showed much thicker cortical bone with a higher BFR at the endocortical surface.
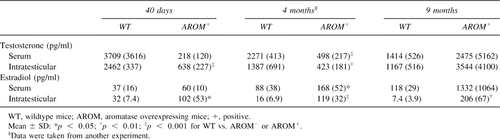
We have previously reported that 4-month-old AROM+ males present increased E2 and reduced T concentrations in serum.23 In this study, we also measured the intratesticular concentrations of E2 and T in 4-month-old animals. As indicated in Table 4, concentrations of T in the serum, as well as in intratesticular fluid, gradually decreased with age in WT mice. However, concentrations of T in AROM+ mice were much lower in young mice, but much higher in aged ones versus WT animals. On the other hand, whereas concentrations of E2 slightly increase in the serum and decrease in intratesticular fluid in male WT mice with age, E2 concentrations in male AROM+ animals were markedly increased in both the serum and intratesticular fluid. The ratio of E2 in the sera of AROM+ versus WT mice was 1.6 at the age of 40 days and 11.3 at the age of 9 months. In intratesticular fluid, it was 3.2 in young mice and 27.8 in aged ones.
Figure 3 shows the bone changes in aged, 9-month-old AROM+ male mice. In contrast to the situation found at 40 days of age, at the age of 9 months, the body weight of the AROM+ mice was increased by 21.2% versus that of WT animals. The difference in femoral length between AROM+ and WT mice did not change over more than 7 months of growth, but the difference in tibial length changed dramatically. In AROM+ males versus WT males, the tibia was 23.2% shorter at the age of 9 months, whereas it was 5.26% shorter at 40 days of age. In addition, the tibias were significantly heavier and had a higher bending strength in AROM+ males compared with the WT animals.
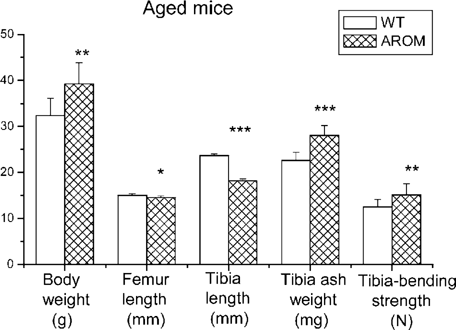
Columns show the changes in body weight, length of femur, length of tibia, tibial ash weight, and bending strength in aged mice. AROM+ animals had a significantly higher body weight with a significantly shorter tibia and femur than WT animals. The difference in tibias between AROM+ and WT mice was dramatically higher than that in femora. However, the shorter tibias of the AROM+ animals had higher ash weight and higher bending strength. Correspondent parameters of young mice are also shown here. Mean ± SD: *p < 0.05; **p < 0.01; ***p < 0.001 for WT vs. AROM+.
BMD measurements from aged males (9 months of age) are presented in Table 5. The densities of trabecular and cortical bone, cortical bone area, and polar moment of inertia of tibias were significantly higher in AROM+ mice than in WT animals. In contrast to the situation found in young AROM+ mice, no differences in tissue areas of proximal tibias or tibial shafts were found in the aged animals. In the femur, all values were markedly higher in AROM+ than in WT animals except for trabecular BMD of the distal femur.
Histomorphometric data in aged mice were consistent with the BMD measurements (Table 6). Furthermore, the data showed that, in contrast to the situation found in young mice, the BFRs at the trabecular bone surfaces and endocortical bone surfaces were significantly higher in the AROM+ mice compared with the WT mice (Figs. 2C and 2D).
DISCUSSION
Aromatization of androgens is one of the two last steps in the biosynthetic pathway of E2 formation from cholesterol. Similarly, testicular T represents a precursor of E2 production in AROM+ males, with enhanced expression of human P450 aromatase. This results in increased circulating and intratesticular E2 concentrations in AROM+ male mice (this study).23 The expression of P450 aromatase in AROM+ mice is, however, low, and no obvious reproductive phenotype has been found in AROM+ female mice, which show normal ovarian and uterine histology and are fertile. However, this study shows a clear impact of P450 aromatase expression in some of the parameters analyzed regarding the female bones at 40 days of age, such as bone growth and tissue area. This suggests that low local E2 formation as a result of the action of P450 aromatase has an impact in female bone physiology. This is in line with the results of previous studies suggesting that, in postmenopausal women and in men, P450 aromatase activity can generate physiologically significant levels of E2 locally without affecting circulating levels of E2.28, 29
As expected, the effects on AROM+ female bone physiology and structure were markedly milder than those observed in the males. AROM+ male mice have previously been shown to present severe structural and functional alterations in their reproductive tissues, such as increased circulating and intratesticular E2 to T concentration ratios combined with Leydig cell hyperplasia, gynecomastia, and undeveloped accessory sex glands.23, 24 Several of these phenotypes are also common features that are caused by perinatal estrogen exposure in males.30-32 A typical feature of fetal estrogen exposure is hypoandrogenization.33 Thus, the low level of T in young adult AROM+ males is not only a result of the enhanced conversion of T to E2, but is also a result of the fact that estrogen action in the males suppresses T production in the testis. Our recent data indicate that a putative reason for the enhanced production of T in 9-month-old AROM+ mice is the activation of testicular macrophages that could produce 25-hydroxycholesterol, this potentially being a direct substrate for T production in the Leydig cells (X Li and M Poutanen, unpublished observations, 2003).
AROM− and AROM+ mice were littermates, and their genotypes were verified by PCR. Some bone parameters of AROM− animals were significantly different from those of WT animals. This is most likely because of the fact that, during the pregnancy and suckling period of 21 days, AROM− mice got more estrogen from their AROM+ mothers than the WT mice.
In this study, we carried out detailed analysis of bone structure and properties in AROM+ male mice and observed marked differences between AROM+ and WT males. Both the young and aged AROM+ males have more trabecular bone in the metaphyseal region and a higher cortical BMD, suggesting that an increased estrogen to androgen ratio increases the bone mass in transgenic males. Estrogens are considered as “antiresorptive” agents, and they inhibit bone resorption by decreasing the activation frequency of the bone remodeling cycle (e.g., in postmenopausal women).34 However, studies with ovariectomized mice showed that low-dose estrogen treatment maintained bone mass after reduction of bone turnover,35, 36 whereas treatment with high doses of estrogens increases bone mass by increasing bone formation rate and can even lead to osteosclerosis.36-38 The AROM+ mouse model shows both of the abovementioned actions of estrogens at different periods of life. In young animals, bone mass was maintained by the enhanced antiresorptive effect of estrogens, which exceeded the estrogen-mediated suppression of bone formation. In aged mice, the effects on bone formation became profound. The reason for these differences, however, is unclear. In young adult AROM+ mice, the level of T is low and that of E2 is increased (this study).23 However, as shown in this study, circulating concentrations of T are increased at the age of 9 months, and accordingly, the level of circulating E2 is highest at this age.
Estrogen influences bone growth at various phases of skeleton maturation. In previous studies, it has been shown that both low and high doses of estrogens possess an inhibitory effect on bone growth at the growth plate in experimental animals.36, 37 Interestingly, in this study, we found that, in young mice (40 days of age), the differences in bone length between AROM+ and WT males were similar in the tibia and femur, but the tibias of aged AROM+ males were relatively much shorter than the femora. The tibias of the AROM+ males were 23.2% shorter than in WT males, whereas the femur length was only 2.7% shorter. This suggests that the tibia and femur respond differently to the hormonal changes between the ages of 40 days and 9 months. The difference could also be related to higher P450arom activity or higher estrogen receptor expression in the tibia, which remains to be studied.
Chondrocytes play an important role in longitudinal bone growth, and it has been shown previously that estrogen treatment reduces the synthetic activity of the chondrocytes.1, 39, 40 However, in this study, we found no differences in the thickness of the whole growth plate or in the thickness of calcified cartilage in the growth plate between the different study groups. Interestingly, the fluorescence double-labeled images clearly showed inhibition of the longitudinal growth rate in the primary spongiosa of the proximal tibia and distal femur of AROM+ mice compared with WT mice. Thus, the results suggest that the growth and maturation of growth plate cartilage is not affected, but the altered bone growth is a result of an effect in the primary spongiosa below and above the cartilage of the proximal tibia and distal femur, respectively.
In summary, transgenic mice bearing the human ubiquitin C promoter/human P450 aromatase fusion gene present increased estrogen biosynthesis in peripheral tissues and increased circulating estradiol levels in male mice. The changes in bone structure in these mice are consistent with the hypothesis that low-dose estrogen treatment inhibits bone resorption and maintains bone mass, whereas high doses of estrogens increase bone mass, mainly through increased bone formation.
Acknowledgements
This work was supported by the Academy of Finland, The Juselius Foundation, and TEKES, Finland.