Follistatin Restricts Bone Morphogenetic Protein (BMP)-2 Action on the Differentiation of Osteoblasts in Fetal Rat Mandibular Cells†
The authors have no conflict of interest
Abstract
We tested whether FS secretion might modulate BMP-2 actions by measuring FS levels and counting bone numbers of rat mandibular cells. In the presence of Dex, BMP-2 stimulated FS secretion at the early phase and augmented bone nodule by neutralizing with FS antibody. We concluded that BMP-2 facilitates FS secretion, and the FS restricts BMP-2 action on osteoblastogenesis.
Introduction: Bone morphogenetic proteins (BMPs) promote the differentiation of osteoprogenitor cells into osteoblasts. Activin A is involved in the regulation of bone formation. Follistatin (FS) antagonizes the bioactivities of BMP and activin A extracellularly.
Materials and Methods: In this study, we tested whether the induction of FS secretion might modulate the effects of BMP-2 on osteoblast development, using the bone nodule-forming cultures of fetal rat mandibular cells.
Results and Conclusions: In the presence of dexamethasone (Dex), BMP-2 stimulated the secretion of FS at the early phase (days 3-9) of the culture. Dex alone had no effect, and BMP-2 alone was less effective than the combination of the two. BMP-4 and -6 had little effect on FS secretion. Activin A inhibited the early upregulation of FS secretion when added with BMP-2 and Dex. In the presence of Dex, BMP-2 increased bone nodule numbers when added to early cultures. The addition of anti-FS antibody to cultures with BMP-2 and Dex augmented bone nodule formation. These results show that BMP-2 facilitates the secretion of FS in the presence of Dex, and the increased FS secretion restricts the action of BMP-2 on osteoblast differentiation.
INTRODUCTION
BONE MORPHOGENETIC PROTEINS (BMPs), members of the transforming growth factor-β (TGF-β) superfamily, are potent local factors that regulate osteoblast differentiation and function.1, 2 BMP-2, -4, and -6 promote the differentiation of osteoprogenitor cells into mature osteoblasts.3-5 The biological activities of BMPs are regulated through their interaction with several antagonistic proteins such as noggin, chordin, gremlin, and follistatin (FS).1, 2 Recent studies have shown that BMPs induce the expression of noggin and gremlin in cultured osteoblasts, suggesting negative feedback loops to regulate BMP activity in the skeletal system.6, 7 Little is known about whether or not FS regulates BMP-induced differentiation of cells in the osteoblast lineage. FS binds to BMPs and neutralizes their activities, forming a trimeric complex of FS, BMP, and the BMP receptor.8, 9 FS is expressed in osteoblastic cells both in vivo and in vitro.10-13 BMP-2 induces the expression of FS in a pre-chondroblastic cell line,14 although little information is available on osteoblastic cells.
In addition to BMPs, FS also binds to activin A, a member of the TGF-β superfamily, and neutralizes activin A bioactivities by interfering with the binding of activin A to its receptors.15, 16 Activin A is involved in the regulation of bone formation.17, 18 Activin A enhances ectopic bone formation when implanted in combination with BMPs.19 Activin A exerts divergent effects on osteoblastic cells in vitro.20-23
In this study, we tested whether the induction of FS secretion modulates the effects of BMP-2 on osteoblast differentiation using a fetal rat mandibular cell culture system. We have previously characterized this culture system and shown the presence of osteoprogenitors under conditions with a physiological concentration of dexamethasone (Dex; 10−7 or 10−8 M).24, 25 Dex exerts synergistic effects on osteoblast differentiation with BMP-2.26 We report here that, in the presence of Dex, BMP-2 causes the early accumulation of FS in the medium, while the addition of anti-FS antibody with BMP-2 and Dex augments bone nodule formation.
MATERIALS AND METHODS
Reagents and antibodies
Recombinant human (rh)BMP-2 was kindly provided by Yamanouchi Pharmaceutical (Tokyo, Japan). rhBMP-4 and -6 were kindly provided by the Genetic Institute (Cambridge, MA, USA). rhactivin A was kindly supplied by Y Eto (Ajinomoto Co., Kawasaki, Japan). Dex was purchased from Sigma (St Louis, MO, USA). Mouse anti-FS monoclonal antibody (mAb), goat anti-FS polyclonal antibody, and rhFS were purchased from R & D Systems (Minneapolis, MN, USA). The reactivity of the anti-FS mAb for rat FS was confirmed by immunostaining, a competitive ELISA, and Western blotting after immunoprecipitation. Normal mouse IgG was obtained from Chemicon (Temecula, CA, USA).
Cell isolation and culture
Cells were isolated from the fetal rat mandible with neutral protease, as described previously.24, 25 Briefly, mandibles of 20- or 21-day-old Wister King A rat fetuses were minced into fragments after removal of the condylar cartilage and connective tissue. The fragments were digested in 0.25% trypsin for 60 minutes, in 0.125% collagenase (Sigma) for 10 minutes, followed by 20 minutes, and finally in 0.25% pronase E (Sigma) for 60 minutes at 37°C. The total number of cells recovered from the final protease digestion was 8 × 104 cells. The cells from the final digestion were seeded into 6-well plates at a density of 5.6 × 103 cells/cm2 and cultured in α-MEM (Gibco BRL, Grand Island, NY, USA) supplemented with 10% FBS (Trace Scientific, Melbourne, Australia) and 100 μg/ml kanamycin sulfate. At subconfluence, 5 days after seeding, the primary cultures were subcultured by 0.003% pronase treatment into 25-cm2 flasks at 6.8 × 103 cells/cm2. After 3 days of culture, the cells were harvested and used for experiments. All cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The medium was changed every 3 days. Animal experiments were conducted in accordance with the Animal Care Guidelines of the Faculty.
Experimental conditions
Cells were seeded into 6-well plates in α-MEM supplemented with 10% FBS and kanamycin sulfate at 5.7 × 103 cells/cm2 and cultured for up to 24 days. The time of seeding was set as day 0. Test agents were added at the same time during days 3-9, the exception being rhFS, which was added 18 h after the addition of BMP-2. The medium (including test agents) was changed every 3 days. Each experiment was performed three or more times independently using cells from different mandibular preparations and each was assayed in duplicate wells.
Bone nodule and alkaline phosphatase assays
On day 24, cells were fixed with 99% ethanol for 10 minutes and stained with alizarin red-S. The number of bone nodules larger than 0.5 mm in diameter was counted with a handy colony counter, as described previously.24 Alkaline phosphatase activity was measured by the colorimetric assay using p-nitrophenol phosphate as a substrate.25
Measurement of FS
Conditioned media were collected every 3 days and concentrated by centrifugal ultrafiltration in Centricon-10 tubes (Amicon, Beverly, MA, USA). The level of FS was measured using the sandwich ELISA Quantikine (R & D systems), according to the manufacturer's protocol. This ELISA measured total FS because preincubation of the samples with excess BMP-2 or activin A had no effect on measured FS levels. The detection limit of this assay was 250 pg.
RESULTS
Effect of BMP-2 and FS on bone nodule formation
Initially, we added BMP-2 (50 ng/ml), Dex (1 × 10−8 M), or both to cultures during days 3-9 and examined bone nodule formation on day 24. BMP-2 alone and Dex alone both increased bone nodule numbers 3-fold (Fig. 1). A combination of BMP-2 plus Dex caused a 6-fold increase in bone nodule numbers (Fig. 1). The stimulatory effects of BMP-2 were dose-dependent, with one-half maximal stimulation at 50 ng/ml (data not shown). Similar results were also obtained when the test agents were added during days 3-18 (Fig. 1). Accordingly, we chose to use BMP-2 at 50 ng/ml and added the test agents during the period of days 3-9 in subsequent experiments.
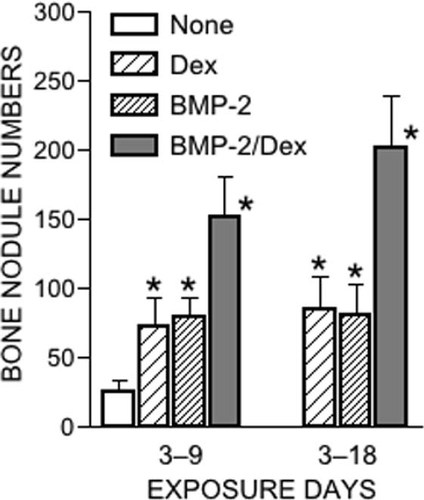
Effect of BMP-2 and Dex on bone nodule formation. Cells were treated with BMP-2 (50 ng/ml), Dex (1 × 10−8 M), BMP-2 plus Dex, or no additives (control) on days 3-9 or 3-18. Results are the means ± SD of seven independent experiments. *Significantly higher than control (p < 0.01).
The addition of FS to cultures either with BMP-2 alone or in combination with Dex caused a decrease in bone nodule numbers dose-dependently (Fig. 2A). In cultures with Dex alone or no additives whatsoever, exogenous FS addition caused no significant changes in bone nodule numbers (Fig. 2B).
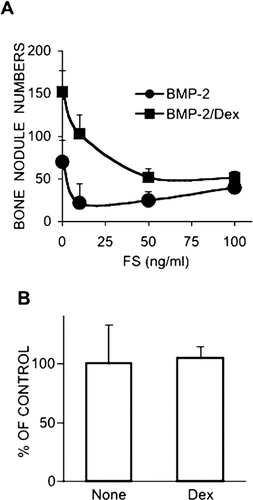
(A and B) Effect of FS on bone nodule formation. (A) Cells were exposed to different concentrations of FS in the presence of BMP-2 (50 ng/ml) alone or in combination with Dex (1 × 10−8 M) on days 3-9. FS was added 18 h after the addition of BMP-2. (B) Cells were exposed to FS (50 ng/ml) with Dex or no additives on days 3-9. In B, the number of bone nodules is expressed as percent control (without FS). Results are the means ± SD of three or more independent experiments. *Significantly lower than cultures without FS (p < 0.01).
Effect of BMP-2, -4, and -6 on FS secretion and its influence on bone nodule formation
To monitor the secretion of FS, we assayed the level of total FS protein present in the 3-day conditioned medium by ELISA. In cultures with no additives or with Dex alone, the amount of FS was low or undetectable until day 9, after which it increased steadily (Fig. 3A). Dex caused a small decrease in the FS level at late time-points. In cultures with BMP-2 alone, the FS amount began to increase on days 6-9 and reached a maximum on days 12-15 before declining (Fig. 3A). In cultures with BMP-2 plus Dex, the FS level was detectable at as early as days 3-6, increased steadily to reach a maximum on days 9-12, and then decreased slightly (Fig. 3A). On days 3-12, a combination of BMP-2 plus Dex caused the highest FS level. On days 15-18, however, BMP-2 plus Dex induced a similar FS level to that seen in the other cultures at the same point. The maximal FS levels were similar among the cultures with BMP-2 plus Dex, BMP-2 alone, and with no additives whatsoever. In contrast to BMP-2, neither BMP-4 nor BMP-6 (50 ng/ml) caused early upregulation of the FS level when added together with Dex (Fig. 3B, cf. Fig. 3A).
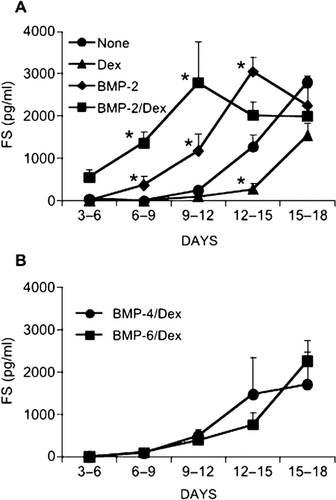
(A and B) Effect of BMP-2, -4, and -6 and Dex on FS secretion. Cells were treated with BMP-2 (50 ng/ml), Dex (1 × 10−8 M), BMP-2 plus Dex, BMP-4 (50 ng/ml) plus Dex, BMP-6 (50 ng/ml) plus Dex, or no additives on days 3-9. The levels of FS in 3-day conditioned media were assayed. Results are the means ± SD of four or more independent experiments. *Significantly different from cultures with no additives at each time-point (p < 0.01).
To examine the influence of secreted FS on bone nodule formation, we added anti-FS mAb to the cultures. As shown in Fig. 4A, anti-FS mAb caused a dose-dependent increase in bone nodule numbers when added together with BMP-2 plus Dex. In cultures with BMP-2 at different concentrations (6.25-75 ng/ml) plus Dex, anti-FS mAb shifted the BMP-2 dose-response curve to the left upside, enhancing bone nodule formation at 25 and 50 ng/ml of BMP-2 (Fig. 4B). In cultures with BMP-2 alone, Dex alone, or no additives whatsoever, however, anti-FS mAb did not cause any increase in bone nodule numbers (Fig. 4C). Similar results were obtained with a polyclonal anti-FS antibody (data not shown). Normal mouse IgG (control) did not affect bone nodule formation (Fig. 4A). In both the presence and the absence of Dex, BMP-4 and BMP-6 caused an increase in bone nodule numbers (Fig. 4C). The addition of anti-FS mAb together with BMP-4 or -6, either in the presence or the absence of Dex, caused no significant change in bone nodule numbers (Fig. 4C). In the presence of anti-FS mAb, the BMP-6 dose-response curve was unaffected (data not shown).
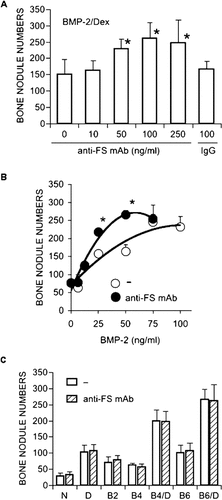
(A-C) Effect of anti-FS mAb on bone nodule formation. (A) Cells were exposed to different concentrations of anti-FS mAb with BMP-2 (50 ng/ml) plus Dex (1 × 10−8 M) on days 3-9. For the control, cells were treated with normal mouse IgG. (B) Cells were exposed to BMP-2 at different concentrations plus Dex (1 × 10−8 M) in the presence or absence of anti-FS mAb (100 ng/ml) on days 3-9. (C) Cells were exposed to anti-FS mAb (100 ng/ml) with no additives (N), Dex (D), BMP-2 (B2), BMP-4 (B4; 50 ng/ml), BMP-4 plus Dex (B4/D), BMP-6 (B6; 50 ng/ml), or BMP-6 plus Dex (B6/D) on days 3-9. Results are the means ± SD of three or more independent experiments. *Significantly higher than cultures without anti-FS mAb (p < 0.025).
We examined the expression of alkaline phosphatase, an osteoblastic cell marker induced by BMP-2. In cultures with BMP-2 and Dex, anti-FS mAb augmented the upregulation by BMP-2 of alkaline phosphatase activity on day 6 (6.3 ± 1.5 and 3.9 ± 1.0 × 103 U/minutes/μg DNA in the presence and absence of anti-FS mAb at 100 ng/ml, respectively, p < 0.05).
Effect of activin A on BMP-2-induced bone nodule formation and FS secretion
When added together with BMP-2 plus Dex, activin A at low doses (1-10 ng/ml) caused an increase in the number of bone nodules (224 ± 30 and 146 ± 23 nodules/well in the presence and absence of activin A at 1 ng/ml, respectively, p < 0.01), whereas at higher doses (50 and 100 ng/ml), it did not cause any change in bone nodule numbers (data not shown). Similarly, activin A caused a biphasic increase in bone nodule numbers when added with BMP-2 alone or no additives (data not shown). In cultures with Dex alone, activin A at both high and low doses caused an increase in bone nodule numbers (data not shown).
Both high and low doses of activin A significantly inhibited the early upregulation of FS levels when added with BMP-2 plus Dex (Fig. 5A). The addition of activin A with BMP-2 maintained the FS amount at a low or undetectable level during days 3-9 (Fig. 5B). Under these two culture conditions, anti-FS mAb caused no increase in bone nodule numbers (data not shown).
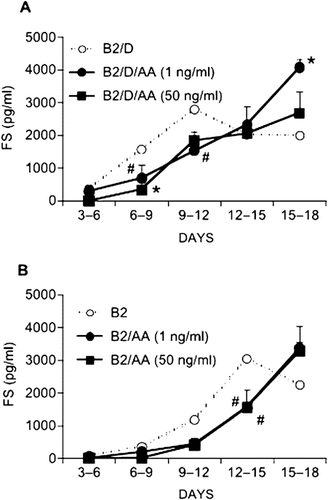
(A and B) Effect of activin A on FS secretion. Cells were treated with activin A (AA; 1 and 50 ng/ml) and BMP-2 (B2; 50 ng/ml) in the (A) presence or (B) absence of Dex (1 × 10−8 M) on days 3-9. The levels of FS in 3-day conditioned media were assayed. Results are the means ± SD of three or more independent experiments. *,#Significantly different from cultures without activin A (*p < 0.01,#p < 0.05).
DISCUSSION
In this study, we show that the induction of FS secretion by BMP-2 restricts the development of osteoblasts in fetal rat mandibular cell cultures. In the absence of BMP-2, FS secretion remained low or undetectable during the early culture periods (days 3-9), and then the levels progressively increased. The early phase represents the proliferation/initial matrix formation stages of the bone nodule formation process.24 BMP-2 stimulated the secretion of FS at the early phase of the culture in the presence of a physiological concentration of Dex. Dex alone had no effect and BMP-2 alone was less effective than the combination of the two. Thus, BMP-2 hastens FS secretion. This is consistent with the kinetics of FS gene expression during osteoblast differentiation.13 FS antagonizes the bioactivities of BMPs extracellularly.8, 9 BMP-2 effectively induced bone nodule formation when added to early cultures, consistent with other reports.4 This induction was negated by the addition of exogenous FS. The blockade of endogenous FS with anti-FS mAb augmented bone nodule formation in cultures with BMP-2 plus Dex. Similar results were observed with the use of fetal rat calvarial cells (unpublished observations). Thus, our results indicate that the early elevation of FS secretion restricts the action of BMP-2 on osteoblast differentiation. In contrast to BMP-2, neither BMP-4 nor BMP-6 facilitated FS secretion, and the addition of anti-FS mAb together with BMP-4 or BMP-6 had no effect on bone nodule formation. Thus, the restriction of osteoblast differentiation by the accelerated FS secretion seems selective to BMP-2. Others have reported a difference between the effects of BMP-2 and -6 on the induction of the BMP antagonist gremlin.7
In addition to acting as a BMP antagonist, FS neutralizes activin A activities.15, 16 FS has a higher affinity for activin A than for BMPs.9 Activin A has been shown to enhance the ectopic bone formation induced by BMP in vivo.19 Whether or not activin A in combination with BMP regulates osteoblast differentiation through FS secretion has not yet been elucidated. In this study, we show that activin A inhibits the early upregulation by BMP-2 of FS secretion. We also show that activin A causes a biphasic effect on BMP-2-induced bone nodule formation, enhancing it at low doses, but having no additive effect at high doses. The addition of anti-FS mAb with activin A plus BMP-2 did not alter bone nodule formation. Thus, our findings indicate that activin A can modulate BMP-2-induced osteoblast differentiation, independently of endogenous FS.
A common feature was the high FS secretion during the late culture periods (days 12-18), regardless of the stimulation with BMP-2, -4, and -6 or activin A. The late phase represents nodule formation and mineralization stages.24 Our findings are consistent with the upregulation of FS gene expression during the late osteoblast differentiation process.13 In contrast, other researchers have reported that FS secretion into the medium remains low throughout osteoblastogenesis in bone marrow cultures.23 This discrepancy may be attributed to differences in the origin of cells (mandible versus bone marrow) or the methodologies used. We detected FS secretion by ELISA and by Western blotting after immunoprecipitation (unpublished observations). The addition of anti-FS mAb to late cultures failed to increase bone nodule formation (unpublished observations), and the role of late FS secretion in osteoblast development remains unclear.
In conclusion, BMP-2 facilitates the secretion of FS in the presence of Dex, and this increased FS secretion restricts the action of BMP-2 on the differentiation of osteoprogenitor cells into osteoblasts. Other researchers have proposed that the BMP antagonists, noggin and gremlin, participate in negative feedback loops to regulate BMP action in the skeletal system.6, 7 Our findings also suggest a feedback regulation whereby BMP-2 stimulates FS secretion to reduce excessive BMP-2 activity. Further studies are needed on the physiological relevance of FS secretion to bone development and on the molecular basis for the regulation of FS secretion.
Acknowledgements
We thank Y Eto for providing the recombinant human activin A. We also thank Yamanouchi Pharmaceutical and the Genetics Institute for providing the recombinant human BMP-2, -4, and -6. This work was supported by Grants for Scientific Research 13672190 and 13307061 from the Ministry of Science, Education, Sports and Culture in Japan. The English used in this manuscript was revised by K Miller (Royal English Language Center, Fukuoka, Japan).




