The Influence of an Insulin-Like Growth Factor I Gene Promoter Polymorphism on Hip Bone Geometry and the Risk of Nonvertebral Fracture in the Elderly: The Rotterdam Study†
The authors have no conflict of interest
Abstract
The absence of the wildtype allele of a promoter polymorphism of the IGF-I gene is associated with increased risk (1.5; 95% CI, 1.1-2.0) of fragility fracture in women (n = 4212) but not in men (n = 2799). An approximation of hip bone geometry (from DXA) suggested the polymorphism is associated with bone strength and stability in gender-specific ways.
Introduction: Previously, we found a CA-repeat promoter polymorphism in the insulin-like growth factor I (IGF-I) gene associated with IGF-I levels and BMD in postmenopausal women, but the relationship with fractures is unclear. In this large population-based study of elderly men and women, we examined the association between this IGF-I promoter polymorphism with parameters of bone geometry and the occurrence of fractures.
Material and Methods: Within the Rotterdam Study, a prospective population-based cohort, the IGF-I polymorphism was analyzed in relation to incident nonvertebral fractures in 2799 men and 4212 women followed on average for 8.6 years. Furthermore, we estimated structural parameters of hip bone geometry indirectly from DXA outputs of the femoral neck in 2372 men and 3114 women. We studied neck width, cortical thickness, and the cortical buckling ratio and the section modulus as indexes of bone stability and bending strength.
Results: Women heterozygotes and noncarriers of the allele had, respectively, 1.2 (95% CI, 1.0-1.5) and 1.5 (95% CI, 1.1-2.0) increased risk of having a fragility fracture at older age compared with homozygotes for the 192-bp allele (p trend = 0.0007). In men, fracture risk was not influenced by the polymorphism. Compared with homozygotes for the 192-bp allele, noncarrier males had ∼1% narrower femoral necks and 2.2% lower section moduli (p trend < 0.05). Noncarrier females had 1.7% thinner cortices and 1.6% higher buckling ratios (p trend < 0.05) but no significant differences in femoral neck widths and section moduli. In women with low body mass index, genotype differences in bone strength (section modulus) and fracture risk were accentuated (p interaction = 0.05). The genotype-dependent differences in hip bone geometry did not fully explain the genotype-dependent differences in fracture risk.
Conclusions: The CA-repeat promoter polymorphism in the IGF-I gene is associated with the risk for fragility fracture at old age in women and with bone structure in both genders.
INTRODUCTION
THE ULTIMATE CLINICAL outcome of osteoporosis is fracture; its occurrence is mainly determined by the strength of bone and the risk of falling. While strength depends on bone geometry and bone material quality, the risk of falling determines the type of fall, the force of impact, and the likelihood of fracture.1, 2 In addition, the specific site of fracture follows specific epidemiological patterns that are influenced by different environmental factors.2
Family history of osteoporotic fracture is a risk factor for future fractures3; indeed, heritability of risk has been estimated to be between 25% and 35%.4, 5 For these reasons, the search for genetic determinants of fracture risk has gained evolving importance, especially after the completion of the Human Genome Project.6-8 In the general population, an individual's genetic susceptibility for fracture has been found to be associated with several common gene variants that have modest but real genetic effects.9, 10 Examples are polymorphisms in the COL1α1,11, 12 VDR,13 and ERα14 genes, all of which have been associated with the risk of fracture. The insulin-like growth factor I (IGF-I) gene has been shown to have an important role in bone metabolism.15 Plasma levels of IGF-I decrease with age both in males and females, and reduced levels have been associated with low BMD,16-18 osteoporosis,19 and fractures.20, 21 Consequently, the IGF-I gene has been considered a strong candidate to explain part of the genetic susceptibility to osteoporotic fracture.
A (CA)n microsatellite repeat polymorphism located in the 5′ promoter region of the IGF-I gene has been described previously.22-24 Earlier, we have studied this polymorphism in relation to several traits and diseases in the Rotterdam study. Birth weight,25, 26 body height, and serum levels of IGF-I after age 55 years27 have been shown to increase with the number of 192-bp alleles in the genotype for this polymorphism. With regard to osteoporosis, we found a small, but significant, gender-specific effect: the presence of the wildtype (192-bp) allele in the genotype was associated with higher BMD and lower rate of BMD decline in postmenopausal women.28 This genotypic effect on BMD suggested a relation between IGF-I activity and BMD decline caused by menopausal estrogen deficiency. Not observing this effect in men could be explained by gender differences in age-related hormonal changes affecting bone turnover rates, bone size, and geometry.
Given our previous findings on the relation of this IGF-I gene promoter polymorphism to BMD, we analyzed the polymorphism in relation to the risk of nonvertebral fracture in elderly men and women. Furthermore, we studied several parameters of hip bone geometry in relation to the risk of hip fracture and in relation to the IGF-I promoter polymorphism.
MATERIALS AND METHODS
Subjects
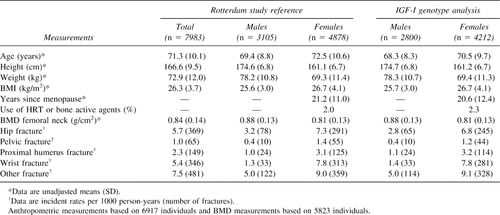
Individuals were derived from the Rotterdam Study (n = 7983), a single-center prospective population-based study of determinants of chronic disabling diseases in the elderly (≥55 years of age). Written informed consent was obtained from every participant. The design and rationale of the study has been described earlier.29 As described previously,28 we have genotyped 87.9% of the participants (n = 7012) for the polymorphism in the promoter region of the human IGF-I gene. Genotyping was not possible in the remaining participants because of technical reasons (1.5%) or the absence of blood samples for DNA isolation (10.6%).
The analysis was performed in two phases. In the first phase, we analyzed IGF-I promoter genotypes (n = 7012) in relation to nonvertebral fracture follow-up data in all genotyped individuals with complete fracture follow-up information (n = 1219 individuals with at least one fracture and 5793 individuals without fracture). In the second phase, we examined at baseline all genotyped individuals with complete BMD and anthropometric measurements for a cross-sectional analysis of hip bone geometry and fracture (n = 5506).
Genotyping
PCR using genomic DNA obtained from peripheral white blood cells was performed using oligonucleotide primers designed to amplify the polymorphic (CA)n repeat, located 1 kb upstream of the human IGF-I gene. Specific genotyping methods and quality control procedures have been described earlier.28 From sequence analysis, it is known that the allele with length 192-bp (“wildtype” in our population) corresponds to 19 CA-repeats (CA(19)).22 Based on the relationship between the polymorphism and serum IGF-I levels, genotypes were assembled from two allele categories as first described by Vaessen et al.27: the 192-bp allele and all other alleles pooled as “non-192-bp” alleles. Individuals were classified in three genotype groups: homozygotes for the 192-bp allele, heterozygotes for the 192-bp allele, and noncarriers of the allele (non-192-bp homozygotes).
Fracture follow-up
Information on incident nonvertebral fractures was collected from baseline (1990-1993) until December 31, 2002, comprising a follow-up period of 8.6 ± 3.4 years. Fracture events were retrieved from computerized records of the general practitioners (GPs) in the research area (covering 80% of the cohort). Research physicians regularly followed participant information in GP's records outside the research area and made an independent review and encoding of all reported events. Subsequently, a medical expert in the field reviewed all coded events for final classification. Additional information on hip fractures was gathered through the Dutch National Hospital Registration. We studied the relationship of the polymorphism to “all types” of fracture. Subsequently, we considered “all fragility” fractures, including hip, pelvic, proximal humerus, and wrist fractures. Furthermore, because wrist fractures occur at a considerable younger age with specific falling patterns,2 we excluded them to study the group with fragility fractures occurring at old age (mean age, >75 years). We also independently analyzed groups with fractures occurring at the hip, pelvis, proximal humerus, or wrist. Finally, we studied groups with “other” nonvertebral fractures including fractures of the rib (n = 59), sternum (n = 9), hand (n = 120), lower leg (n = 63), ankle (n = 55), metatarsus (n = 55), and foot (n = 58). Vertebral fractures were not considered in this study.
Measurements
Between 1990 and 1993, participants were invited to come to the research center for a baseline clinical examination. Height (cm) and weight (kg) were measured in standing position wearing indoor clothes and without shoes. Body mass index (BMI; kg/m2) was calculated as weight divided by the square of height. BMD measurements (g/cm2) of the proximal femur were performed by DXA using a Lunar DPX-L densitometer (Lunar Radiation, Madison, WI, USA). Methods, quality assurance, accuracy, and precision issues of the DXA measurements have been described previously.30
Bone geometry computational method
Parameters of structural geometry of the hip were calculated indirectly from conventional DXA outputs obtained from the femoral neck region of interest (ROI) and are shown in Fig. 1. Geometry parameters can be estimated from the BMD and the bone outer diameter using models of the cross-section represented by the ROI.31 The average diameter of the femoral neck was obtained by dividing the bone area by the width of the region of interest (usually 1.5 cm). To assess the reliability of this approximation of bone geometry, we compared the femoral neck diameter obtained from this calculation with that measured with the DXA ruler tool as recently described by Duan et al.,32 in 30 scans randomly selected from all 5674 available DXA scans. Femoral neck diameter approximated from bone area had a single measured percent CV of 10.6 (n = 30) and 10.8 (n = 5674) compared with 9.6 obtained when measured directly (n = 30) with the DXA ruler tool.
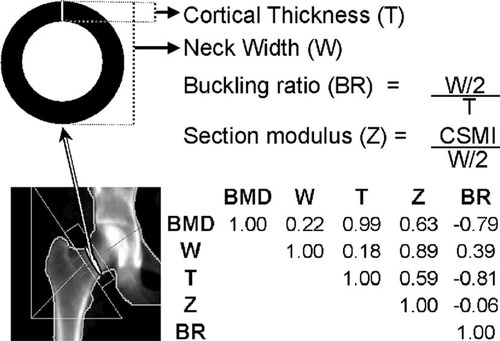
Graphical correspondence and correlations of the hip bone geometry parameters. All Pearson's correlations are significant (p < 0.00001). CSMI, cross-sectional moment of inertia.
We used BMD and femoral neck diameter to approximate femoral neck geometry parameters that are conventionally extracted directly from the mass distribution using hip structural analysis.33 The mathematical calculation and the underlying assumptions regarding the geometry and structure of bone have been reported earlier.32, 34, 35 In summary, the approximation method assumes that the bone within the femoral neck region has the configuration of a uniform right circular cylinder (tube), with a proportion of cortical mass of 60% and an effective density of hydroxyapatite in fully mineralized bone tissue of ∼1.05 g/cm3.31 As shown in Fig. 1, because the specific parameters considered in the analysis are obtained from a computational algorithm, by definition they are not independent from each other. In addition to BMD, we considered the neck width (cm) and the estimated cortical thickness (mm); the section modulus (cm3), an index of bending strength related to the radial distribution of the bone mass (computed as the ratio of the cross-sectional moment of inertia CSMI to the outer radius of the cross-section); and the cortical buckling ratio (BR), an index of bone geometrical instability (computed as the ratio of the radius of the cross-section to the cortical thickness). In engineering, this instability threshold is reached when buckling ratios exceed a factor of ∼10.36 Both geometry estimates and genotypes were obtained in a total of 5506 individuals.
Statistical analysis
Genotype and allele frequencies of the IGF-I promoter polymorphism were determined, and the genotype frequencies were tested for Hardy-Weinberg equilibrium proportions using the GENEPOP-package.37
In both phases we stratified the analyses by gender. For the analysis of fracture follow-up data, we estimated incident rates overall and by genotype. For the time-to-event or fracture-free survival analysis, we specified age (in years) as the underlying time variable instead of follow-up time to analyze the effect of chronological age on fracture. To do this we took into account delayed entry (left truncation) by using the counting process notation of S-PLUS V.6.0. We estimated cumulative probabilities of fracture events after age 55 years using the Kaplan-Meier method. Crude and adjusted hazard ratios were estimated using COX proportional-hazards models. The assumption of proportionality of hazards was verified for all covariates. Interactions between genotype and covariates were examined. In addition, the risk of fracture was analyzed in BMI strata defined by tertiles and by the following BMI definition: low (underweight; BMI < 20 kg/m2), normal (BMI = 20-25 kg/m2), and high (overweight, including obesity; BMI > 25 kg/m2). For the analysis of hip bone geometry parameters, multiple linear regression was used to compare adjusted means between individuals with and without hip fracture and to model the relation of genotype with bone geometry and anthropometric variables. Bone geometry parameters were also studied through cross-sectional strata across age decades starting at the age of 55 years and pooling together all individuals older than age 75 years to preserve sufficient power for the analysis. The influence of BMI on the relation was analyzed in strata as described previously for the risk of fracture. Adjustments were done for age, weight, and height. Trend analysis assuming an underlying additive genetic model38 was done for the presence of zero, one, or two copies of the associated allele, incorporating the genotype variable as a continuous term in a multiple linear regression model. Finally, model assumptions were verified, and model residuals were checked for goodness-of-fit. If not stated otherwise, analyses were performed using the SPSS-package V.11 (SPSS, Chicago, IL, USA).
RESULTS
Genotype frequencies of women and men in the bone geometry study (n = 5506) were very similar to those observed in all the 7012 genotyped individuals of the Rotterdam Study (44% in homozygotes, 44% in heterozygotes, and 12% in noncarriers of the 192-bp allele) and were in Hardy-Weinberg equilibrium (HWE) proportions.
Table 1 compares baseline characteristics and fracture incident rates observed during the follow-up period in women and men included in the IGF-I genotype analysis (n = 7012) with those observed in the reference population (n = 7983). On average, individuals included in the IGF-I genotype analyses were ∼2 years younger. All other characteristics did not show significant differences, including BMD levels and use of concurrent estrogen replacement therapy (HRT) or bone active agents in women. IGF-I genotypes were available for >92% and 88% of men and women with fracture. Fracture incident rates in individuals with genotypes are not significantly different from those observed in the reference population.
IGF-I genotype and fracture risk
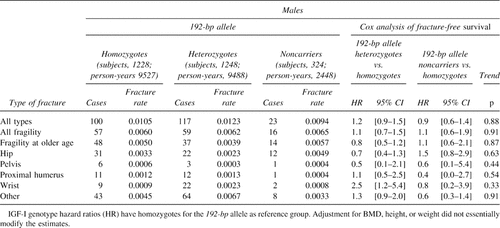
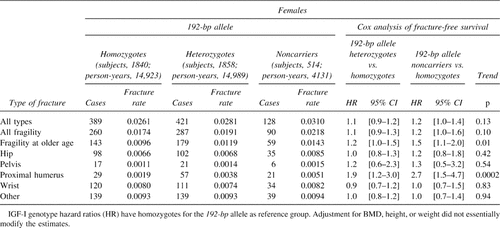
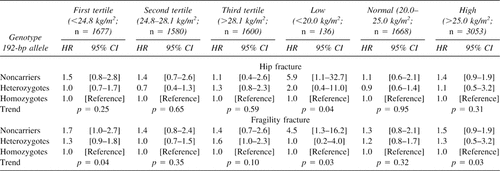
The influence of genotype on fracture rates is shown in Tables 2 and 3. In women, noncarriers of the 192-bp allele have ∼1.2 times increased risk of “all-type” and “fragility fracture” compared with homozygotes for the 192-bp allele. When pooling hip, pelvic, and proximal humerus fractures together as “fragility fractures occurring at older age,” noncarrier women have 1.5 times increased risk compared with those homozygous for the 192-bp allele. In Fig. 2A, the fracture-free survival analysis shows that this higher proportion of noncarrier women with “fragility fracture at older age” is consistent through age with a significant allele-dose effect (p = 0.007). In these three types of fracture (hip, pelvis, and proximal humerus), noncarriers tend to have higher incidence rates than their heterozygous and homozygous counterparts, with a significant allele dose effect at the proximal humerus (Tables 2 and 3). In men, there were no significant difference in fracture rates among IGF-I genotypes, as shown in Fig. 2B for fragility fractures at older age, although the genotype coefficient was not significantly different to that observed in women (p = 0.80). In both genders, no consistent fracture rate differences were observed for wrist and other types of fractures. Whether or not high-impact fractures (i.e., skull fractures; n = 40) were included in the latter group did not modify the risk estimates. Adjustment for BMD, height and weight, BMI, or hip geometry parameters did not essentially change the risk estimates (i.e., in women the risk of fragility fractures at older age was 1.45 [95% CI, 0.9, 2.1] and 1.30 [95% CI, 0.9, 1.7] for noncarriers and heterozygotes compared with homozygotes for the 192-bp allele, respectively). When evaluating interactions between the IGF-I genotype and BMI in women (Table 4), the allele-dose effect for the risk of hip and fragility fracture at older age was accentuated in the lower tertile and “low” BMI strata. The interaction between “low” BMI (<20 kg/m2) and the noncarrier genotype was significant both for hip (p = 0.04) and fragility (p = 0.05) fractures. This means that women with low BMI and who are noncarriers have an increased risk of hip and fragility fracture at older age, which is greater than that observed by the sum of having very low BMI or being a noncarrier alone. In this BMI stratum (which represents only ∼5% of the women), compared with homozygote carriers, women who don't carry any 192-bp allele have 5.9 (95% CI, 1.1-32.7) and 4.5 (95% CI, 1.3-16.2) times greater increased risk of hip and fragility (at older age) fracture, respectively (Table 4). In men, the evaluation of such interactions was limited by the small number of fractures.
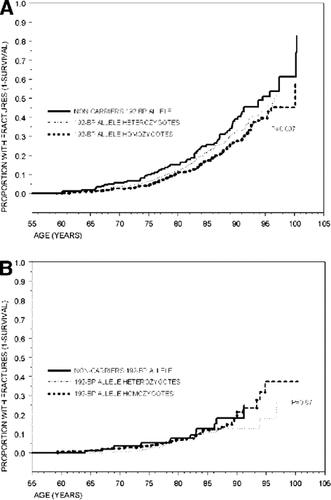
(A) Proportion of fragility (hip, pelvis, and proximal humerus) fractures with age according to IGF-I promoter genotypes in women. (B) Proportion of fragility (hip, pelvis, and proximal humerus) fractures with age according to IGF-I promoter genotypes in men.
Hip bone geometry and fracture risk
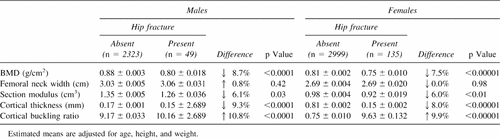
When hip bone geometry was examined in relation to the occurrence of hip fracture (Table 5) in both genders, individuals with hip fracture had on average ∼8% significantly lower BMD levels (p < 0.00001), 6% lower section moduli (p = 0.003), 9% thinner cortices (p < 0.00001), and 10% higher buckling ratios (p < 0.00001) compared with nonfractured individuals. No differences were observed in neck width. When analyzing the overall effect of age on bone geometry (data not shown), there was no apparent change in femoral neck width across age strata, whereas there was a decrease in section modulus (p < 0.00001), a decrease in cortical thickness (p < 0.00001), and an increase in cortical buckling ratio (p < 0.00001).
Hip bone geometry by IGF-I genotype
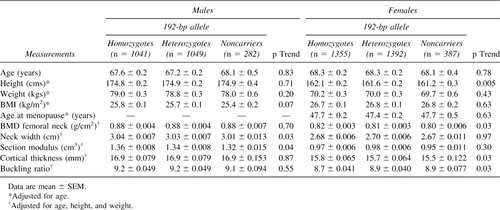
Anthropometric and bone geometry parameters were evaluated across IGF-I promoter genotypes (Table 6). Men who do not carry the 192-bp allele had significantly shorter femoral neck widths and lower section moduli with evidence for an allele dose effect. Noncarrier women had significant lower height, BMD and cortical thickness, and increased buckling ratios, also with evidence for an allele dose effect. In both genders, section modulus was 2-3% lower in noncarriers compared with homozygotes for the 192-bp allele, with mean differences that were borderline significant (p < 0.10).
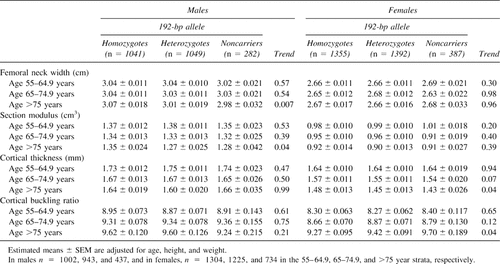
Bone geometry estimates across age strata and by IGF-I genotype are presented for men and women in Table 7. In men, the allele dose effects observed for femoral neck width and section modulus are driven by the group older than 75 years. In women, differences in cortical thickness and buckling ratio are suggested after age 65 years, but significant trends are present only in the group older than 75 years.
When evaluating bone geometry parameters in relation to different BMI levels in women, a substantial genotype effect on bone strength was observed. Genotype differences in section modulus decreased with increasing BMI (data not shown) and are accentuated in the “low” BMI stratum (p trend = 0.0003). In this stratum (n = 67), noncarriers and heterozygotes for the 192-bp allele had 30.7% (p = 0.002) and 20.0% (p = 0.02) lower section modulus compared with homozygotes for the 192-bp allele (0.93 ± 0.05 SE). Although not significant, a similar pattern was observed in men (data not shown).
DISCUSSION
This population-based study in elderly individuals showed that the absence of the wildtype (192-bp) allele in a (CA)n repeat polymorphism in the promoter region of the IGF-I gene is associated with increased risk of nonvertebral fracture in women, in particular with “fragility” fractures occurring at older age (hip, pelvis, and proximal humerus). Although no evidence for an effect of the polymorphism on fracture risk was seen in men, an effect cannot be completely ruled out because risk effect sizes were not significantly different between genders. In addition, the IGF-I promoter polymorphism was associated with hip bone geometry in gender- and age-specific ways, but the genotype-dependent differences in hip bone geometry did not fully explain the genotype-dependent differences in fracture risk.
These results on fracture and bone geometry in women are in line with our previous findings where the absence of the 192-bp allele was associated with lower BMD levels and greater rate of bone loss in women.28 In this study, female noncarriers had thinner (estimated) cortices and higher buckling ratios, especially in women older than 75 years. This pattern is consistent with the observation that noncarriers were more likely to suffer fragility. Nevertheless, the IGF-I genotype-dependent fracture risk does not seem to be mediated by genotype-dependent differences in hip bone geometry, considering that adjustment for either baseline BMD or bone geometry parameters did not essentially modify the genotype effect on the risk of fragility fracture. This could suggest that other IGF-dependent differences extrinsic to bone (i.e., effects on lean mass, cushion adiposity, neurological fitness) may be involved in the complex pathway leading to fracture. Differences in hip bone geometry are observed in men older than 75 years. Noncarriers of the 192-bp allele show wider femoral necks with higher section moduli, but consistent with the observation that buckling ratios were not higher in noncarriers, the genotype did not discriminate fragility fractures in men. This suggests that the presence of the 192-bp allele seems to cause men to have comparative wider and (resulting) stronger necks, but with no increase in cortical thickness and no (resulting) increase in stability.
One important question deals with the mechanism for achieving these effects on bone geometry. Genetically determined differences in IGF-I levels could be an explanation. As shown previously, decreased IGF-I levels are associated with decreased BMD in both genders,16-18 with gender differences that could partially be explained by IGF-I-mediated changes in sex steroid bioavailability.18, 39, 40 Similarly, it has been postulated that IGF-I levels could be involved in the sexual dimorphism observed for the changes in bone geometry with aging.41, 42 In addition, we have reported earlier that this promoter polymorphism influences the age-related decline in IGF-I levels.43 Furthermore, we have found that the presence of the 192-bp allele was associated with increased total IGF-I serum levels and with body height in a random sample of the Rotterdam Study.27 However, given the population-based approach of our association study, we cannot determine if the 192-bp promoter polymorphism is directly involved in the regulation of IGF-I expression or if it is in linkage disequilibrium with another variant in the IGF-I gene locus that affects IGF-I gene function. Therefore, the specific mechanisms underlying the functionality of the polymorphism merit further research.
Although we observed that height in women increases with the presence of the 192-bp allele as already reported,27 differences in bone strength and instability are not attributable to differential body size because the IGF-I genotype-dependent differences in bone geometry remained after adjustment for height (in addition to age and weight).
Our findings in women with low BMI (<20 kg/m2), where differences in fracture risk and bone strength (section modulus) across genotypes become more evident, suggest that differences in body weight could influence the IGF-I-mediated adaptation of bone to mechanical stimuli. This so-called “mechanosensitivity” has been thought to play a role in the development of postmenopausal osteoporosis44, 45 and is thought to be genetically determined.46 Furthermore, this relationship of decrease loading affecting IGF-I action on bone formation is in concordance with findings recently reported in mouse models where skeletal unloading was found to induce resistance to IGF-I on bone formation47 by inhibiting the activation of the IGF-I signaling pathways.48 In addition, differential production and/or activity of IGF-I will be more evident in this extreme stratum of frail women with expected low IGF-I, estrogen levels, and/or increased morbidity.
In a recent report, Ahlborg et al.35 found a relation between the extent of endocortical resorption and periosteal apposition in the distal radius of postmenopausal women using a crude approximation of bone geometry similar to the one we have used. Interestingly, their results suggest that (postmenopausal) estrogen deficiency removes a constraint on increasing periosteal apposition (i.e., bone expansion), a phenomenon that compensates for endocortical bone loss by maintaining the section modulus.41 It could be hypothesized that if IGF-I mediates bone expansion directly, differential IGF-I bioavailability across genotypes will influence bone instability and consequent fracture susceptibility in postmenopausal women. That is, after menopause, noncarriers of the 192-bp allele will reach instability faster as a consequence of rapidly expanding diameter in bones with already thin cortices. However, the structural data do not seem to support this hypothesis because femoral neck width was not detectably greater in women without the 192-bp allele, but this could be explained by measurement limitations as discussed below.
There are some limitations to our study. Although the approximation method for estimating femoral neck geometry has been used previously,32, 35 it is crude and relies on several assumptions. Nevertheless, with the exception of neck width, our bone geometry estimations show expected patterns for sex, age, and weight.41 We also show that differences in geometric parameters significantly discriminate fractured individuals. Except for neck width, our findings are consistent with previous reports using DXA,49 film radiography,50 and CT,51 which show wider necks, thinner cortices, lower strength (section moduli or cross-sectional moments of inertia), and increased instability (buckling ratios). However, it is important that our findings on bone geometry using the approximation method are verified by more direct measurements of geometry either by hip structure analysis33 or by a high-resolution 3-D method.
Another limitation of our study is that the analysis of bone geometry was cross-sectional. Thus, the relationship is weakened by the fact that most fracture events occurred on average 8.2 years later than baseline geometry measurements. Furthermore, age-specific effects on bone geometry parameters should be interpreted cautiously because this type of analysis is prone to age cohort effects. Our inability to detect subtle increases in femoral neck width through age strata (widely observed elsewhere32, 35,49, 52, 53) suggests lack of precision. This was also indicated by our substudy on 30 random scans, where we found the femoral neck width derived from bone area to be less precise than the ruler method. This lack of precision may also be the reason why we were unable to observe a relationship between femoral neck width and the occurrence of hip fracture. Similarly, a direct measurement of bone geometry within a longitudinal study design should allow a better assessment of how changes in bone geometry relate to fracture incidence.
A further limitation might be information and selection biases. We expect the bias in fracture follow-up information to be small because fracture incidence rates of our study are similar to those reported earlier in our population within shorter follow-up periods.54 Moreover, genotypes were absent in only ∼10% of all fracture cases. With regard to the BMD and geometry analysis, there could be selection bias because individuals were selected based on having complete BMD measurements and IGF-I genotypes. However, as discussed earlier,28 the effect seems to be random, because IGF-I allele frequencies were similar to those observed in other white populations.55
Considering that we have evaluated multiple endpoints in our study, multiple testing concerns could arise. Nevertheless, a more stringent significance threshold (p < 0.01) in that respect would not affect the interpretation of our fracture analysis. An argument against considering our findings prone to a type I error is the fact that the direction of the effect seems invariably consistent, in that noncarriers of the 192-bp allele show unfavorable patterns of bone geometry as well as greater fracture risk compared with homozygotes for the allele. As reported earlier for the IGF-I genotype effect on BMD,28 the relationship between the IGF-I genotype effect on bone geometry parameters is expected to be real, but modest, as for most genetic effects in complex diseases.9
In summary, this population-based study provides evidence to implicate genetically determined levels of (local or systemic) IGF-I in the complex pathway leading to fragility fracture. We found an IGF-I gene promoter polymorphism associated with increased risk of “fragility” fractures occurring at older age (hip, pelvis, and proximal humerus). Noncarriers of the 192-bp allele had 1.5 (95% CI, 1.1-2.0) times increased risk of having this type of fragility fracture. In males, no association was observed with the risk of nonvertebral fracture. Hip bone geometry was associated to the polymorphism in gender-specific ways but does not explain the IGF-I genotype effect on fracture risk. Women noncarriers of the 192-bp allele had femoral necks with thinner cortices and higher instability (buckling ratios). In men, femoral neck width and section modulus increased with the number of 192-bp (wildtype) alleles in the genotype, but no apparent differences in bone instability were observed.
Acknowledgements
This work was supported by the Netherlands Organization for Scientific Research (NWO) project 014-90-001, Oxagen Limited, and was part of Fernando Rivadeneira's MSc Training Program in Clinical Epidemiology at Pontificia Universidad Javeriana, who was partially supported by Grant 98-002-522-11 of the International Clinical Epidemiology Network (INCLEN). The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The contributions of the general practitioners and pharmacists of the Ommoord district to the Rotterdam Study are greatly acknowledged. We thank A Bertoli, T Rademaker, L Testers, J Vergeer-Drop, and R Oskamp for help in genotyping and data management.