Altered enteric expression of the homeobox transcription factor Phox2b in patients with diverticular disease
Abstract
Background
Diverticular disease, a major gastrointestinal disorder, is associated with modifications of the enteric nervous system, encompassing alterations of neurochemical coding and of the tyrosine receptor kinase Ret/GDNF pathway. However, molecular factors underlying these changes remain to be determined.
Objectives
We aimed to characterise the expression of Phox2b, an essential regulator of Ret and of neuronal subtype development, in the adult human enteric nervous system, and to evaluate its potential involvement in acute diverticulitis.
Methods
Site-specific gene expression of Phox2b in the adult colon was analysed by quantitative polymerase chain reaction. Colonic specimens of adult controls and patients with diverticulitis were subjected to quantitative polymerase chain reaction for Phox2b and dual-label immunochemistry for Phox2b and the neuronal markers RET and tyrosine hydroxylase or the glial marker S100β.
Results
The results indicate that Phox2b is physiologically expressed in myenteric neuronal and glial subpopulations in the adult enteric nervous system. Messenger RNA expression of Phox2b was increased in patients with diverticulitis and both neuronal, and glial protein expression of Phox2b were altered in these patients.
Conclusions
Alterations of Phox2b expression may contribute to the enteric neuropathy observed in diverticular disease. Future studies are required to characterise the functions of Phox2b in the adult enteric nervous system and to determine its potential as a therapeutic target in gastrointestinal disorders.
Key summary
- Diverticulitis is associated with an enteric neuropathology and alterations of the Ret/GDNF pathway.
- Phox2b is an essential regulator of Ret during enteric nervous system development.
- Expression of Phox2b was altered in enteric neurons and in enteric glial cell subpopulations in patients with diverticulitis.
- These findings suggest that Phox2b may play a role in intestinal homeostasis and that its dysregulation may be associated with gastrointestinal disorders in adulthood.
Introduction
Unlike all other peripheral organs, the gastrointestinal tract possesses its own intrinsic nervous system which directly controls digestive homeostasis and functions. This enteric nervous system (ENS) is composed at the cellular level of enteric neurons and enteric glial cells (EGCs), which both contribute to the regulation of gastrointestinal functions including the transport of ions, water and nutrients through the intestinal barrier, as well as to intestinal motility.1,2 Gastrointestinal disorders are often associated with alterations of the ENS, including enteric neurodegeneration or altered neurochemical coding.3
This is particularly the case in diverticular disease (DD), one of the most prevalent gastrointestinal disorders in the elderly in western societies.4,5 In spite of its wide distribution and potentially lethal complications, the pathogenesis of DD remains quite under-researched. In particular, aetiological factors as well as factors underlying the evolution of the disease are still largely unknown. Importantly, DD was recently shown to be associated with an enteric neuropathy including hypoganglionosis of the ENS,6 alterations of the neurochemical coding,7 as well as disturbed neurotrophic pathways such as the GDNF/Ret system.8 However, less is known about the molecular factors underlying these changes.
Regulation of enteric neuronal plasticity has mainly been studied during embryonic development. Both enteric neurons and EGCs arise from common progenitor cells, which migrate into the developing gut, proliferate and differentiate into EGCs and enteric neurons, which ultimately give rise to more than 15 different neuronal subtypes.9 Molecular pathways underlying these processes rely on complex regulatory networks, involving different signaling mediators, such as the receptor tyrosine kinase Ret and its main ligand GDNF, as well as many transcription factors.2 Among these factors, the homeobox transcription factor Phox2b is a main regulator of neuronal subtype specification. Importantly, normal ENS development strictly relies on the genetic interaction between Ret and Phox2b.10 In particular, Phox2b has been shown to regulate Ret expression directly,11 and mutations of Ret or Phox2b are associated with defective ENS development leading to aganglionosis in both mice and humans (Hirschsprung's disease). Within the ENS, Phox2b is first expressed in enteric precursor cells and its expression remains in enteric neurons and at a lower level in EGCs after differentiation. Furthermore, Phox2b directly regulates the development of serotoninergic12 and dopaminergic neurons in the peripheral nervous system.13,14
Alterations of the GDNF pathway have been shown to play important functions after birth in the ENS.15 In particular, the GDNF/Ret pathway is involved in the regulation of intestinal inflammatory disorders in adults.16 More recently, we showed that the GDNF/Ret signaling pathway is similarly altered in the myenteric plexus of patients with DD,17 indicating that alteration of the regulatory networks involved in ENS development may contribute to gastrointestinal disorders during adulthood. Although Phox2b has been identified as a candidate gene for Crohn's disease in humans,18 its expression in the human adult ENS and its potential involvement of Phox2b in DD has not been investigated so far. In this study, we first characterised the expression in the human adult ENS and further assessed its potential alterations in patients with DD.
Materials and methods
Patients and tissue source
The following patients and control groups were included in this retrospective study: patients (n = 20, eight women, 12 men, mean age 68.10 years) who underwent partial colectomy for non-obstructive colorectal carcinoma were used as controls. Anorectal evacuation and colonic motility disorders were previously excluded. Full-thickness specimens were harvested from the sigmoid colon at a safe distance (>5 cm) from the tumour. Patients who underwent sigmoid resection/left hemicolectomy (n = 20, 13 women, seven men, mean age 62.95 years) for symptomatic diverticulitis were assigned to the DD group. Surgery was carried out electively during symptom-free intervals after one or more acute events of diverticulitis. Patients with emergency surgery for peritonitis due to open perforation or with complications due to stenosis have been excluded from the study. Full-thickness specimens were harvested from sites distant to inflamed diverticula. All surgical specimens were immediately transferred to the laboratory for further processing.
Tissue processing and fluorescence immunohistochemistry
Specimens were transferred into phosphate-buffered saline (PBS) (pH 7.2) at 37°C. Full-thickness rectangular tissue blocks (30 mm × 10 mm) were pinned out flat on a cork plate by fine needles without artificial stretching or shortening thereby preserving the original size. After fixation (4% paraformaldehyde in PBS) for 24 hours and dehydration tissue blocks were transferred into paraffin wax and cut into 6 µm thick sections for immunohistochemistry.
Tissue sections of control and DD specimens were pre-treated with citrate buffer (pH 6.0, 95°C water bath) for 25 minutes followed by overnight incubation with either mouse anti-RET (Imgenex, San Diego, USA), rabbit anti-Phox2b (provided by Jean-François Brunet, Ecole Normale Paris, France), mouse anti-S100β (Millipore, Darmstadt, Germany) or mouse anti-tyrosine hydroxylase (TH) (Sigma, Munich, Germany) diluted in antibody diluent (Invitrogen, Karlsruhe, Germany) as primary antibodies. Secondary antibodies, including anti-rabbit AlexaFluor488, anti-rabbit Alexafluor546, anti-mouse AlexaFluor488 and anti-mouse Alexafluor546 (all from Life Technologies) were diluted in antibody diluent (Life Technologies) and incubated for 2 hours at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Roche, Mannheim, Germany). To correct for unspecific signals, blank controls were performed by processing adjacent sections for each tissue block omitting primary antibodies. Image acquisition was performed on a fluorescence inverted microscope (Axiovert 200 M, Zeiss, Jena, Germany) coupled to a digital camera (AxioCam MR3 (monochrome), Zeiss, Germany) under the software Axiovision (version 4.7, Zeiss, Germany). Quantitation of TH fluorescent intensity was performed as follow. Briefly, for each individual, the mean grey value of five myenteric ganglia and five corresponding blank myenteric ganglia in which primary antibody was omitted were measured using the same acquisition time and magnification parameters for each respective staining set. Background correction was performed by subtracting the mean grey value of the blank control to the corresponding target specimen. Fluorescent intensity measurement and cell number quantitation were performed using the software Fiji.19
Site-specific real-time quantitative polymerase chain reaction
For mRNA expression analyses, the muscularis propria, mucosa and submucosa were carefully isolated by manual dissection from full-thickness biopsies of the colonic wall. After preparation, specimens were immediately frozen in isopentane and stored at –70° until further processing. Myenteric ganglia were isolated using laser capture microdissection (LCM), as previously described (Böttner et al., 2010). Briefly, cryosections (14 µm thickness) were placed on membrane-coated slides (polyethylene naphtalate, 1 µm; Carl Zeiss MicroImaging GmbH, Göttingen, Germany). Myenteric ganglia were identified under a microscope (Axiovert, Zeiss) after staining with cresyl violet. For each individual, 2 mm2 of tissue from myenteric ganglia were excised by LCM and collected by laser pressure catapulting (P.A.L.M. Microlaser Technologies, Bernried, Germany) in the cap of 0.5 ml reaction tubes.
Extraction of total RNA from the muscularis propria, mucosa, submucosa or LCM-isolated myenteric ganglia was performed using the NucleoSpin Kit (Machery-Nagel, Düren) according to the manufacturer's recommendations. Reverse transcription was carried out using random hexamer primer (GE Healthcare, Freiburg, Germany), 0.5 mM dNTPs (Promega, Mannheim, Germany), 0.01 M dithiothreitol (DTT), 1 × reaction buffer, in combination with 150 U Superscript II Reverse Transcriptase (Invitrogen). The annealing, elongation, and denaturation steps were carried out at 25°C for 10 minutes, at 42°C for 50 minutes and at 70°C for 15 minutes, respectively.
Real-time quantitative polymerase chain reaction (qPCR) was performed using the qPCR Master Mix (Eurogentec, Köln, Germany) according to supplier recommendations and run on an ABI Prism 7500 fast Real-Time PCR System (Applied Biosystems, CA, USA) for 40 cycles. The housekeeping gene HPRT was used for normalisation. The following primers/probe sets were used: Hs00243679_m1 (Life Technologies) for Phox2b and TGAACGTCTTGCTCGAGATGTG, CCAGCAGGT CAGCAAAGAATTT and TGGGAGGCCATCACA TTGTAGCC for HPRT.
Statistical analyses
Statistical analysis of fluorescence quantitation and mRNA expression data were performed using the Graphpad software (PrismTM, San Diego, CA, USA). Grubbs’ test was performed to detect and exclude significant outliers from the analyses. Normal distribution was tested using the D’Agostino and Pearson omnibus normality test. Student's t-test was performed for groups following a normal distribution and the non-parametric Mann–Whitney U-test was used in other cases. Differences were considered significant for P < 0.05. All results are expressed as mean ± SEM.
Results
Expression and distribution of Phox2b in the human myenteric plexus
Expression of Phox2b was first determined on RNA isolated from human adult colonic mucosa, submucosa, muscularis propria and LCM-isolated myenteric ganglia using qPCR (Figure 1). Expression of Phox2b mRNA was detected in all compartments of the colonic wall; however, with the highest level in myenteric ganglia compared to the other layers, e.g. 96.6 ± 19.5-fold expression in comparison to colonic mucosal tissue. Distribution and localisation of Phox2b were further characterised in the myenteric plexus by immunohistochemistry in control adult colonic specimens. Co-staining for Phox2b and the pan-neuronal marker HuC/D was performed on full-thickness colonic sections (Figure 2(a–d)). We observed a clear nuclear expression of Phox2b in most HuC/D myenteric neurons. Interestingly, we also observed few HuC/D-positive neurons in which the expression of Phox2b appeared to be restricted to neuronal somata. A faint Phox2b staining could also be detected within smaller nuclei in the surrounding neurophil reminiscent of EGCs. To confirm that these cells were EGCs, we performed co-staining for Phox2b and the glial marker S100β (Figure 2(e–h)). In the ENS, S100β is essentially localised along glial processes but also stains a significant proportion of enteric glial nuclei.20 Interestingly, all S100β-positive nuclei also expressed Phox2b (Figure 2(e–h) arrows), whereas EGCs in which the expression of S100β was restricted to glial processes remained Phox2b-negative (Figure 2(e–h) arrowheads).
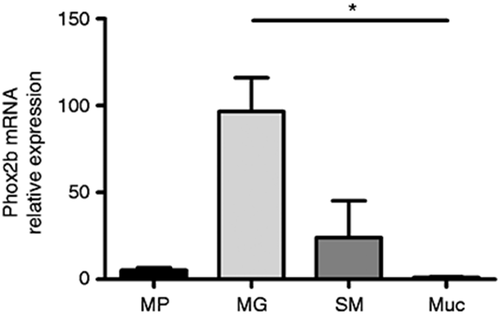
Site-specific expression of Phox2b mRNA in adult human colon. Analysis of site-specific mRNA expression profile of Phox2b in the muscularis propria (MP), in myenteric ganglia (MG) isolated by laser capture microdissection, in the submucosa (SM) and mucosa (Muc) of the adult human colon determined by quantitative polymerase chain reaction. Expression was normalised to the housekeeping gene HPRT (n = 3–4 per group; non-parametric analysis of variance followed by Dunn's post-test, P < 0.05).
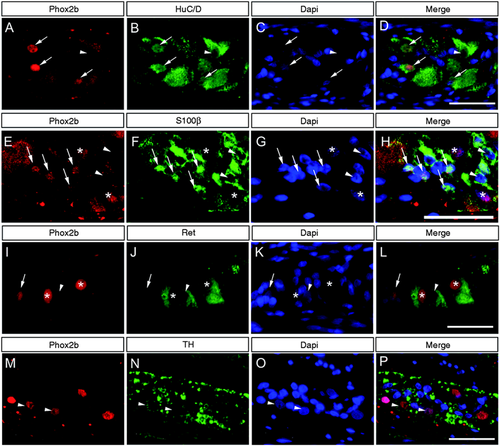
Localisation of Phox2b in myenteric ganglia of adult human colon. Co-expression of Phox2b (a, e, i, m) and the pan-neuronal marker HuC/D (b), the glial marker S100β (f) and the neuronal markers Ret (j) and tyrosine hydroxylase (TH) (n) were analysed in the adult human myenteric plexus by immunohistochemistry. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (c, g, k, o) and corresponding merges are indicated (d, h, l, p). (a–d) Most HuC/D neurons display nuclear expression of Phox2b (arrows), whereas expression of Phox2b was restricted to neuronal somata in a minority of HuC/D neurons (arrowhead). (e–h) Besides S100β-negative enteric neurons (indicated by asterisks) Phox2b expression was also observed in S100β-positive nuclei of enteric glial cells (EGCs) (arrows). EGCs in which S100β expression was restricted to glial processes did not express Phox2b (arrowheads). (i–l) Immunopositive signals for Ret were localised in neuronal somata of myenteric neurons. The large majority of Ret-positive neurons displayed a robust nuclear staining for Phox2b (asterisks), whereas weak Phox2b staining was restricted to the neuronal somata in a few Ret-positive neurons (arrowheads). The presence of Phox2b-positive neurons that were not immunoreactive for Ret was also observed (arrows). (m–p) TH immunoreactive signals were essentially localised along the neuronal processes. Few neuronal somata displayed immunopositive signals for TH (arrowheads). Scale bars = 20 µm.
Ret expression is directly regulated by Phox2b during ENS development. Although largely described as a pan-neuronal marker, we recently showed that a minority of enteric neurons do not express Ret in the human colon.21
In order to determine whether Ret expression coincides with the expression of Phox2b, we further performed co-localisation experiments for these two proteins (Figure 2(i–l)). Nuclear expression of Phox2b was observed in a large majority of Ret-positive neurons. However, expression of Phox2b remained undetectable in the nuclei of approximately 20% of Ret-positive cells. Interestingly, the expression of Phox2b was also clearly detected in a few neuronal cells that were not immunoreactive for Ret (Figure 2(i–l) arrows).
Phox2b is a main regulator of adrenergic neuron development in the peripheral nervous system. Because enteric neurons do not express dopamine beta hydroxylase, it was postulated that TH-positive neurons are mainly dopaminergic in the ENS.22,23 Thus, co-staining for TH and Phox2b was performed (Figure 2(m–p)). Although TH-positive neurons are present only in a limited number in the ENS, TH-positive neuronal somata could be detected in the myenteric plexus. From the tissue analysed, all TH-positive neurons appeared to co-express Phox2b.
Expression and distribution of Phox2b protein in patients with diverticulitis
In order to analyse the potential involvement of Phox2b in DD, we characterised its expression in LCM-isolated myenteric ganglia from patients with DD compared to controls using qPCR (Figure 3(a)). Because Phox2b is a direct regulator of Ret and Ret expression appears to be downregulated in DD,8 we hypothesised that the expression of Phox2b may similarly be diminished in colonic myenteric ganglia of patients with DD. However, Phox2b mRNA expression was significantly increased by 2.0 ± 0.4-fold in isolated myenteric ganglia of patients with DD in comparison to controls (Figure 3(a)). In order to determine which cellular compartment may contribute to this altered expression of Phox2b, protein expression of Phox2b was further analysed using immunohistochemistry in the colonic myenteric plexus of patients with DD and compared to controls (Figure 3(b–h)). Whereas the proportion of Ret-positive myenteric neurons displaying nuclear expression of Phox2b was as high as 82.4 ± 3.4% in controls, this proportion was significantly decreased to 52.6 ± 9.3% in patients with DD (Figure 3(b)). Furthermore, the expression of Phox2b appeared more prominent in the neuronal somata and the myenteric neuropil of these patients (Figure 3(c–h)). Interestingly, this expression in the neuropil of the myenteric ganglia co-localised with the glial marker S100β (Figure 4(a–f)), and the proportion of Phox2b-positive EGCs was significantly increased from 36.3 ± 3.8% in controls to 53.7 ± 5.3% in patients with diverticulitis (Figure 4(g)). Finally, the intensity of expression of TH was determined using immunohistochemistry. TH expression shows a tendency to be decreased by 24.1 ± 8.4% in patients with DD in comparison to controls (Figure 5(a–c)).
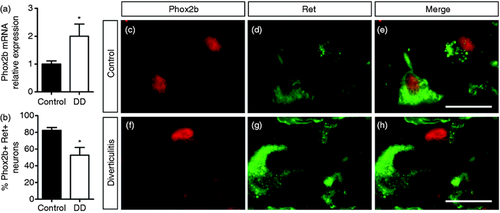
Altered expression of Phox2b in myenteric ganglia of patients with diverticular disease (DD). (a) Expression of Phox2b at mRNA level as determined by quantitative polymerase chain reaction (qPCR) analysis in laser capture microdissection (LCM)-isolated myenteric ganglia was significantly increased by 2.0 ± 0.4-fold in patients with DD compared to controls. Expression was normalised to the housekeeping gene HPRT (n = 8–10 per group; unpaired t-test, P < 0.05). (b) Quantification of the proportion of myenteric neurons co-expressing Phox2b and Ret (n = 7–8 per group; Mann–Whitney U-test; P < 0.05). (c–h) Co-expression of Phox2b (c, f) and Ret (d, g) and corresponding merges (e, h) in the colonic myenteric plexus of controls (c–e) and of patients with DD (f–h) as determined by immunohistochemistry. Scale bars = 20 µm.
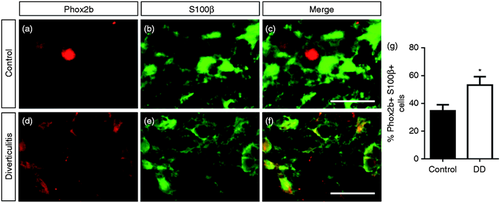
Altered co-expression of Phox2b and S100β in myenteric glial cells in patients with diverticular disease (DD). (a–f) Co-expression of Phox2b (a, d) and S100β (b, e) and corresponding merge (c, f) in myenteric ganglia of controls (a–c) and patients with DD (d–f) was determined using immunohistochemistry. Scale bars = 20 µm. (g) Quantitation of myenteric enteric glial cells (EGCs) co-expressing Phox2b and S100β (n = 7 per group; Mann–Whitney U-test; P < 0.05).
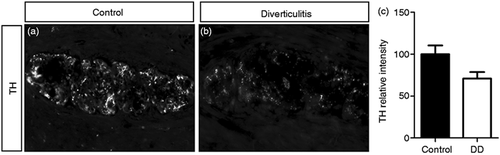
Expression of tyrosine hydroxylase (TH) in the myenteric ganglia of patients with diverticular disease (DD). (a, b) Expression of TH in the colonic myenteric plexus of controls (a) and patients with DD (b) was determined by immunohistochemistry. (c) Quantitation of TH relative signal intensity in the myenteric plexus of controls and patients with DD (n = 10–11 per group; Mann–Whitney U-test).
Discussion
DD has been associated with alterations of the ENS including hypoganglionosis, altered neurochemical coding and diminished expression of the neurotrophic factor GDNF and its receptor Ret.6-8 In an attempt to identify factors potentially underlying these alterations, we characterised in this study the expression of the homeobox transcription factor Phox2b in the human adult ENS and showed that its expression is altered in DD.
To our knowledge, no detailed characterisation of Phox2b expression has been performed in the adult human ENS so far. We could demonstrate that Phox2b is expressed in myenteric neurons of the adult human colon. Site-specific gene expression analysis revealed that the highest mRNA levels were found in the myenteric plexus compared to other compartments of the gut wall, e.g. submucosa, mucosa or muscle layers. Interestingly, although a large majority of myenteric neurons expressed Phox2b with a nuclear staining pattern, we also observed Phox2b-negative neurons as well as enteric neurons with Phox2b expression restricted to neuronal somata. The level of Phox2b expression within neuronal nuclei appeared to be of variable intensity. Although similar results have been reported in the zebrafish,24 further work is required to determine the physiological significance of this observation.
Accumulating data have helped identify Phox2b as a main regulator of Ret expression during ENS development.11,25,26 Our group recently confirmed that Ret is expressed in human adult enteric neurons, although not all neurons within the human ENS express Ret.21 Here we show that a large majority of Ret-positive neurons express Phox2b. However, a minority of Ret-positive neurons remained negative for Phox2b, and Phox2b expression was similarly observed in Ret-negative neurons, indicating that, beside Phox2b, other factors might contribute to the maintenance of Ret expression in enteric neurons during adulthood.
Phox2b is a direct regulator of Ret which has been shown to be downregulated in DD.8 In accordance with this observation, we found that the proportion of Ret-positive neurons co-expressing Phox2b was decreased in DD.
In contrast to this result, the overall gene expression level of Phox2b in myenteric ganglia was increased in DD, which may be due to the increased expression of Phox2b in the surrounding intraganglionic EGCs. Indeed, often perceived as a strict neuronal marker, evidence including initial immunohistochemistry analyses in mice and data from zebrafish have shown that Phox2b is maintained at a low level in EGCs,24,27 and we observed a faint but clear localisation of Phox2b in EGC nuclei. The presence of several EGC subpopulations has recently been reported.28 Our group and others recently demonstrated that EGCs are also altered in DD.6,29,30 Interestingly, Phox2b was expressed in a subpopulation of S100β-positive EGCs and the proportion of these cells was increased in patients with DD. Although further work is required to determine whether these subpopulations represent different functional entities, these data bring further evidence regarding the impairment of EGCs in DD.
Enteric dopaminergic neurons are important regulators of intestinal motility.31,32 Because Phox2b is an important regulator of adrenergic neuron development, we assessed whether altered Phox2b expression may be associated with modifications of this neuronal population in patients with DD. However, we observed only limited alterations of TH expression in these patients, suggesting that dopaminergic neuronal subpopulations may contribute to a lesser extent to the intestinal motility disorder observed in these patients than the alterations of other neuronal subpopulations, including the serotoninergic system.7
The succession of events leading to DD remains largely unclear, and further work is required to determine whether alteration of Phox2b expression is a cause or consequence of the pathophysiological processes taking place in DD. Nonetheless, because of its involvement in the regulation of the development of both enteric neurons and glial cells, it is tempting to speculate that the dysregulation of Phox2b may lead to the altered expression of Ret observed in DD. Whereas both alterations of the ENS6-8,33 and of the intestinal musculature34 have been identified in patients with DD, our observations reinforce the view that ENS alterations may contribute substantially to the pathogenesis of DD. Moreover, our data indicate that factors essential for the development of the ENS, such as Phox2b, may also be altered in the adult ENS thereby contributing to gastrointestinal disorders in this population.
In conclusion, our data suggest that Phox2b may play an important role in intestinal homeostasis not only during development but also during adulthood, and that its dysregulation may be associated with gastrointestinal disorders. Future studies will help to characterise fully the functions of Phox2b in the adult human ENS under physiological and pathological conditions and determine whether Phox2b and related factors could be used as potential therapeutic targets or biomarkers in these pathologies.
Acknowledgements
The authors would like to thank Karin Stengel, Inka Geurink and Bettina Facompré (Institute of Anatomy, Christian-Albrechts-University of Kiel) for their excellent technical assistance.
Declaration of conflicting interests
The authors declare that they have no conflict of interest.
Funding
This work was supported by research grants from the German Research Society (Deutsche Forschungsgemeinschaft, DFG WE 2366/4-3) and the Faculty of Medicine, University of Kiel (F356920). The funding source has no role in the study design, management of data and writing of the paper.
Ethics approval
Collection and analysis of these tissues has received approval from the local ethics committee of the Faculty of Medicine, Kiel University, Germany (B299/07, title: ‘Histological, molecular and functional characterisation of neuromuscular alterations in patients with gastro-intestinal motility disorders’, approval date 8 November 2007) in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
All patients gave their written informed consent.




