Diagnostic delay does not influence survival of pancreatic cancer patients
Abstract
Background
Most pancreatic ductal adenocarcinoma patients present with advanced disease. Whether it is possible to increase survival by earlier diagnosis is unclear.
Objective
The purpose of this study was to investigate the association between presenting complaints and risk factors for pancreatic cancer with diagnostic delay, stage and survival.
Methods
This was a single-centre retrospective cohort study. Consecutive patients were interviewed and data on demographics, medical history, risk factors and complaints leading to pancreatic ductal adenocarcinoma diagnosis and disease stage were recorded. Diagnostic delay was considered as time between first complaint and diagnosis. Patients received appropriate treatments and their outcome was recorded in a dedicated database. The Chi-square test for comparison of categorical variables and the Mann–Whitney test for continuous variables were employed with Bonferroni corrections. Correlation between continuous variables was evaluated by means of the Spearman correlation coefficient. Survival analysis was performed with the Kaplan–Meier method and a log-rank test.
Results
The median diagnostic delay for 477 pancreatic ductal adenocarcinoma patients was two months (interquartile range 1–5), being significantly shorter for patients presenting with jaundice compared with those with pain, weight loss, diabetes (p < 0.001). The global rate of metastatic disease at diagnosis was 40%, being only 22% in those presenting with jaundice. The median diagnostic delay, however, was not significantly different among disease stages but was significantly longer in patients with a body mass index>25 kg/m2. The median survival time was seven months. Factors associated with worse survival at the multivariable analysis were older age (hazard ratio 1.02 per year), metastatic disease (hazard ratio 2.12) and pain as presenting complaint (hazard ratio 1.32), while diagnostic delay was not.
Conclusion
While some complaints are associated with a shorter diagnostic delay and less advanced disease stage, we could not demonstrate that delay is associated with survival, possibly suggesting that prevention rather than early recognition is important to tackle pancreatic cancer lethality.
Key summary
Summarise the established knowledge on this subject
- Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease, and is usually diagnosed at advanced stages not amenable to curative treatments. Most complaints caused by PDAC are unspecific and might not be recognised promptly, leading to diagnostic delay.
- A shorter delay between the first presenting complaints and the detection of the disease should help in improving the prognosis of PDAC patients, but data on the association between presenting complaints, diagnostic delay, disease stage and survival in patients with PDAC are extremely limited and heterogeneous.
- Awareness campaigns also underline the role of risk factors for PDAC occurrence, such as family history of PDAC, smoking, diabetes, overweight or obesity and excessive alcohol consumption. It is, therefore, conceivable that subjects carrying these risk factors require more prompt investigation, but to the best of our knowledge, there are no data investigating whether these factors might influence the diagnostic delay and, hence, the stage of the disease at diagnosis and its prognosis.
- The aim of this study was to investigate the association between presenting complaints of the disease, the diagnostic delay and tumour stage at time of diagnosis and survival. The secondary aim was to evaluate whether risk factors for PDAC occurrence are associated with its presentation, with the disease stage at diagnosis and with survival.
What are the significant and/or new findings of this study?
- In the largest cohort of 477 PDAC patients for a study of this kind, we found that while some presenting complaints, such as jaundice, have a lower diagnostic delay with a lower rate of metastatic disease, the hypothesis that diagnostic delay is associated with survival was rejected.
- Among the investigated risk factors for PDAC, overweight was associated with an increased diagnostic delay.
- Factors associated with worse survival at the multivariable analysis were older age, metastatic disease at diagnosis and pain as presenting complaint, while an association with diagnostic delay was not confirmed.
- These data support the view that prevention rather than early recognition is important to tackle PDAC lethality as the disease is too aggressive when complaints appear.
Background
Pancreatic ductal adenocarcinoma (PDAC) is the 12th most common cancer worldwide1 and currently the third leading cause of cancer-related death,2 but projections to 2030 estimate that it will become the second leading cause.3 The prognosis of patients with PDAC is poor, with a five-year survival rate of only about 8%.4 This is mainly due to the fact that most patients present with advanced disease not amenable to surgical resection, which remains the only potentially curative treatment.5 Indeed, outside of screening programmes that are currently limited to research protocols conducted on small populations of high-risk individuals,6,7 the disease is usually diagnosed as a result of signs and symptoms that are often unspecific and unrecognised, possibly determining diagnostic delay. The key question concerning early detection is whether or not it is possible to increase survival by identifying those patients whose complaints and risk factors suggest a diagnosis of PDAC.
The most common presenting complaints of PDAC are obstructive jaundice, abdominal pain, new-onset diabetes and weight loss.8 While jaundice is strictly related to the site of the disease in the head of the pancreas and usually leads to early referral for evaluation of the biliary tract and the pancreas, the other complaints might be subtle and often lead to other investigations or medical care that are not focused on the pancreas.9
Campaigns aimed at increasing the awareness of the population and of primary care physicians toward the initial symptoms of PDAC have been undertaken with the aim of allowing diagnosis at early stages of the disease.10 However, although one can intuitively hypothesise that a shorter delay between the first presenting complaints and the detection of the disease should help in improving the prognosis of PDAC patients, data on the association between presenting complaints, diagnostic delay, disease stage and survival in patients with PDAC are extremely limited and heterogeneous.11,12 Awareness campaigns also underline the role of risk factors for PDAC occurrence, such as family history of PDAC smoking, diabetes, overweight or obesity and excessive alcohol consumption.10,13 It is, therefore, conceivable that subjects with these risk factors require more prompt investigation, but to the best of our knowledge, there are no data investigating whether these factors might influence the diagnostic delay and, hence, the stage of the disease at diagnosis and its prognosis.
The primary aim of the present study was to investigate the association between presenting complaints of the disease, the diagnostic delay and tumour stage at time of diagnosis and survival.
The secondary aim was to evaluate whether risk factors for PDAC occurrence are associated with its presentation, with the disease stage at diagnosis and with survival.
Patients and methods
Study design
A retrospective, single centre cohort study was conducted at the Digestive and Liver Disease Unit of S. Andrea Hospital, University Sapienza of Rome, Italy. All consecutive patients with histologically confirmed PDAC, prospectively recorded between July 2005–April 2018, were enrolled upon institutional review board approval of a database of pancreatic cancer clinical data (protocol number 251/2012). Written, informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Patients were interviewed at the time of diagnosis by trained medical doctors who filled in a specific questionnaire to collect data on demographics, medical history, risk factors and symptoms or signs that led to PDAC diagnosis. No proxies were interviewed.
Investigated risk factors
The following variables were collected: sex, age, tobacco and alcohol intake, body mass index (BMI), family history of cancer and history of diabetes, as previously described.14 Ever smokers were defined as subjects reporting >6 months of smoking or >100 cigarettes smoked during their lifetime. The following data about smoking were recorded for each patient: age at smoking initiation, mean daily number of smoked cigarettes and total years of smoking. For former smokers, the number of years since quitting smoking was also recorded. Ever-alcohol drinkers were defined as such if they drank at least a mean of 12.5 g of alcohol per day for at least one year, or a lower amount for >1 year. One glass of wine, one pint (or can) of beer and one shot of hard liquor were all considered equal to one alcohol unit (∼12.5 g of alcohol). The number of mean alcohol units per day drunk by patients was recorded. Subjects were asked about the cancer history of first- and second-degree relatives’. When available, data on the number of family members with cancer, type(s) of cancer and age at cancer diagnosis were recorded.
Presenting complaints
As far as presenting complaints are concerned, the patients were asked about the very first complaint they could recall preceding the diagnosis. Specifically, they were asked whether they had experienced jaundice, weight loss (of at least 5 kg or of 5% or their usual adult weight), significant epigastric and/or back pain, new-onset diabetes (diagnosed within the 12 months before diagnosis) or other symptoms. In cases where no complaints were reported and the lesion was diagnosed during imaging procedures performed for other indications, the diagnosis was deemed incidental. Patients diagnosed during specific surveillance protocols for high-risk individuals were excluded. The symptoms and signs were considered the first complaint by the time of first presentation and not by their severity or request of first medical observation, as previously reported.15 The diagnostic delay was considered as the time between the first reported complaint and the histological diagnosis of PDAC.
Tumour stage and patients’ outcome
Data about tumour stage at diagnosis were also recorded and patients were classified accordingly as with: (a) resectable disease, (b) locally advanced or borderline resectable, (c) metastatic disease as defined previously.16 Patients received appropriate medical treatments and their outcome was recorded in a dedicated prospective database.
Statistical analysis
Continuous variables are presented as mean (±standard deviation (SD)) when the distribution is not skewed or as median and interquartile range (IQR; 25th–75th percentiles) if the distribution is skewed.
Chi square was employed for comparison of categorical variables, and the Mann–Whitney test for continuous variables with post-hoc Bonferroni correction for pairwise comparisons. Correlation between continuous variables was evaluated by means of the Spearman correlation coefficient. Overall survival (OS) was defined as the time between diagnosis and date of death. Survival analysis was performed using the Kaplan–Meier method, and the results were compared using a log-rank test. Risk factors were expressed as hazard ratio (HR) (95% confidence interval (CI)). The analysis of risk factors for prediction of survival was performed with univariable and multivariable analysis using a Cox proportional hazards regression model. The multivariable model was constructed by the ‘enter’ method, after including all variables which had significant results in the univariable analysis. Tests of statistical significance and confidence intervals were two-sided; a value of p < 0.05 was considered to be statistically significant. A dedicated software (Medcalc 12.1, Belgium) was used throughout the study.
Results
Study cohort
Of 512 consecutive patients seen in the study period, 10 (1.9%) were not histologically confirmed, seven (1.4%) refused to be interviewed and six (1.1%) were too ill to take part and provide information and were therefore excluded; 12 patients (2.3%) with a follow-up shorter than three months were also excluded. The participation rate was therefore 93%, and the final study population consisted of 477 PDAC cases. Their mean age was 68 years (±11.3), 245 (51.4%) were males. Concerning the disease stage at diagnosis, 144 (30.5%) had a resectable disease, 140 (29.5%) had a locally advanced or borderline resectable disease and the majority had a distant metastasis (190 patients, 40%).
Regarding the distribution of major risk factors for pancreatic cancer, 298 (62.5%) were ever-smokers, 283 (62.2%) had a BMI>25, 214 (44.9%) were ever-alcohol drinkers, 74 (15.5%) had a previous history of diabetes mellitus type II and 39 (8.2%) had a first degree family history of PDAC (see Table 1).
| Mean age at diagnosis (years) | 68 (±11.3) | |
| Male sex | 245/477 | (51.4%) |
| Female sex | 232/477 | (48.6%) |
| Disease stage at diagnosisa | ||
| Resectable | 144/474 | (30.4%) |
| Locally advanced/borderline resectable | 140/474 | (29.6%) |
| Distant metastasis | 190/474 | (40%) |
| Factors associated with PDAC risk | ||
| 1st degree family history of PDAC | 39/477 | (8.2%) |
| Ever-smokers | 298/477 | (62.5%) |
| Ever-alcohol drinker | 214/477 | (44.9%) |
| Previous diabetes mellitus | 74/477 | (15.5%) |
| BMI > 25 (kg/m2) | 283/455 | (62.2%) |
| Median diagnostic delay (months) | 2 (1-5) |
- BMI: body mass index; IQR: interquartile range; PDAC: pancreatic ductal adenocarcinoma; SD: standard deviation.
- Data are expressed as number (%), as mean (±SD) or as median (IQR; 25th–75th percentiles).
- a Disease stage was deemed uncertain in three cases.
Association between presenting complaints, diagnostic delay and stage
Disease presented with jaundice in 77 (16.2%) patients, with weight loss in 178 (37.6%) patients, with pain in 119 (25.1%) patients, and new onset diabetes was the first complaint in 45 (9.5%) cases. A minority of cases (26, 5.5%) reported other less specific complaints and 29 (6.1 %) had incidental diagnosis (see Table 2). When the relationship between the first presenting complaint and stage at diagnosis was analysed, the results suggested a significant association (Chi square test, p = 0.001 with contingency coefficient 0.215). In detail, when performing pairwise comparisons between the rate of patients with jaundice presenting with metastatic disease at the time of diagnosis and the other five subgroups of presenting complaints (Bonferroni correction, 0.05/5 = 0.01), the results were significantly different between the subgroup of patients with jaundice compared with those presenting with pain (p = 0.00004) or with weight loss (p = 0.0011), but not compared with those with incidental diagnosis (p = 0.44) or diabetes (p = 0.13) or other complaints (p = 0.12).
| Jaundice | Incidental | Diabetes | Other | Weight loss | Pain | Total | |
|---|---|---|---|---|---|---|---|
| Median delay | 1 (1–1) | – | 4 (3–9) | 1 (1–3.5) | 4 (2–8) | 2 (1–4) | |
| Resectable | 36 (46.8%) | 11 (37.9%) | 12 (26.7%) | 8 (30.8%) | 49 (27.6%) | 28 (23.5%) | 144 |
| Borderline/locally advanced | 24 (31.1%) | 9 (31%) | 17 (37.7%) | 8 (30.8%) | 52 (29.2%) | 30 (25.2%) | 140 |
| Metastatic | 17 (22.1%) | 9 (31%) | 16 (35.6%) | 10 (38.4%) | 77 (43.2%) | 61 (51.3%) | 190 |
| Totala | 77 | 29 | 45 | 26 | 178 | 119 | 474 |
- Data are expressed as number (%) or as median (interquartile range).
- a Disease stage was deemed uncertain in three cases.
We also performed a logistical regression analysis with metastatic disease at diagnosis as outcome and the different presenting complaints as explanatory variables and while jaundice (odds ratio (OR) 0.36; 95% CI 0.20–0.65) was associated with a decreased risk of metastatic disease, and pain with an increased risk (OR 1.84; 95% 1.21–2.80), all other presenting complaints were not significantly associated with metastatic disease.
Overall, the median diagnostic delay was two months (1–5). The median diagnostic delay (see Table 2) was different among the subgroups with different presenting complaints (incidental diagnosis were excluded from this comparison), being significantly shorter for patients presenting with jaundice (one month, IQR 1–1) when compared with those presenting with pain, weight loss, diabetes (p < 0.001 for all groups with p value set at 0.0125 with Bonferroni correction but not compared with those reporting ‘other complaints’ (p = 0.03).
The median diagnostic delay was of one month (IQR 1–4) in patients with resectable disease, being significantly shorter compared with the median of three months (1–6) in patients with a locally advanced or borderline resectable disease (p = 0.014) and of 2.5 months (1–5) in patients with distant metastases (p = 0.0021 with p value set at 0.016 with Bonferroni correction), while there was no difference between those two latter groups (p = 0.924).
Association of risk factors for pancreatic cancer with diagnostic delay
The median diagnostic delay was significantly longer in patients with a BMI > 25 kg/m2 (three months, IQR 1–6) compared with those with a BMI≤25 kg/m2 (one month, IQR 1–4; p = 0.0006), while all the other investigated risk factors were not associated with delay (see Table 3). In fact, there was no difference in terms of diagnostic delay between patients with and without family history of PDAC, nor between ever-smokers and never-smokers, ever-alcohol drinkers and never-alcohol drinkers, and patients with or without previous history of diabetes.
| Risk factor | Diagnostic delay | p Value |
|---|---|---|
| BMI > 25 | 3 (1–6) | 0.0006 |
| BMI ≤ 25 | 1 (1–4) | |
| PDAC FD family history | 3 (1–4) | 0.28 |
| No PDAC FD family history | 2 (1–5) | |
| Ever smoker | 2 (1–6) | 0.63 |
| Never smoker | 2 (1–5) | |
| Ever alcohol | 2 (1–6) | 0.06 |
| Never alcohol | 2 (1–4) | |
| Previous diabetes | 2 (1–5) | 0.62 |
| No diabetes | 2 (1–5) |
- Data are expressed as median (interquartile range).
- BMI: body mass index; FD: first degree.
Factors associated with survival
During a median follow-up of eight months (IQR 4–18), there were 369 deaths with a median survival time of seven months (IQR 3–15). The median survival was 12.5 months (4.5–28) for patients with resectable disease, 10 months (6–16.5) for those with locally advanced disease and five months (2–11) for those with metastatic disease. The median survival according to the first reported complaint was 11 months (IQR 6.75–22) for patients with an incidental diagnosis, nine months (3–18) for patients presenting with jaundice, six months (3–15) for those presenting with weight loss, 10 months (4–18) for those presenting with pain, 8.5 months (4–17) for those presenting with pain and 10 months (8–17) for those reporting other less common complaints. At the Cox regression analysis (see Table 4), factors associated with worse OS at the univariable analysis were age at diagnosis (HR 1.02 per year, 95% CI 1.01–1.03, p < 0.0001), metastatic disease at diagnosis (HR 2.17, 95% CI 1.75–2.69, p < 0.0001), pain as first presenting complaint (HR 2.24, 95% CI 1.2–4.18, p = 0.02) and BMI > 25 kg/m2 (HR 1.26, 94% CI 1.01–1.56, p = 0.03).
| Factor | Univariable analysis HR (95% CI; p value) | Multivariable analysis HR (95% CI; p value) |
|---|---|---|
| Male sex | 1.09 (0.89–1.34; p = 0.39) | – |
| Age (per increasing year) | 1.024 (1.01–1.03; p < 0.0001) | 1.027 (1.01–1.03; p < 0,0001) |
| Metastatic disease at diagnosis | 2.17 (1.75–2.69; p < 0.0001) | 2.12 (1.69–2.66; p < 0.0001) |
| CA19.9 > 37 | 1.25 (0.92–1.70; p = 0.14) | – |
| Jaundice | 1.01 (0.82–1.24; p = 0.92) | – |
| Weight loss | 1.21 (0.97–1.49; p = 0.07) | – |
| Pain | 2.24 (1.20–4.18; p = 0.02) | 1.32 (1.06–1.65; p = 0.01) |
| New onset diabetes | 0.96 (0.73–1.28; p = 0.82) | – |
| Incidental | 0.74 (0.47–1.14; p = 0.16) | – |
| 1st degree family history of PDAC | 1.01 (0.70–1.47; p = 0.92) | – |
| Ever-smoker | 0.91 (0.74–1.12; p = 0.40) | – |
| Ever-alcohol drinker | 0.90 (0.73–1.10; p = 0.32) | – |
| Previous diabetes mellitus | 1.27 (0.92–1.69; p = 0.10) | – |
| BMI > 25 (kg/m2) | 1.26 (1.01–1.56; p = 0.03) | 1.22 (0.98–1.51; p = 0.07) |
| Diagnostic delay (per month) | 1.004 (0.98–1.02; p = 0.69) | – |
- BMI: body mass index; CI: confidence interval; HR: hazard ratio; PDAC: pancreatic ductal adenocarcinoma.
At multivariable analysis, increasing age (HR 1.027 per year, 95% CI 1.01–1.03; p < 0,0001), metastatic disease at diagnosis (HR 2.12, 95% CI 1.69–2.66; p < 0.0001) and the presence of pain as presenting complaint (HR 1.32, 95% CI 1.06–1.65; p = 0.01) were confirmed to be associated with worse OS (see Figure 1). Notably, the diagnostic delay was not associated with OS. Also, there was no significant correlation between the diagnostic delay and OS (r = –0.0098; 95% CI –0.09–0.08, p = 0.82). Only 10 patients (10/577) survived more than five years (2.1%). Their median diagnostic delay was two months, being no different from that of the whole study population. Four patients presented with jaundice, four patients with weight loss and two patients with diabetes. We could not identify factors associated with this particularly favourable outcome. We also investigated the possible association between diagnostic delay and survival in the Cox regression analysis in the three subgroups of patients with different disease stage at diagnosis and this resulted in no association with survival either in the 144 patients with resectable disease (HR 1.00; 95% 0.96–1.04; p = 0.84), in the 140 patients with locally advanced (HR 0.98; 95% CI 0.94–1.02; p = 0.54), or in the 190 patients with metastatic disease (HR 0.99; 95% CI 0.96–1.02).
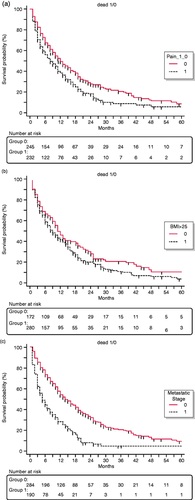
Kaplan–Meier curves highlighting the association of worse overall survival with (a) pain as first presenting complaint, (b) body mass index (BMI)>25 and (c) metastatic stage.
Discussion
Given the high mortality rate of PDAC, the reduction of the delay between the very first clinical complaint and diagnosis and treatment is considered a critical issue. Attempts to increase the awareness of the population and of primary care physicians with regard to the most common symptoms and signs of the disease have been increasingly made.10,15 However, whether such a policy can effectively lead to diagnosis at an earlier stage and to an increase in survival is unclear.
In the present study, the association between presenting complaints caused by the disease, the diagnostic delay, risk factors and outcome of consecutive patients with PDAC were investigated.
The study population included 477 patients, 40% with metastatic disease, 30% with locally advanced/borderline resectable disease and 30% with resectable disease at diagnosis. The median diagnostic delay between the very first complaint that patients recalled and diagnosis was two months. The commonest presenting complaint was weight loss which was reported by 37% of patients, followed by pain (25%) and jaundice (16%). New-onset diabetes was the first sign of the disease in 9% of patients and the diagnosis was incidental in only 6% of cases. These results suggest that most initial complaints associated with PDAC are unspecific, as the most specific and alarming sign, jaundice, was reported as initial complaint by only 16% of patients.
The primary aim of the study was to investigate whether the different presenting complaints were associated with different diagnostic delays and, hence, with different stages of disease and survival. It is, indeed, unclear whether shortening the window from the first presenting complaint to the diagnosis results in better clinical outcomes. Interestingly, while (as expected) some presenting complaints such as jaundice were associated with a significantly shorter diagnostic delay and with a lower rate of advanced disease at diagnosis, the overall survival of patients presenting with jaundice was no longer than that observed in other subgroups. The longest diagnostic delays were observed in patients reporting new-onset diabetes and weight loss with significant differences compared with jaundice, with a somehow intermediate interval when pain was the first complaint. These differences were not unexpected and similar findings have been reported by others. Porta and colleagues17 investigated 185 consecutively enrolled PDAC patients and reported that an increased symptom-to-diagnosis interval was associated with a more advanced stage at diagnosis and that patients with jaundice more often had localised disease. However, they did not investigate the survival of patients after diagnosis.
Walter et al.15 reported that there was only a non-significant difference in the diagnostic interval from the first symptom to diagnosis comparing patients without (mean delay 108 days) or with metastatic disease (mean delay 136 days; p = 0.2) at diagnosis. Also, Apollos and colleagues9 found that delays due to pre-diagnostic investigations for unspecific symptoms were not associated with worse survival.
The secondary aim of the present study was to investigate the association between the most common factors linked with the risk of developing PDAC, such as smoking, overweight, alcohol consumption, first degree family history of PDAC and previous history of diabetes, and the diagnostic delay and prognosis. Notably, overweight patients were found to have a longer diagnostic delay compared with those with normal weight. None of the other risk factors was associated with the diagnostic delay. This is, to the best of our knowledge, the first attempt to investigate this association, thus interpretation of these findings can only be speculative. It is possible both that overweight patients might take more time to seek medical attention for symptoms such as weight loss, abdominal pain and diabetes or that physicians might consider the symptoms with less promptness in overweight subjects. Also, trans-abdominal ultrasonography that is commonly performed as first-line investigation might have limited accuracy in overweight patients. As we have no data on the composition of the total diagnostic delay into the ‘patient interval’ (time from very first complaint to first presentation to a physician) and the ‘health system interval’ (time from first presentation and final diagnosis) we can only hypothesise that all of these factors might have some relevance. Notably, a BMI > 25 before symptoms onset was also a borderline significant factor associated with worse survival.
The reduction of the delay between the very first complaint reported by the patient and the diagnosis is based upon the ultimate goal of prolonging survival. Interestingly, in the present cohort of 477 patients, the factors associated with survival at the multivariable regression analysis were metastatic disease at diagnosis (HR 2.1), age at diagnosis (HR 1.02 per year) and pain as first presenting complaint (HR 1.3), while none of the other investigated factors, including the diagnostic delay, influenced survival. Pain is a frequent symptom in PDAC patients. It has a multifactorial genesis, deriving both from mechanical obstruction of the pancreatic duct by the tumour mass and by its direct contact with nerve plexus and by a specific form of neuropathic pain.18 Pain might be associated with a worse prognosis either because it is caused by the retropancreatic extension of the tumour or because neuroinvasion is an independent factor associated with worse prognosis.19
Our finding that the association between diagnostic delay and survival was not confirmed is not totally unexpected. Whether a decrease in the diagnostic delay from first complaint to diagnosis might help improving the prognosis of PDAC patients is, indeed, a debated issue. Gobbi and colleagues12 investigated the association between survival and diagnostic delay in 147 patients with PDAC and found that time to diagnosis had an influence on survival. On the contrary, Apollos et al.9 reported that delays caused by pre-diagnostic investigations due to unspecific gastrointestinal complaints did not appear to contribute to the poor prognosis of pancreatic cancer in 153 patients. Similar findings were reported by Jooste et al.20 who examined the prognosis of 345 PDAC patients and found that, similarly to what we found, jaundice and the absence of metastasis were associated with a shorter diagnostic delay. However, when they performed a survival analysis on 298 patients, after adjustment for other factors, especially disease stage, delay was not associated with prognosis.
In another very recent study, Suzuky et al.21 reported data on detection-to-diagnosis and diagnosis-to-treatment waiting times of 149 PDAC patients, finding that these intervals have no influence on the prognosis.
The present study has several strengths. It is by far the largest study on the topic, examining 477 consecutively enrolled PDAC patients seen in one centre, with available data on presenting complaints, disease features and prognosis. Also, it is the first study to investigate the possible influence of factors associated with an increased risk of pancreatic cancer both on the diagnostic delay and on the prognosis of the disease.
Among the study weaknesses is its retrospective nature, with comparisons that can only be done in an indirect manner, its design that does not allow a conclusion on the mechanisms of the observed associations, and the lack of a distinction of the total delay between the ‘patient interval’ (time from very first complaint to first presentation to a physician) and the ‘health system interval’ (time from first presentation and final diagnosis). Also, regarding the survival analysis, we could not analyse the interval from diagnosis to actual time of treatment initiation or the type of treatment due to lack of data. However, as the study was of a single centre with a certain homogeneity of treatment according to stage, we believe it is unlikely that this variable might be a bias for the present results. At any rate, as the study enrolment took place over a long time-span between 2005–2018, during which novel intensified chemotherapy regimens became available, it would have been interesting to evaluate whether the effect of diagnostic delay on prognosis is different in patients that received these novel treatments. Unfortunately, the analysis of the association between symptoms and signs, diagnostic delay and specific treatments was not possible due to lack of data and is beyond the scope of this article. Finally, the present results should be considered with caution as, although use of the first complaint is an adequate approach to understanding the presentation of these patients, this can be difficult to interpret in clinical practice, and the rejection of the hypothesis of a significant association between diagnostic delay and survival in the present analysis does not mean that this association is absent.
The present results fit well with the common feeling of experts, as recently suggested by a survey on different cancer types listing PDAC among tumours for which a mortality benefit from expedited diagnosis of symptomatic cases seems unlikely.22 PDAC is a lethal disease because, in most cases, the disease has spread to metastatic sites either macroscopically or microscopically at the time of the first complaint, posing a strong rationale for a more diffuse use of chemotherapy even in resectable cases.23 In the past few years, it has been postulated that as patients with PDAC present to their primary care physicians multiple times during the year before the diagnosis, and sometimes with unique symptoms and signs, specific tools to help primary care physicians decisions or use of screening biomarkers might result in early diagnosis and survival benefits.24 In terms of health policies and research priorities, our findings might instead support the need for primary prevention strategies tackling factors such as smoking, overweight and excessive alcohol intake, and further research on optimization of treatments. Also, as recent findings report a non-negligible rate of germline mutations in sporadic PDAC cases,25 it is possible that in the future there will be an increase in the numbers of subjects with an early diagnosis thanks to surveillance protocols that seem to guarantee increased survival.26 Although the present results should be considered with caution given the many intrinsic limitations, they suggest that prevention might still be more rewarding in the case of pancreatic cancer than relying on complaints for an early diagnosis which is rarely early enough.
Declaration of conflicting interests
The authors declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Ethics approval
The study was approved by the local institutional review board (protocol number 251/2012).
Informed consent
Written, informed consent was obtained from each patient.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.